Merkel Cell Carcinoma: An Updated Review Focused on Bone and Bone Marrow Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology of MCC
4. Etiology and Risk Factors for MCC
4.1. Two (Viral- and UV-Related) Driving Mechanisms for MCC Onset
4.2. The Debate of MCC Cell of Origin
5. Clinical Features and Diagnosis of MCC
6. Staging System (AJCC Eighth Edition) and Prognostic Factors
7. Bone and Bone Marrow Metastases in MCC
7.1. Type of Bone Metastases
7.2. Pattern of Metastatic Spread and Association Between Primary MCC and BMs
7.3. Clinical and Demographic Data
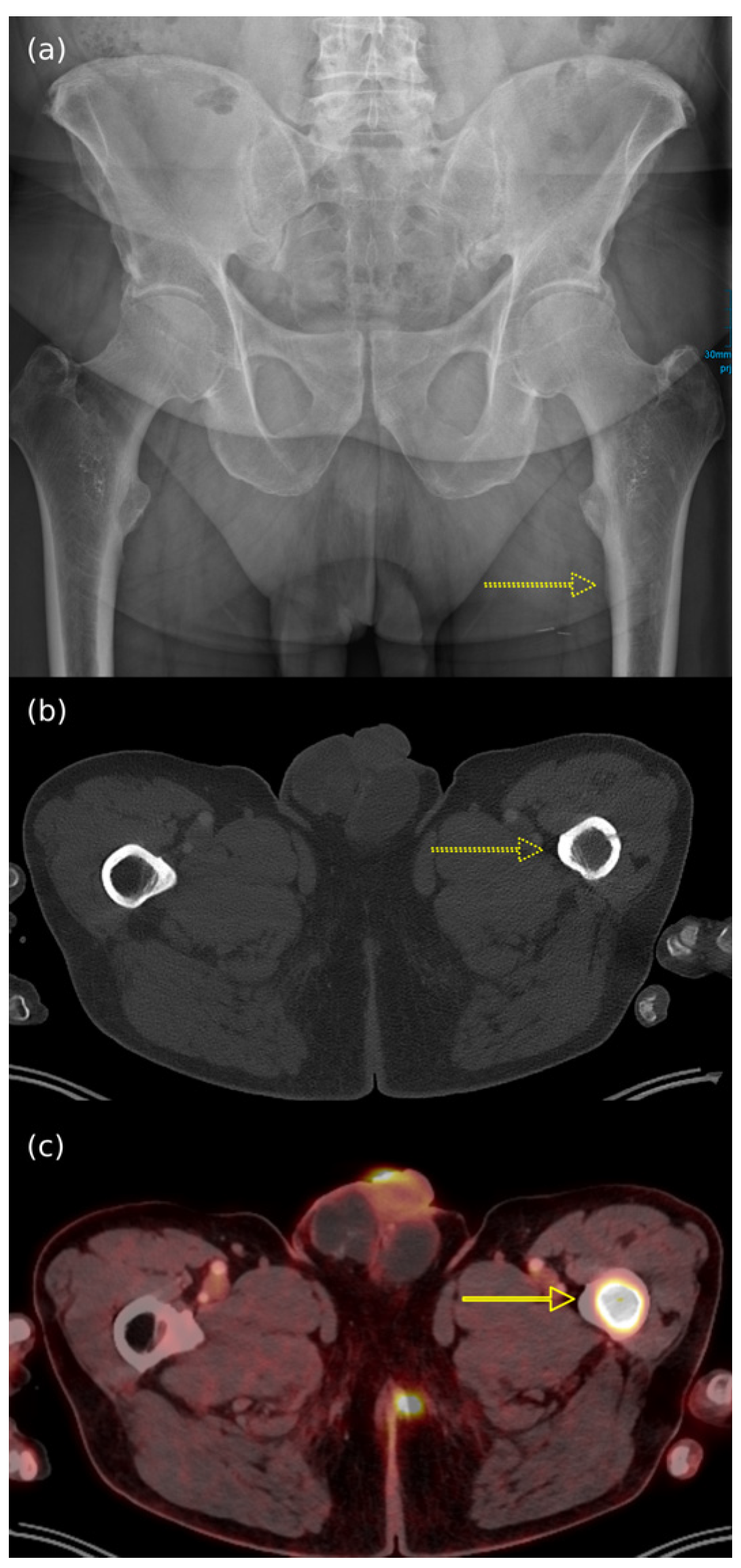
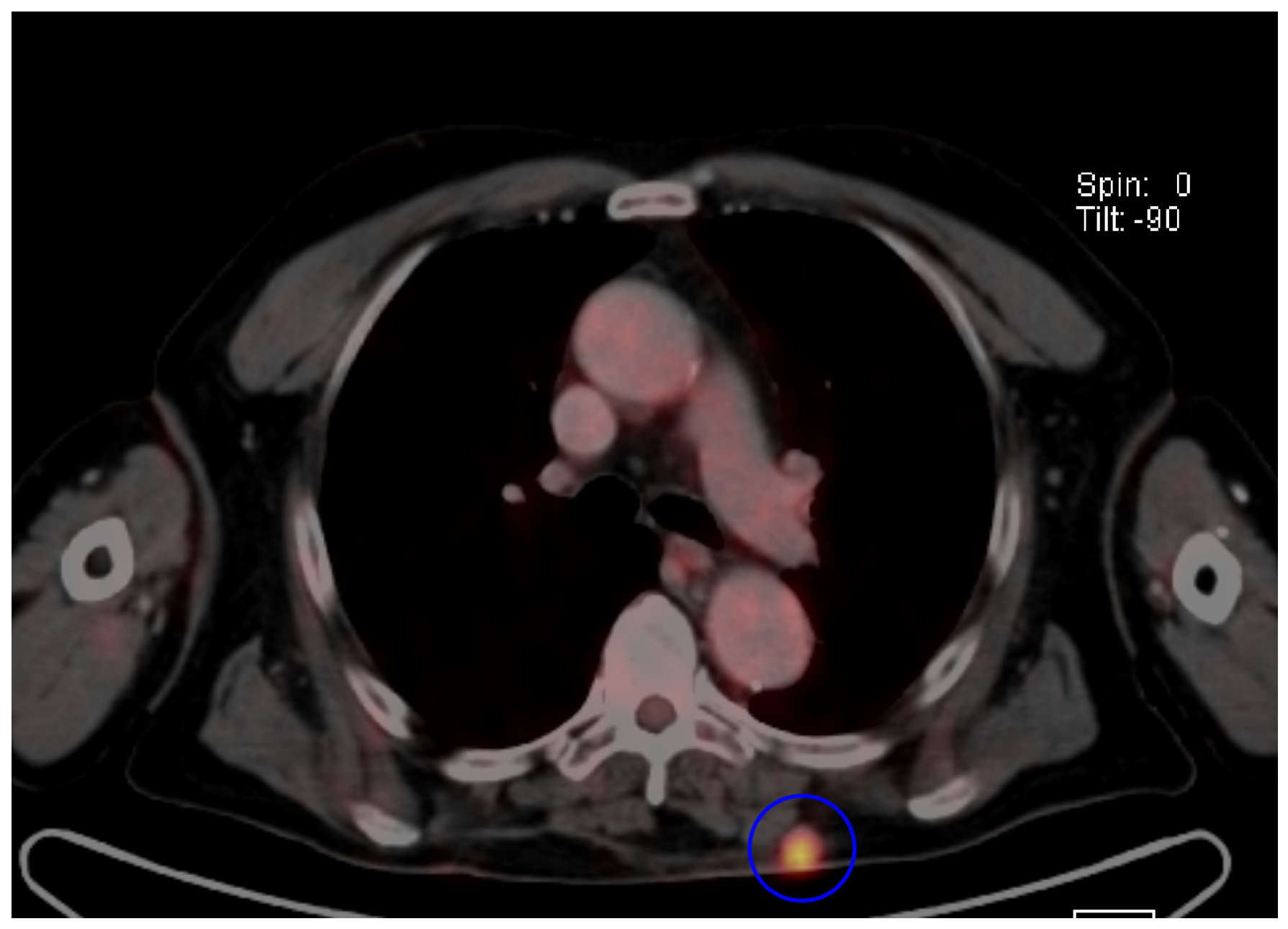
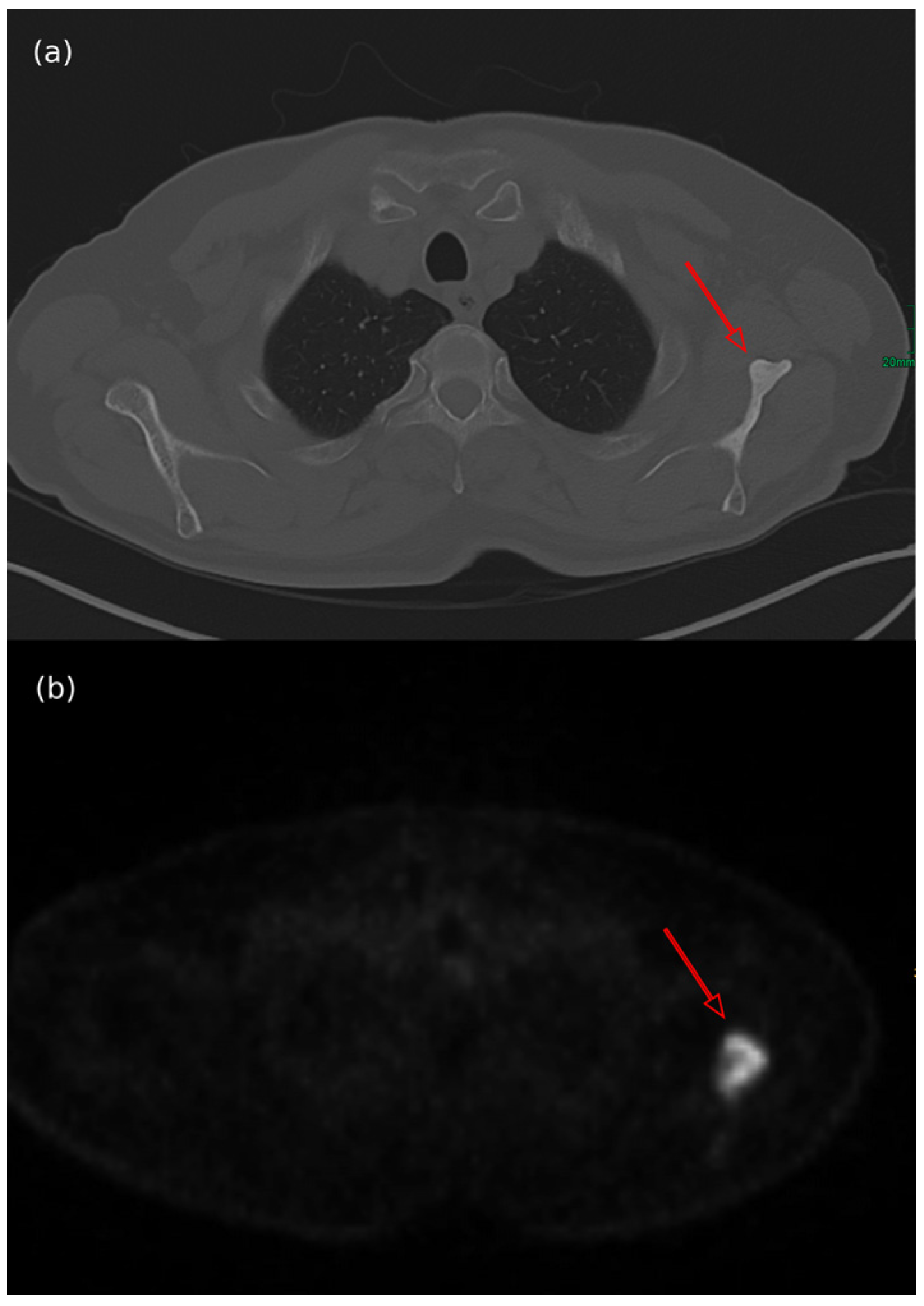
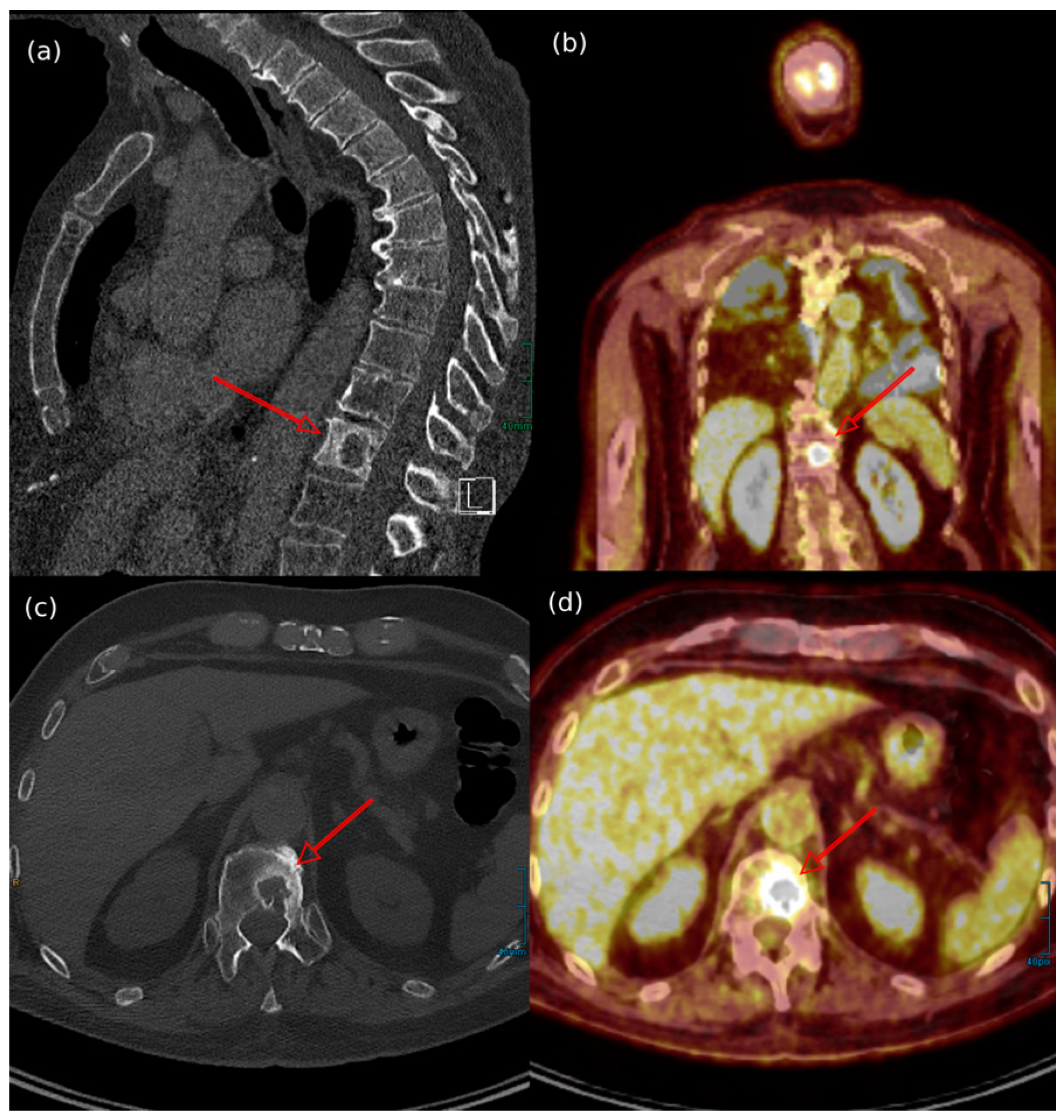
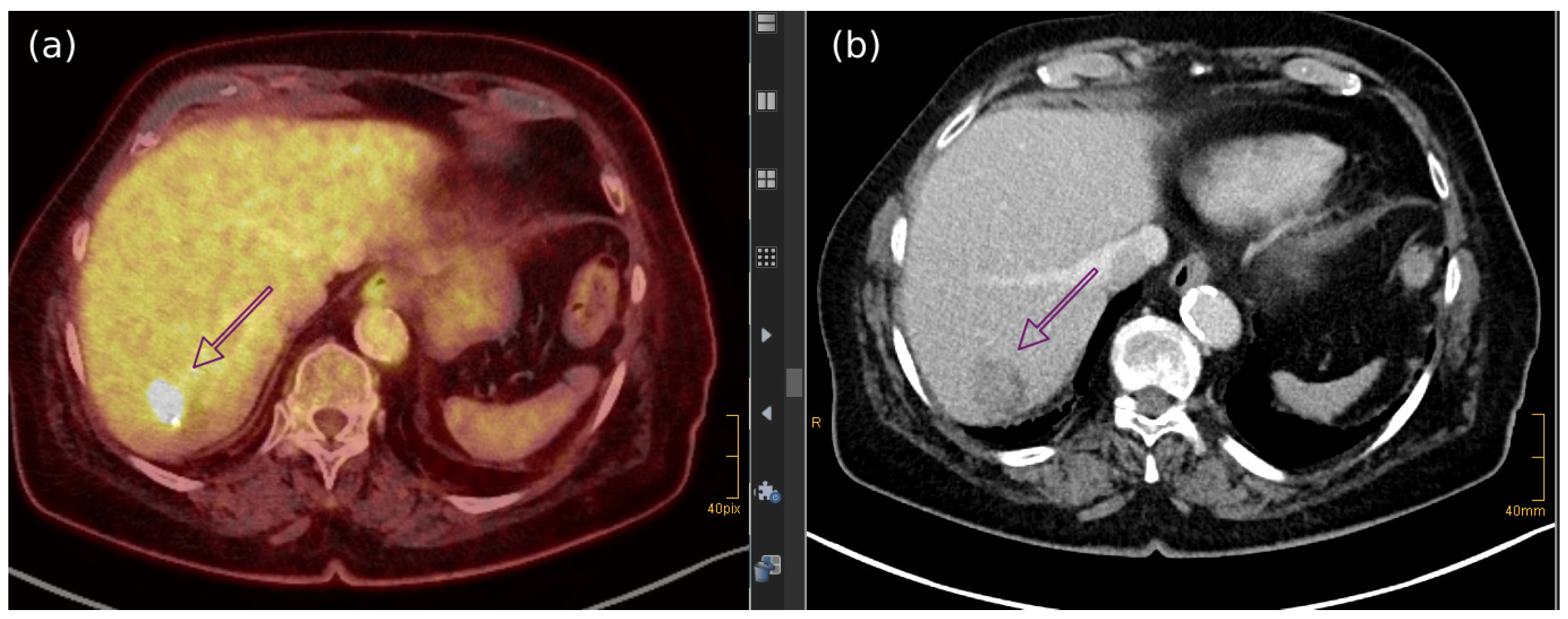
7.4. Imaging Features of MCC Across Different Diagnostic Techniques
7.5. Treatment of Metastatic Bone/Bone Marrow MCC
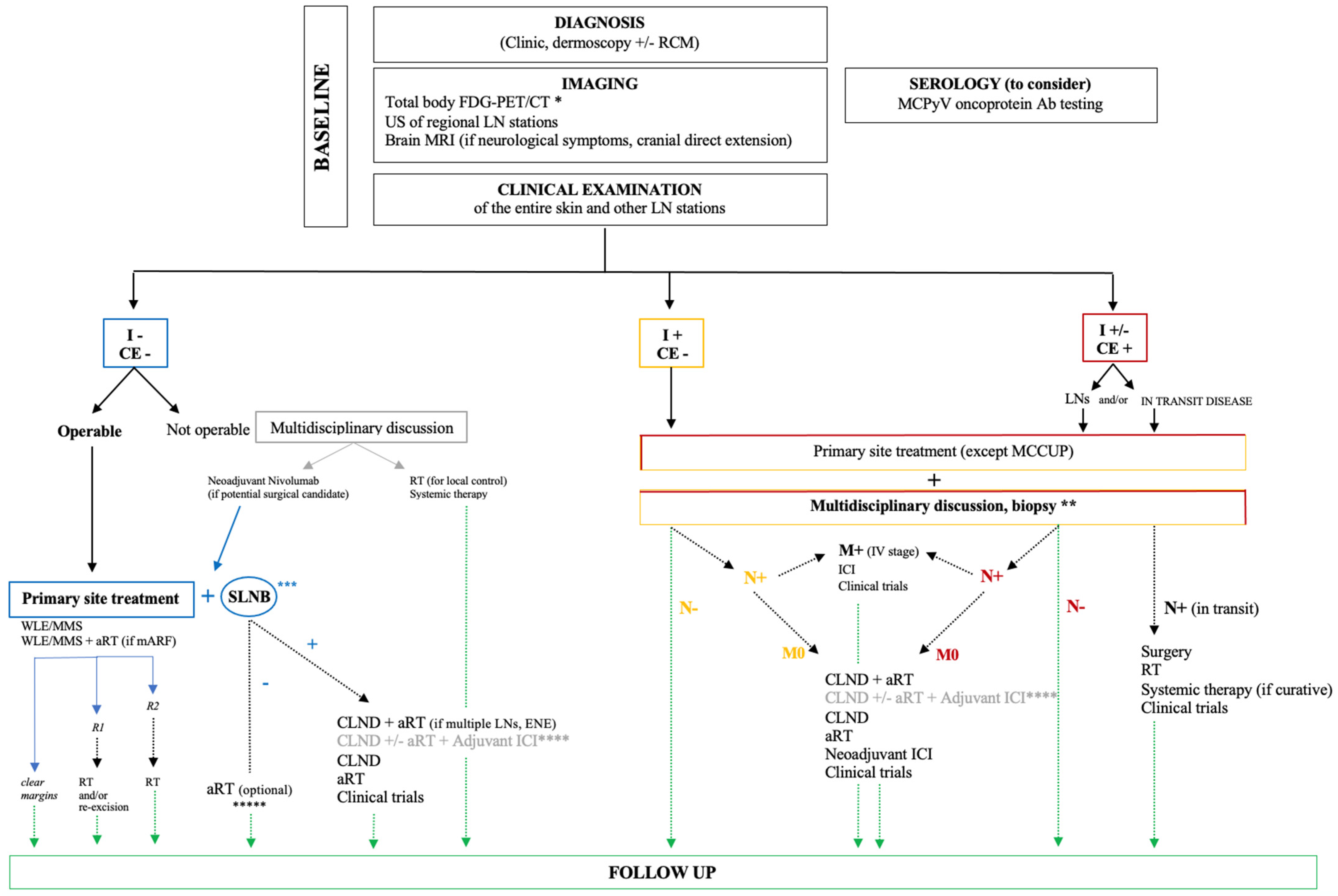
8. MCC General Management
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| ALK | Anaplastic Lymphoma Kinase |
| AKT1 | v-akt murine thymoma viral oncogene homolog 1 |
| ARID1 | AT-Rich Interactive Domain-Containing Protein 1 |
| ATM | Ataxia-Telangiectasia Mutated |
| ASXL1 | Additional Sex Combs-Like 1 |
| BCOR | BCL6 Corepressor |
| BRCA1/2 | Breast Cancer 1/2 |
| CDX2 | Caudal-type Homeobox 2 |
| DNA | Deoxyribonucleic Acid |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| EMA | European Medicines Agency |
| EP400 complex | E1A-binding protein p400 complex |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FAT1 | FAT Atypical Cadherin 1 |
| FDA | Food and Drug Administration |
| GATA3 | GATA Binding Protein 3 |
| Gy | Gray |
| HRAS | Harvey Rat Sarcoma Viral Oncogene Homolog |
| HIV | Human Immunodeficiency Virus |
| HMB45 | Human Melanoma Black 45 |
| HR | Hazard Ratio |
| ICI | Immune Checkpoint Inhibitor |
| IQR | Interquartile Range |
| ISL1 | ISL LIM Homeobox 1 |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| KMT2 | Lysine Methyltransferase 2 |
| KRT | Keratin |
| LSD1 | Lysine-Specific Demethylase 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MDM2 | Mouse Double Minute 2 homolog |
| MSH2 | MutS Homolog 2 |
| MYCL | v-myc avian myelocytomatosis viral oncogene lung carcinoma-derived homolog |
| NCCN | National Comprehensive Cancer Network |
| NKX3.1 | NK3 Homeobox 1 |
| NOTCH1 | Notch homolog 1 |
| PAX5 | Paired Box 5 |
| Piezo2 | Piezo-type mechanosensitive ion channel component 2 |
| PD-1/-L1 | Programmed Death-1/Programmed Death-Ligand 1 |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PI3K | Phosphoinositide 3-Kinase |
| POU3F2 | POU Class 3 Homeobox 2/BRN2 |
| PRAME | Preferentially Expressed Antigen in Melanoma |
| SATB2 | Special AT-rich Sequence-Binding Protein 2 |
| SD | Standard Deviation |
| SMARCA4 | SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin Subfamily A, Member 4 |
| SOX | SRY-related HMG-box family of transcription factors |
| STIR | Short Tau Inversion Recovery |
| SUV | Standardized Uptake Value |
| TdT | Terminal deoxynucleotidyl Transferase |
| VP1 | Viral Protein 1 |
References
- Dika, E.; Pellegrini, C.; Lambertini, M.; Patrizi, A.; Ventura, A.; Baraldi, C.; Cardelli, L.; Mussi, M.; Fargnoli, M.C. Merkel cell carcinoma: An updated overview of clinico-pathological aspects, molecular genetics and therapy. Eur. J. Dermatol. 2021, 31, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bolotin, D.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; et al. NCCN® Guidelines Insights: Merkel Cell Carcinoma, Version 1.2024. J. Natl. Compr. Cancer Netw. 2024, 22, e240002. [Google Scholar] [CrossRef] [PubMed]
- Lugowska, I.; Becker, J.C.; Ascierto, P.A.; Veness, M.; Blom, A.; Lebbe, C.; Migliano, E.; Hamming-Vrieze, O.; Goebeler, M.; Kneitz, H.; et al. Merkel cell carcinoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2024, 9, 102977. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.L.; Aristei, C.; Becker, J.C.; Blom, A.; Bataille, V.; Dreno, B.; Del Marmol, V.; Forsea, A.M.; Fargnoli, M.C.; Grob, J.J.; et al. Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline—Update 2022. Eur. J. Cancer 2022, 171, 203–231. [Google Scholar] [CrossRef]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/explorer/ (accessed on 23 December 2024).
- Lewis, C.W.; Qazi, J.; Hippe, D.S.; Lachance, K.; Thomas, H.; Cook, M.M.; Juhlin, I.; Singh, N.; Thuesmunn, Z.; Takagishi, S.R.; et al. Patterns of distant metastases in 215 Merkel cell carcinoma patients: Implications for prognosis and surveillance. Cancer Med. 2020, 9, 1374–1382. [Google Scholar] [CrossRef]
- Kim, E.Y.; Liu, M.; Giobbie-Hurder, A.; Bahar, F.; Khaddour, K.; Silk, A.W.; Thakuria, M. Patterns of initial distant metastases in 151 patients undergoing surveillance for treated Merkel cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1202–1212. [Google Scholar] [CrossRef]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef]
- Li, Z.; Ji, W.; Hu, Q.; Zhu, P.; Jin, Y.; Duan, G. Current status of Merkel cell carcinoma: Epidemiology, pathogenesis and prognostic factors. Virology 2024, 599, 110186. [Google Scholar] [CrossRef]
- Mohsen, S.T.; Price, E.L.; Chan, A.W.; Hanna, T.P.; Limacher, J.J.; Nessim, C.; Shiers, J.E.; Tron, V.; Wright, F.C.; Drucker, A.M. Incidence, mortality and survival of Merkel cell carcinoma: A systematic review of population-based studies. Br. J. Dermatol. 2024, 190, 811–824. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; Schrama, D.; Ugurel, S. Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates. Am. J. Clin. Dermatol. 2024, 25, 541–557. [Google Scholar] [CrossRef]
- Paulson, K.G.; Nghiem, P. One in a hundred million: Merkel cell carcinoma in pediatric and young adult patients is rare but more likely to present at advanced stages based on US registry data. J. Am. Acad. Dermatol. 2019, 80, 1758–1760. [Google Scholar] [CrossRef]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef]
- Scotti, B.; Vaccari, S.; Maltoni, L.; Robuffo, S.; Veronesi, G.; Dika, E. Clinic and dermoscopy of genital basal cell carcinomas (gBCCs): A retrospective analysis among 169 patients referred with genital skin neoplasms. Arch. Dermatol. Res. 2024, 316, 307. [Google Scholar] [CrossRef] [PubMed]
- Rapparini, L.; Alessandrini, A.; Scotti, B.; Dika, E. Invasive penile glans Squamous Cell Carcinoma (peSCC) and Reflectance Confocal Microscopy (RCM): Is it a valuable alternative to histopathology? Skin Res. Technol. 2024, 30, e13564. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Rodríguez-Perálvarez, M.L.; Pageaux, G.P.; Sanchez-Fueyo, A.; Feng, S. Liver transplantation immunology: Immunosuppression, rejection, and immunomodulation. J. Hepatol. 2023, 78, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Jallah, B.P.; Kuypers, D.R.J. Impact of Immunosenescence in Older Kidney Transplant Recipients: Associated Clinical Outcomes and Possible Risk Stratification for Immunosuppression Reduction. Drugs Aging 2024, 41, 219–238. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Kervarrec, T.; Appenzeller, S.; Samimi, M.; Sarma, B.; Sarosi, E.M.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Blom, A.; Benethon, N.; et al. Merkel Cell Polyomavirus–Negative Merkel Cell Carcinoma Originating from In Situ Squamous Cell Carcinoma: A Keratinocytic Tumor with Neuroendocrine Differentiation. J. Investig. Dermatol. 2022, 142 Pt A, 516–527. [Google Scholar] [CrossRef]
- Tolstov, Y.L.; Pastrana, D.V.; Feng, H.; Becker, J.C.; Jenkins, F.J.; Moschos, S.; Chang, Y.; Buck, C.B.; Moore, P.S. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 2009, 125, 1250–1256. [Google Scholar] [CrossRef]
- Goh, G.; Walradt, T.; Markarov, V.; Blom, A.; Riaz, N.; Doumani, R.; Stafstrom, K.; Moshiri, A.; Yelistratova, L.; Levinsohn, J.; et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016, 7, 3403–3415. [Google Scholar] [CrossRef]
- Tsai, K.Y. The Origins of Merkel Cell Carcinoma: Defining Paths to the Neuroendocrine Phenotype. J. Investig. Dermatol. 2022, 142, 507–509. [Google Scholar] [CrossRef]
- Nirenberg, A.; Steinman, H.; Dixon, J.; Dixon, A. Merkel cell carcinoma update: The case for two tumours. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Samimi, M.; Guyétant, S.; Sarma, B.; Chéret, J.; Blanchard, E.; Berthon, P.; Schrama, D.; Houben, R.; Touzé, A. Histogenesis of Merkel Cell Carcinoma: A Comprehensive Review. Front. Oncol. 2019, 9, 451. [Google Scholar] [CrossRef]
- Samimi, M.; Kervarrec, T.; Touze, A. Immunobiology of Merkel cell carcinoma. Curr. Opin. Oncol. 2020, 32, 114–121. [Google Scholar] [CrossRef]
- Miller, R.W.; Rabkin, C.S. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol. Biomark. Prev. 1999, 8, 153–158. [Google Scholar]
- Howard, R.A.; Dores, G.M.; Curtis, R.E.; Anderson, W.F.; Travis, L.B. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; McKee, P.H.; Mayer, R.J. Merkel cell carcinoma. J. Clin. Oncol. 2002, 20, 588–598. [Google Scholar] [CrossRef]
- Wong, S.Q.; Waldeck, K.; Vergara, I.A.; Schröder, J.; Madore, J.; Wilmott, J.S.; Colebatch, A.J.; De Paoli-Iseppi, R.; Li, J.; Lupat, R.; et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015, 75, 5228–5234. [Google Scholar] [CrossRef]
- Youlden, D.R.; Youl, P.H.; Soyer, H.P.; Fritschi, L.; Baade, P.D. Multiple primary cancers associated with Merkel cell carcinoma in Queensland, Australia, 1982–2011. J. Investig. Dermatol. 2014, 134, 2883–2889. [Google Scholar] [CrossRef]
- Sahi, H.; Savola, S.; Sihto, H.; Koljonen, V.; Bohling, T.; Knuutila, S. RB1 gene in Merkel cell carcinoma: Hypermethylation in all tumors and concurrent heterozygous deletions in the polyomavirus-negative subgroup. APMIS 2014, 122, 1157–1166. [Google Scholar] [CrossRef]
- Harms, P.W.; Vats, P.; Verhaegen, M.E.; Robinson, D.R.; Wu, Y.M.; Dhanasekaran, S.M.; Palanisamy, N.; Siddiqui, J.; Cao, X.; Su, F.; et al. The Distinctive Mutational Spectra of Polyoma-virus-Negative Merkel Cell Carcinoma. Cancer Res. 2015, 75, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.A.; Verhaegen, M.E.; Joseph, M.K.; Harms, K.L.; Harms, P.W. Merkel cell carcinoma: Updates in tumor biology, emerging therapies, and preclinical models. Front. Oncol. 2024, 14, 1413793. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Hayashi, K.; Matsushita, M.; Nonaka, D.; Kohashi, K.; Kuwamoto, S.; Umekita, Y.; Oda, Y. Merkel cell polyomavirus-negative Merkel cell carcinoma is associated with JAK-STAT and MEK-ERK pathway activation. Cancer Sci. 2022, 113, 251–260. [Google Scholar] [CrossRef]
- Portilla, N.; Alzate, J.P.; Sierra, F.A.; Parra-Medina, R. A Systematic review and Meta-Analysis of the survival and clinicopathological features of p63 expression in Merkel cell carcinoma. Australas. J. Dermatol. 2020, 61, e276–e282. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, R.C.; Carter, M.D.; Pasternak, S.; Fleming, K.E.; Gaston, D.; Legge, A.; Ly, T.Y.; Walsh, N.M. Relationship between p63 and p53 expression in Merkel cell carcinoma and corresponding abnormalities in TP63 and TP53: A study and a proposal. Hum. Pathol. 2021, 117, 31–41. [Google Scholar] [CrossRef]
- Walsh, N.M.; Saggini, A.; Pasternak, S.; Carter, M.D.; Fleming, K.; Ly, T.Y.; Doucette, S. p63 expression in Merkel cell carcinoma: Comparative immunohistochemistry invokes TAp63 as the dominant isoform involved. Hum. Pathol. 2020, 97, 60–67. [Google Scholar] [CrossRef]
- Harms, P.W.; Verhaegen, M.E.; Hu, K.; Hrycaj, S.M.; Chan, M.P.; Liu, C.J.; Grachtchouk, M.; Patel, R.M.; Udager, A.M.; Dlugosz, A.A. Genomic evidence suggests that cutaneous neuroendocrine carcinomas can arise from squamous dysplastic precursors. Mod. Pathol. 2022, 35, 506–514. [Google Scholar] [CrossRef]
- Ríos-Viñuela, E.; Mayo-Martínez, F.; Nagore, E.; Millan-Esteban, D.; Requena, C.; Sanmartín, O.; Llombart, B. Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review. Cancers 2024, 16, 411. [Google Scholar] [CrossRef]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef]
- An, K.P.; Ratner, D. Merkel cell carcinoma in the setting of HIV infection. J. Am. Acad. Dermatol. 2001, 45, 309–312. [Google Scholar] [CrossRef]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I.; International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Czapiewski, P.; Biernat, W. Merkel cell carcinoma—Recent advances in the biology, diagnostics and treatment. Int. J. Biochem. Cell Biol. 2014, 53, 536–546. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 2018, 37, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Tilling, T.; Moll, I. Which Are the Cells of Origin in Merkel Cell Carcinoma? J. Skin Cancer 2012, 2012, 680410. [Google Scholar] [CrossRef]
- Verhaegen, M.E.; Harms, P.W.; Van Goor, J.J.; Arche, J.; Patrick, M.T.; Wilbert, D.; Zabawa, H.; Grachtchouk, M.; Liu, C.J.; Hu, K.; et al. Direct cellular reprogramming enables development of viral T antigen-driven Merkel cell carcinoma in mice. J. Clin. Investig. 2022, 132, e152069. [Google Scholar] [CrossRef]
- Ikeda, R.; Cha, M.; Ling, J.; Jia, Z.; Coyle, D.; Gu, J.G. Merkel cells transduce and encode tactile stimuli to drive aβ-Afferent impulses. Cell 2014, 157, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Patel, R.M.; Verhaegen, M.E.; Giordano, T.J.; Nash, K.T.; Johnson, C.N.; Daignault, S.; Thomas, D.G.; Gudjonsson, J.E.; Elder, J.T.; et al. Distinct gene expression profiles of viral- and nonviral-associated merkel cell carcinoma revealed by transcriptome analysis. J. Investig. Dermatol. 2013, 133, 936–945. [Google Scholar] [CrossRef]
- Van Keymeulen, A.; Mascre, G.; Youseff, K.K.; Harel, I.; Michaux, C.; De Geest, N.; Szpalski, C.; Achouri, Y.; Bloch, W.; Hassan, B.A.; et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 2009, 187, 91–100. [Google Scholar] [CrossRef]
- Gambichler, T.; Mohtezebsade, S.; Wieland, U.; Silling, S.; Höh, A.K.; Dreißigacker, M.; Schaller, J.; Schulze, H.J.; Oellig, F.; Kreuter, A.; et al. Prognostic relevance of high atonal homolog-1 expression in Merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 43–49. [Google Scholar] [CrossRef]
- Fradette, J.; Godbout, M.J.; Michel, M.; Germain, L. Localization of Merkel cells at hairless and hairy human skin sites using keratin 18. Biochem. Cell Biol. 1995, 73, 635–639. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, S.; Lu, Y. A case of multiple recurrent facial Merkel cell carcinomas: Treatment and imaging findings. Asian J. Surg. 2023, 46, 2548–2549. [Google Scholar] [CrossRef] [PubMed]
- González-Vela, M.D.C.; Curiel-Olmo, S.; Derdak, S.; Beltran, S.; Santibañez, M.; Martínez, N.; Castillo-Trujillo, A.; Gut, M.; Sánchez-Pacheco, R.; Almaraz, C.; et al. Shared Oncogenic Pathways Implicated in Both Virus-Positive and UV-Induced Merkel Cell Carcinomas. J. Investig. Dermatol 2017, 13, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hesbacher, S.; Pfitzer, L.; Wiedorfer, K.; Angermeyer, S.; Borst, A.; Haferkamp, S.; Scholz, C.J.; Wobser, M.; Schrama, D.; Houben, R. RB1 is the crucial target of the Merkel cell polyomavirus Large T antigen in Merkel cell carcinoma cells. Oncotarget 2016, 7, 32956–32968. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Devine, W.P.; Pekmezci, M.; Umetsu, S.E.; Krings, G.; Federman, S.; Cho, S.J.; Saunders, T.A.; Jen, K.Y.; Bergsland, E.; et al. Identification of high-risk human papillomavirus and Rb/E2F pathway genomic alterations in mutually exclusive subsets of colorectal neuroendocrine carcinoma. Mod. Pathol. 2019, 32, 290–305. [Google Scholar] [CrossRef]
- Meder, L.; König, K.; Ozretic, L.; Schultheis, A.M.; Ueckeroth, F.; Ade, C.P.; Albus, K.; Boehm, D.; Rommerscheidt-Fuss, U.; Florin, A.; et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int. J. Cancer 2016, 138, 927–938. [Google Scholar] [CrossRef]
- Syder, A.J.; Karam, S.M.; Mills, J.C.; Ippolito, J.E.; Ansari, H.R.; Farook, V.; Gordon, J.I. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 4471–4476. [Google Scholar] [CrossRef]
- Haigis, K.; Sage, J.; Glickman, J.; Shafer, S.; Jacks, T. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J. Biol. Chem. 2006, 281, 638–647. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Wright, M.C.; Bolock, A.M.; Geng, X.; Maricich, S.M. Ectopic Atoh1 expression drives Merkel cell production in embryonic, postnatal and adult mouse epidermis. Development 2015, 142, 2533–2544. [Google Scholar]
- Morrison, K.M.; Miesegaes, G.R.; Lumpkin, E.A.; Maricich, S.M. Mammalian Merkel cells are descended from the epidermal lineage. Dev. Biol. 2009, 336, 76–83. [Google Scholar] [CrossRef]
- Perdigoto, C.N.; Bardot, E.S.; Valdes, V.J.; Santoriello, F.J.; Ezhkova, E. Embryonic maturation of epidermal merkel cells is controlled by a redundant transcription factor network. Development 2014, 141, 4690–4696. [Google Scholar] [CrossRef]
- Thibault, K. Evidence of an epithelial origin of Merkel cell carcinoma. Mod. Pathol. 2022, 35, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, J.K.; Sheu, K.M.; Wang, L.; Balanis, N.G.; Nguyen, K.; Smith, B.A.; Cheng, C.; Tsai, B.L.; Cheng, D.; et al. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 2018, 362, 91–95. [Google Scholar] [CrossRef]
- Park, K.S.; Liang, M.C.; Raiser, D.M.; Zamponi, R.; Roach, R.R.; Curtis, S.J.; Walton, Z.; Schaffer, B.E.; Roake, C.M.; Zmoos, A.F.; et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle 2011, 10, 2806–2815. [Google Scholar] [CrossRef]
- Thanguturi, S.; Tallet, A.; Miquelestorena-Standley, E.; Coco, C.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Blom, A.; Saiag, P.; Beneton, N.; Bens, G.; et al. Investigation of the RB1-SOX2 axis constitutes a tool for viral status determination and diagnosis in Merkel cell carcinoma. Virchows Arch. 2022, 480, 1239–1254. [Google Scholar] [CrossRef]
- Gravemeyer, J.; Spassova, I.; Verhaegen, M.E.; Dlugosz, A.A.; Hoffmann, D.; Lange, A.; Becker, J.C. DNA-methylation patterns imply a common cellular origin of virus- and UV-associated Merkel cell carcinoma. Oncogene 2022, 41, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Aljundi, M.; Appenzeller, S.; Samimi, M.; Maubec, E.; Cribier, B.; Deschamps, L.; Sarma, B.; Sarosi, E.M.; Berthon, P.; et al. Polyomavirus-Positive Merkel Cell Carcinoma Derived from a Trichoblastoma Suggests an Epithelial Origin of this Merkel Cell Carcinoma. J. Investig. Dermatol. 2020, 140, 976–985. [Google Scholar] [CrossRef]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Krump, N.A.; MacDonald, M.; You, J. Merkel Cell Polyomavirus Infection of Animal Dermal Fibroblasts. J. Virol. 2018, 92, e01610-17. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Zur Hausen, A.; Rennspiess, D.; Winnepenninckx, V.; Speel, E.J.; Kurz, A.K. Early B-Cell differentiation in merkel cell carcinomas: Clues to cellular ancestry. Cancer Res. 2013, 73, 4982–4987. [Google Scholar] [CrossRef]
- Scotti, B.; Cama, E.; Venturi, F.; Veronesi, G.; Dika, E. Clinical and dermoscopic features of Merkel cell carcinoma: Insights from 16 cases, including two reflectance confocal microscopy evaluation and the identification of four representative MCC subtypes. Arch. Dermatol. Res. 2025, 317, 587. [Google Scholar] [CrossRef]
- Suárez, A.L.; Louis, P.; Kitts, J.; Busam, K.; Myskowski, P.L.; Wong, R.J.; Chen, C.S.; Spencer, P.; Lacouture, M.; Pulitzer, M.P. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J. Am. Acad. Dermatol. 2015, 73, 968–975. [Google Scholar] [CrossRef]
- McGowan, M.A.; Helm, M.F.; Tarbox, M.B. Squamous cell carcinoma in situ overlying merkel cell carcinoma. J. Cutan. Med. Surg. 2016, 20, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, X.; Huang, H.; Zhang, L.; Song, Z.; Yang, X.; Lei, S.; Zhai, Z. Merkel cell carcinoma overlapping Bowen’s disease: Two cases report and literature review. J. Cancer Res. Clin. Oncol. 2024, 150, 217. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Zhao, L.; Johnson, B.; Wang, X.; Carskadon, S.; Palanisamy, N.; Rhodes, D.R.; Mannan, R.; Vo, J.N.; Choi, J.E.; et al. Virus-positive Merkel Cell Carcinoma Is an Independent Prog-nostic Group with Distinct Predictive Biomarkers. Clin. Cancer Res. 2021, 27, 2494–2504. [Google Scholar] [CrossRef]
- Mir, R.; Sciubba, J.J.; Bhuiya, T.A.; Blomquist, K.; Zelig, D.; Friedman, E. Merkel cell carcinoma arising in the oral mucosa. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yom, S.S.; Rosenthal, D.I.; El-Naggar, A.K.; Kies, M.S.; Hessel, A.C. Merkel cell carcinoma of the tongue and head and neck oral mucosal sites. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 761–768. [Google Scholar] [CrossRef]
- Longo, F.; Califano, L.; Mangone, G.M.; Errico, M.E. Neuroendocrine (Merkel cell) carcinoma of the oral mucosa: Report of a case with immunohistochemical study and review of the literature. J. Oral Pathol. Med. 1999, 28, 88–91. [Google Scholar] [CrossRef]
- de Arruda, J.A.A.; Mesquita, R.A.; Canedo, N.H.S.; Agostini, M.; Abrahão, A.C.; de Andrade, B.A.B.; Romañach, M.J. Merkel cell carcinoma of the lower lip: A case report and literature review. Oral Oncol. 2021, 113, 105019. [Google Scholar] [CrossRef]
- Islam, M.N.; Chehal, H.; Smith, M.H.; Islam, S.; Bhattacharyya, I. Merkel Cell Carcinoma of the Buccal Mucosa and Lower Lip. Head Neck Pathol. 2018, 12, 279–285. [Google Scholar] [CrossRef]
- Baker, P.; Alguacil-Garcia, A. Moderately differentiated neuroendocrine carcinoma in the floor of the mouth: A case report. J. Oral Maxillofac. Surg. 1999, 57, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Smitha, R.S.; Punnya, V.A. Merkel cell carcinoma of the alveolar mucosa in a young adult: A rare case report. Br. J. Oral Maxillofac. Surg. 2010, 48, 48–50. [Google Scholar] [CrossRef]
- Inoue, T.; Shimono, M.; Takano, N.; Saito, C.; Tanaka, Y. Merkel cell carcinoma of palatal mucosa in a young adult: Immunohistochemical and ultrastructural features. Oral Oncol. 1997, 33, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, I.; Nandi, A.; Roy, R. Primary Merkel cell carcinoma of the oral mucosa in a young adult male: Report of a rare case. Indian J. Pathol. Microbiol. 2015, 58, 214–216. [Google Scholar] [PubMed]
- Tomic, S.; Warner, T.F.; Messing, E.; Wilding, G. Penile Merkel cell carcinoma. Urology 1995, 45, 1062–1065. [Google Scholar] [CrossRef]
- Best, T.J.; Metcalfe, J.B.; Moore, R.B.; Nguyen, G.K. Merkel cell carcinoma of the scrotum. Ann. Plast. Surg. 1994, 33, 83–85. [Google Scholar] [CrossRef]
- Cwynar, M.; Chmielik, E.; Cwynar, G.; Ptak, P.; Kowalczyk, K. Vulvar Merkel cell carcinoma combined with squamous cell carcinoma of the vulva. Ginekol. Polska 2024, 95, 502–503. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Tahseen, A.I.; Vaudreuil, A.M.; Caponetti, G.C.; Huerter, C.J. Clinical features and treatment of vulvar Merkel cell carcinoma: A systematic review. Gynecol. Oncol. Res. Pract. 2017, 4, 2. [Google Scholar] [CrossRef]
- Dalle, S.; Parmentier, L.; Moscarella, E.; Phan, A.; Argenziano, G.; Thomas, L. Dermoscopy of merkel cell carcinoma. Dermatology 2012, 224, 140–144. [Google Scholar] [CrossRef]
- Koumaki, D.; Evangelou, G.; Katoulis, A.C.; Apalla, Z.; Lallas, A.; Papadakis, M.; Gregoriou, S.; Lazaridou, E.; Krasagakis, K. Dermoscopic characteristics of Merkel cell carcinoma. BMC Cancer 2024, 24, 785. [Google Scholar] [CrossRef]
- Ho, J.; Collie, C.J. What’s new in dermatopathology 2023: WHO 5th edition updates. J. Pathol. Transl. Med.-Cine 2023, 57, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Ronen, S.; Czaja, R.C.; Ronen, N.; Pantazis, C.G.; Iczkowski, K.A. Small Cell Variant of Metastatic Melanoma: A Mimicker of Lymphoblastic Leukemia/Lymphoma. Dermatopathology 2019, 6, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Sobanko, J.F.; Etzkorn, J.R.; Shin, T.M.; Giordano, C.N.; McMurray, S.L.; Walker, J.L.; Zhang, J.; Miller, C.J.; Higgins, H.W., 2nd. Merkel Cell Carcinoma. Dermatol. Clin. 2023, 41, 101–115. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kataria, S.P.; Yadav, A.K. Expression of Neuron-Specific Enolase and Other Neuroendocrine Markers is Correlated with Prognosis and Response to Therapy in Non-Hodgkin Lymphoma. J. Lab. Physicians 2022, 14, 427–434. [Google Scholar] [CrossRef]
- Bobos, M.; Hytiroglou, P.; Kostopoulos, I.; Karkavelas, G.; Papadimitriou, C.S. Immunohistochemical distinction between merkel cell carcinoma and small cell carcinoma of the lung. Am. J. Dermatopathol. 2006, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Tallet, A.; Miquelestorena-Standley, E.; Houben, R.; Schrama, D.; Gambichler, T.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Mod. Pathol. 2019, 32, 499–510. [Google Scholar] [CrossRef]
- Pasternak, S.; Carter, M.D.; Ly, T.Y.; Doucette, S.; Walsh, N.M. Immunohistochemical profiles of different subsets of Merkel cell carcinoma. Hum. Pathol. 2018, 82, 232–238. [Google Scholar] [CrossRef]
- Miner, A.G.; Patel, R.M.; Wilson, D.A.; Procop, G.W.; Minca, E.C.; Fullen, D.R.; Harms, P.W.; Billings, S.D. Cytokeratin 20-negative Merkel cell carcinoma is infrequently associated with the Merkel cell polyomavirus. Mod. Pathol. 2015, 28, 498–504. [Google Scholar] [CrossRef]
- Miller, E.; Biesemier, A.; Coomes, D.M.; Raghavan, S.S. PRAME Expression in Merkel Cell Carcinoma. Am. J. Surg. Pathol. 2024, 48, 1270–1276. [Google Scholar] [CrossRef]
- Raghavan, S.S. Response to Expanding Insights into PRAME Expression in Merkel Cell Carcinoma. Am. J. Surg. Pathol. 2025, 49, 637–638. [Google Scholar] [CrossRef]
- Ricci, C.; Altavilla, M.V.; Corti, B.; Pasquini, E.; Presutti, L.; Baietti, A.M.; Amorosa, L.; Balbi, T.; Baldovini, C.; Ambrosi, F.; et al. PRAME Expression in Mucosal Melanoma of the Head and Neck Region. Am. J. Surg. Pathol. 2023, 47, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Szumera-Cieckiewicz, A.; Massi, D.; Cassisa, A.; Krzyzinski, M.; Dudzisz-Sledz, M.; Biecek, P.; Rutkowski, P.; Marszalek, A.; Hoang, M.P.; Donizy, P. SATB2, CKAE1/AE3, and synaptophysin as a sensitive immunohistochemical panel for the detection of lymph node metastases of Merkel cell carcinoma. Virchows Arch. 2024, 484, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Veija, T.; Koljonen, V.; Bohling, T.; Kero, M.; Knuutila, S.; Sarhadi, V.K. Aberrant expression of ALK and EZH2 in Merkel cell carcinoma. BMC Cancer 2017, 17, 236. [Google Scholar] [CrossRef]
- Santoro, F.; Maletta, F.; Parente, R.; Fissore, J.; Tampieri, C.; Santoro, L.; Birocco, N.; Picciotto, F.; Quaglino, P.; Volante, M.; et al. Clinical-Pathological Evaluation and Prognostic Analysis of 228 Merkel Cell Carcinomas Focusing on Tumor-Infiltrating Lymphocytes, MCPYV Infection and ALK Expression. Endocr. Pathol. 2022, 33, 289–303. [Google Scholar] [CrossRef]
- Erickson, L.A.; Papouchado, B.; Dimashkieh, H.; Zhang, S.; Nakamura, N.; Lloyd, R.V. Cdx2 as a marker for neuroendocrine tumors of unknown primary sites. Endocr. Pathol. 2004, 15, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xiao, L.; Zhang, M.; Kamat, A.M.; Siefker-Radtke, A.; Dinney, C.P.; Czerniak, B.; Guo, C.C. Small cell carcinoma of the urinary bladder: A clinicopathological and immunohistochemical analysis of 81 cases. Hum. Pathol. 2018, 79, 57–65. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Gopalan, A.; Al-Ahmadie, H.; Chen, Y.B.; Sarungbam, J.; Sirintrapun, S.J.; Tickoo, S.K.; Reuter, V.E.; Fine, S.W. Neuroendocrine differentiation in the setting of prostatic carcinoma: Contemporary assessment of a consecutive series. Histopathology 2022, 81, 246–254. [Google Scholar] [CrossRef]
- Ricci, C.; Morandi, L.; Righi, A.; Gibertoni, D.; Maletta, F.; Ambrosi, F.; Agostinelli, C.; Uccella, S.; Asioli, S.; Sessa, F.; et al. PD-1 (PDCD1) promoter methylation in Merkel cell carcinoma: Prognostic relevance and relationship with clinico-pathological parameters. Mod. Pathol. 2019, 32, 1359–1372. [Google Scholar] [CrossRef]
- Ricci, C.; Morandi, L.; Ambrosi, F.; Righi, A.; Gibertoni, D.; Maletta, F.; Agostinelli, C.; Corradini, A.G.; Uccella, S.; Asioli, S.; et al. Intron 4–5 hTERT DNA Hypermethylation in Merkel Cell Carcinoma: Frequency, Association with Other Clinico-pathological Features and Prognostic Relevance. Endocr. Pathol. 2021, 32, 385–395. [Google Scholar] [CrossRef]
- Ricci, C.; Righi, A.; Ambrosi, F.; Gibertoni, D.; Maletta, F.; Uccella, S.; Sessa, F.; Asioli, S.; Pellilli, M.; Maragliano, R.; et al. Prognostic Impact of MCPyV and TIL Subtyping in Merkel Cell Carcinoma: Evidence from a Large European Cohort of 95 Patients. Endocr. Pathol. 2020, 31, 21–32. [Google Scholar] [CrossRef]
- Trinidad, C.M.; Torres-Cabala, C.A.; Prieto, V.G.; Aung, P.P. Update on eighth edition American Joint Committee on Cancer classification for Merkel cell carcinoma and histopathological parameters that determine prognosis. J. Clin. Pathol. 2019, 72, 337–340. [Google Scholar] [CrossRef]
- Singh, N.; Alexander, N.A.; Lachance, K.; Lewis, C.W.; McEvoy, A.; Akaike, G.; Byrd, D.; Behnia, S.; Bhatia, S.; Paulson, K.G.; et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: Analysis of 584 patients. J. Am. Acad. Dermatol. 2021, 84, 330–339. [Google Scholar] [CrossRef]
- Hawryluk, E.B.; O’Regan, K.N.; Sheehy, N.; Guo, Y.; Dorosario, A.; Sakellis, C.G.; Jacene, H.A.; Wang, L.C. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: A study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J. Am. Acad. Dermatol. 2013, 68, 592–599. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours—Towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, H.; Urist, M.M.; Fiveash, J.; Heslin, M.J.; Bland, K.I.; Beenken, S.W. Multimodality Treatment of Merkel Cell Carcinoma: Case Series and Literature Review of 1024 Cases. Ann. Surg. Oncol. 2001, 8, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef]
- McEvoy, A.M.; Lachance, K.; Hippe, D.S.; Cahill, K.; Moshiri, Y.; Lewis, C.W.; Singh, N.; Park, S.Y.; Thuesmunn, Z.; Cook, M.M.; et al. Recurrence and Mortality Risk of Merkel Cell Carcinoma by Cancer Stage and Time from Diagnosis. JAMA Dermatol. 2022, 158, 382–389. [Google Scholar] [CrossRef]
- Pectasides, D.; Pectasides, M.; Economopoulos, T. Merkel cell cancer of the skin. Ann. Oncol. 2006, 17, 1489–1495. [Google Scholar] [CrossRef]
- Song, Y.; Azari, F.S.; Tang, R.; Shannon, A.B.; Miura, J.T.; Fraker, D.L.; Karakousis, G.C. Patterns of Metastasis in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 519–529. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Bryce-Alberti, M.; Portmann-Baracco, A.; Castillo-Flores, S.; Pretell-Mazzini, J. Treatment and survival outcomes in metastatic Merkel cell carcinoma: Analysis of 2010 patients from the SEER database. Cancer Treat. Res. Commun. 2022, 33, 100665. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.J.; Na, H.; Cho, H.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Lee, W.J. Clinicopathological characteristics and prognosis of Merkel cell carcinoma: A single-center retrospective study in Korea. J. Cancer Res. Clin. Oncol. 2023, 149, 10065–10074. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef] [PubMed]
- Maloney, N.J.; Nguyen, K.A.; Bach, D.Q.; Zaba, L.C. Sites of distant metastasis in Merkel cell carcinoma differ by primary tumor site and are of prognostic significance: A population-based study in the Surveillance, Epidemiology, and End Results database from 2010 to 2016. J. Am. Acad. Dermatol. 2021, 84, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.J.; Cao, D.S.; Zhao, J.; Zhu, B.Z.; Xie, J. Frequency and prognosis of metastasis to liver, lung, bone and brain from Merkel cell carci-noma. Future Oncol. 2020, 16, 1101–1113. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shabaik, A.S. Metastatic Merkel cell carcinoma involving the bone marrow with chronic lymphocytic leukemia mimicking Richter transformation. Blood 2013, 122, 2776. [Google Scholar] [CrossRef]
- Keow, J.; Kwan, K.F.; Hedley, B.D.; Hsia, C.C.; Xenocostas, A.; Chin-Yee, B. Merkel cell carcinoma mimicking acute leukemia. Int. J. Lab. Hematol. 2024, 46, 761–763. [Google Scholar] [CrossRef]
- Goepfert, H.; Remmler, D.; Silva, E.; Wheeler, B. Merkel cell carcinoma (endocrine carcinoma of the skin) of the head and neck. Arch. Otolaryngol. 1984, 110, 707–712. [Google Scholar] [CrossRef]
- Haykal, T.; Towfiq, B. Merkel cell carcinoma with intramedullary spinal cord metastasis: A very rare clinical finding. Clin. Case Rep. 2018, 6, 1181–1182. [Google Scholar] [CrossRef]
- Barkdull, G.C.; Healy, J.F.; Weisman, R.A. Intracranial spread of Merkel cell carcinoma through intact skull. Ann. Otol. Rhinol. Laryngol. 2004, 113, 683–687. [Google Scholar] [CrossRef]
- Khaddour, K.; Liu, M.; Kim, E.Y.; Bahar, F.; Lôbo, M.M.; Giobbie-Hurder, A.; Silk, A.W.; Thakuria, M. Survival outcomes in patients with de novo metastatic Merkel cell carcinoma according to site of metastases. Front. Oncol. 2024, 14, 1444590. [Google Scholar] [CrossRef]
- Payne, M.M.; Rader, A.E.; McCarthy, D.M.; Rodgers, W.H. Merkel cell carcinoma in a malignant pleural effusion: Case report. Cytojournal 2004, 1, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abul-Kasim, K.; Söderström, K.; Hallsten, L. Extensive central nervous system involvement in Merkel cell carcinoma: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 35. [Google Scholar] [CrossRef]
- Pennisi, G.; Talacchi, A.; Tirendi, M.N.; Giordano, M.; Olivi, A. Intradural extramedullary cervical metastasis from Merkel cell carcinoma: A case report and literature review. Chin. Neurosurg. J. 2022, 8, 38. [Google Scholar] [CrossRef]
- Leão, I.; Marinho, J.; Costa, T. Long-term response to avelumab and management of oligoprogression in Merkel cell carcinoma: A case report. World J. Clin. Cases 2021, 9, 4829–4836. [Google Scholar] [CrossRef]
- Lentz, S.R.; Krewson, L.; Zutter, M.M. Recurrent Neuroendocrine (Merkel Cell) Carcinoma of the Skin Presenting as Marrow Failure in a Man with Systemic Lupus Erythematosus. Med. Pediatr. Oncol. 1993, 21, 137–141. [Google Scholar] [CrossRef]
- Khan, A.; Adil, S.; Estalilla, O.C.; Jubelirer, S. Bone marrow involvement with Merkel cell carcinoma. BMJ Case Rep. 2020, 13, e234234. [Google Scholar] [CrossRef]
- Morris, K.L.; Williams, B.; Kennedy, G.A. Heavy bone marrow involvement with metastatic Merkel cell tu-mour in an immunosuppressed renal transplant recipient. Br. J. Haematol. 2005, 128, 133. [Google Scholar] [CrossRef]
- Kressin, M.K.; Kim, A.S. Metastatic Merkel cell carcinoma in the bone marrow of a patient with plasma cell myeloma and therapy-related myelodysplastic syndrome. Int. J. Clin. Exp. Pathol. 2012, 5, 1007–1012. [Google Scholar] [PubMed]
- Durmus, O.; Gokoz, O.; Saglam, E.A.; Ergun, E.L.; Gulseren, D. A rare involvement in skin cancer: Merkel cell carcinoma with bone marrow infiltration in a kidney transplant recipient. Clin. Med. J. R. Coll. Physicians Lond. 2023, 23, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, I.; Sato-Matsumura, K.C.; Fujita, Y.; Natsuga, K.; Ujiie, H.; Tomita, Y.; Kato, N.; Kondo, M.; Ohnishi, K. Leukaemic dissemination of Merkel cell carcinoma in a patient with systemic lupus erythematosus. Clin. Exp. Dermatol. 2008, 33, 270–272. [Google Scholar] [CrossRef]
- Highland, B.; Morrow, W.P.; Arispe, K.; Beaty, M.; Maracaja, D. Merkel Cell Carcinoma with Extensive Bone Marrow Metastasis and Peripheral Blood Involvement: A Case Report with Immunohistochemical and Mutational Studies. Appl. Immunohistochem. Mol. Morphol. 2024, 32, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Smadja, N.; De Gramont, A.; Gonzalez-Canali, G.; Louvet, C.; Wattel, E.; Krulik, M. Cytogenetic Study in a Bone Marrow Metastatic Merkel Cell Carcinoma. Cancer Genet. Cytogenet. 1991, 51, 85–87. [Google Scholar] [CrossRef]
- Le Gall-Ianotto, C.; Coquart, N.; Ianotto, J.C.; Guillerm, G.; Grall, C.; Quintin-Roue, I.; Marion, V.; Greco, M.; Berthou, C.; Misery, L. Pancyto-paenia secondary to bone marrow dissemination of Merkel cell carcinoma in a patient with Waldenström macroglobulinaemia. Eur. J. Dermatol. 2011, 21, 126–127. [Google Scholar]
- Kobrinski, D.A.; Choudhury, A.M.; Shah, R.P. A case of locally-advanced Merkel cell carcinoma progressing with disseminated bone marrow metastases. Eur. J. Dermatol. 2018, 28, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Folyovich, A.; Majoros, A.; Jarecsny, T.; Pánczél, G.; Pápai, Z.; Rudas, G.; Kozák, L.; Barna, G.; Béres-Molnár, K.A.; Vadasdi, K.; et al. Epileptic Seizure Provoked by Bone Metastasis of Chronic Lymphoid Leukemia and Merkel Cell Carcinoma. Case Rep. Med. 2020, 2020, 4318638. [Google Scholar] [CrossRef]
- Vlad, R.; Woodlock, T.J. Merkel Cell Carcinoma after Chronic Lymphocytic Leukemia: Case Report and Literature Review. Am. J. Clin. Oncol. Cancer Clin. Trials 2003, 26, 531–534. [Google Scholar] [CrossRef]
- Goodwin, C.R.; Mehta, A.I.; Adogwa, O.; Sarabia-Estrada, R.; Sciubba, D.M. Merkel Cell Spinal Metastasis: Management in the Setting of a Poor Prognosis. Glob. Spine J. 2015, 5, 39–43. [Google Scholar] [CrossRef]
- Madden, N.; Thomas, P.; Johnson, P.; Anderson, K.; Arnold, P. Thoracic Spinal Metastasis of Merkel Cell Carcinoma in an Immunocompromised Patient: Case Report. Evid.-Based Spine-Care J. 2013, 4, 54–58. [Google Scholar] [CrossRef]
- Moayed, S.; Maldjianb, C.; Adam, R.; Bonakdarpour, A. Magnetic resonance imaging appearance of metastatic Merkel cell carcinoma to the sacrum and epidural space. Magn. Reson. Imaging 2000, 18, 1039–1042. [Google Scholar] [CrossRef]
- Nguyen, B.D.; McCullough, A.E. Isolated Tibial Metastasis from Merkel Cell Carcinoma. Radiol. Case Rep. 2007, 2, 88. [Google Scholar] [CrossRef]
- Kamijo, A.; Koshino, T.; Hirakawa, K.; Saito, T. Merkel cell carcinoma with bone metastasis: A case report. J. Orthop. Sci. 2002, 7, 574–577. [Google Scholar] [CrossRef]
- Pectasides, D.; Moutzourides, G.; Dimitriadis, M.; Varthalitis, J.; Athanassiou, A. Chemotherapy for Merkel cell carcinoma with car-boplatin and etoposide. Am. J. Clin. Oncol. 1995, 18, 418–420. [Google Scholar] [CrossRef]
- Pilotti, S.; Rilke, F.; Bartoli, C.; Grisotti, A. Clinicopathologic correlations of cutaneous neuroendocrine Merkel cell carcinoma. J. Clin. Oncol. 1988, 6, 1863–1873. [Google Scholar] [CrossRef]
- Principe, D.R.; Clark, J.I.; Emami, B.; Borowicz, S. Combined radio-immunotherapy leads to complete clinical regression of stage IV Merkel cell carcinoma. BMJ Case Rep. 2019, 12, e230518. [Google Scholar] [CrossRef]
- Vijay, K.; Venkateswaran, K.; Shetty, A.P.; Rajasekaran, S. Spinal extradural metastasis from Merkel cell carcinoma: A rare cause of paraplegia. Eur. Spine J. 2008, 17, 267–270. [Google Scholar] [CrossRef]
- Ng, G.; Lenehan, B.; Street, J. Metastatic Merkel cell carcinoma of the spine. J. Clin. Neurosci. 2010, 17, 1069–1071. [Google Scholar] [CrossRef]
- Turgut, M.; Gökpinar, D.; Barutça, S.; Erkuş, M. Lumbosacral metastatic extradural Merkel cell carcinoma causing nerve root compression—Case report. Neurol. Med.-Chir. 2002, 42, 78–80. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.; Meng, M.B. Merkel cell carcinoma with lymph node metastasis in the absence of a primary site: Case report and literature review. Oncol. Lett. 2012, 4, 1329–1334. [Google Scholar] [CrossRef]
- Maugeri, R.; Giugno, A.; Giammalva, R.G.; Gulì, C.; Basile, L.; Graziano, F.; Iacopino, D.G. A thoracic vertebral localization of a metastasized cutaneous Merkel cell carcinoma: Case report and review of literature. Surg. Neurol. Int. 2017, 8, 190. [Google Scholar] [CrossRef][Green Version]
- Chao, T.C.; Park, J.M.; Rhee, H.; Greager, J.A. Merkel cell tumor of the back detected during pregnancy. Plast. Reconstr. Surg. 1990, 86, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Turgut, M.; Baka, M.; Yurtseven, M. Metastatic Merkel cell carcinoma to the sacrum and epidural space: Case report. Magn. Reson. Imaging 2004, 22, 1340. [Google Scholar] [CrossRef]
- Tam, C.S.; Turner, P.; McLean, C.; Whitehead, S.; Cole-Sinclair, M. ‘Leukaemic’ presentation of metastatic Merkel cell carcinoma. Br. J. Haematol. 2005, 129, 446. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, C.; Woollons, A.; Ross, J.; Price, M.; Wojnarowska, F.; Morris, P.J.; Wall, S.; Bunker, C.B. Merkel cell carcinoma arising after thera-peutic immunosuppression. Br. J. Dermatol. 1997, 137, 637–641. [Google Scholar] [CrossRef]
- Park, J.S.; Park, Y.M. Cervical Spinal Metastasis of Merkel Cell Carcinoma. Neurospine 2009, 6, 197–200. [Google Scholar]
- Fang, J.; Xu, Q. Differences of osteoblastic bone metastases and osteolytic bone metastases in clinical features and molecular characteristics. Clin. Transl. Oncol. 2015, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Høilund-Carlsen, P.F.; Hess, S.; Werner, T.J.; Alavi, A. Cancer metastasizes to the bone marrow and not to the bone: Time for a paradigm shift! Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 893–897. [Google Scholar] [CrossRef]
- O’Sullivan, G.J.; Carty, F.L.; Cronin, C.G. Imaging of bone metastasis: An update. World J. Radiol. 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Akaike, G.; Akaike, T.; Fadl, S.A.; Lachance, K.; Nghiem, P.; Behnia, F. Imaging of merkel cell carcinoma: What imaging experts should know. Radiographics 2019, 39, 2069–2084. [Google Scholar] [CrossRef]
- Litofsky, N.S.; Smith, T.W.; Megerian, C.A. Merkel cell carcinoma of the external auditory canal invading the intracranial compartment. Am. J. Otolaryngol. 1998, 19, 330–334. [Google Scholar] [CrossRef]
- Scampa, M.; Kalbermatten, D.F.; Oranges, C.M. Demographic and Clinicopathological Factors as Predictors of Lymph Node Metastasis in Merkel Cell Carcinoma: A Population-Based Analysis. J. Clin. Med. 2023, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Mandal, A. An Extremely Rare Case of Metastatic Merkel Carcinoma of the Liver. Cureus 2021, 13, e19659. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakamura, M.; Kato, H.; Morita, A. Distant recurrence of Merkel cell carcinoma after spontaneous regression. J. Dermatol. 2019, 46, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, V.; Owen, I.D.; Nuli, B.; Xiao, P.Q. Neuroendocrine (Merkel cell) carcinoma of the retroperitoneum with no identifiable primary site. World J. Surg. Oncol. 2007, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Sandoval, O.A.; Cuellar-Hubbe, M.; Lino-Silva, L.S.; Salcedo-Hernández, R.A.; López-Basave, H.N.; Padilla-Rosciano, A.E.; Le-ón-Takahashi, A.M.; Herrera-Gómez, Á. Primary retroperitoneal Merkel cell carcinoma: Case report and literature review. Int. J. Surg. Case Rep. 2016, 19, 21–24. [Google Scholar] [CrossRef]
- Rossini, D.; Caponnetto, S.; Lapadula, V.; De Filippis, L.; Del Bene, G.; Emiliani, A.; Longo, F. Merkel cell carcinoma of the retroperitoneum with no identifiable primary site. Case Rep. Oncol. Med. 2013, 2013, 131695. [Google Scholar] [CrossRef]
- Durastante, V.; Conte, A.; Brollo, P.P.; Biddau, C.; Graziano, M.; Bresadola, V. Merkel Cell Carcinoma with Gastric Metastasis, a Rare Presentation: Case Report and Literature Review. J. Gastrointest. Cancer 2023, 54, 309–315. [Google Scholar] [CrossRef]
- Vaiciunaite, D.; Beddell, G.; Ivanov, N. Merkel cell carcinoma: An aggressive cutaneous carcinoma with rare metastasis to the thyroid gland. BMJ Case Rep. 2019, 12, e228273. [Google Scholar] [CrossRef]
- Santandrea, G.; Borsari, S.; Filice, A.; Piana, S. Merkel Cell Carcinoma: Mind the Genital Metastatic Sites! Dermatol. Pract. Conceptual. 2023, 13, e2023213. [Google Scholar] [CrossRef]
- Zijlker, L.P.; Bakker, M.; van der Hiel, B.; Bruining, A.; Klop, W.M.C.; Zuur, C.L.; Wouters, M.W.J.M.; van Akkooi, A.C.J. Baseline ultrasound and FDG-PET/CT imaging in Merkel cell carcinoma. J. Surg. Oncol. 2023, 127, 841–847. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.J. Diagnostic Test Accuracy of 18F-FDG PET or PET/CT in Merkel Cell Carcinoma. Clin. Nucl. Med. 2022, 47, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, D.; Choi, Y.S.; Kim, E.K.; Kim, T.E. Merkel Cell Carcinoma of the Trunk: Two Case Reports and Imaging Review. J. Korean Soc. Radiol. 2023, 84, 1134–1139. [Google Scholar] [CrossRef]
- Kirienko, M.; Gelardi, F.; Fiz, F.; Bauckneht, M.; Ninatti, G.; Pini, C.; Briganti, A.; Falconi, M.; Oyen, W.J.G.; van der Graaf, W.T.A.; et al. Personalised PET imaging in oncology: An umbrella review of meta-analyses to guide the appropriate radiopharmaceutical choice and indication. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Wu, N.; Qian, T.; Li, X.; Wan, D.Q.; Li, C.; Li, Y.; Wu, Z.; Wang, X.; Liu, J.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Rohren, E.M.; Madewell, J.E.; Hamaoka, T.; Theriault, R.L.; Yu, T.K.; Lewis, V.O.; Ma, J.; Stafford, R.J.; Tari, A.M.; et al. Imaging bone metastases in breast cancer: Techniques and recommendations for diagnosis. Lancet Oncol. 2009, 10, 606–614. [Google Scholar] [CrossRef]
- Uchida, N.; Sugimura, K.; Kajitani, A.; Yoshizako, T.; Ishida, T. MR imaging of vertebral metastases: Evaluation of fat saturation imaging. Eur. J. Radiol. 1993, 17, 91–94. [Google Scholar] [CrossRef]
- Girard, R.; Djelouah, M.; Barat, M.; Fornès, P.; Guégan, S.; Dupin, N.; Soyer, P.; Hoeffel, C. Abdominal metastases from Merkel cell carcinoma: Prevalence and presentation on CT examination in 111 patients. Diagn. Interv. Imaging 2022, 103, 41–48. [Google Scholar] [CrossRef]
- Li, X.; Wu, N.; Zhang, W.; Liu, Y.; Ming, Y. Differential diagnostic value of 18F-FDG PET/CT in osteolytic lesions. J. Bone Oncol. 2020, 24, 100302. [Google Scholar] [CrossRef]
- Patel, P.Y.; Dalal, I.; Griffith, B. [18F]FDG-PET Evaluation of Spinal Pathology in Patients in Oncology: Pearls and Pitfalls for the Neuroradiologist. American Journal of Neuroradiology. Am. J. Neuroradiol. 2022, 43, 332–340. [Google Scholar] [CrossRef]
- Hong, S.B.; Choi, S.H.; Kim, K.W.; Park, S.H.; Kim, S.Y.; Lee, S.J.; Lee, S.S.; Byun, J.H.; Lee, M.G. Diagnostic performance of [18F]FDG-PET/MRI for liver metastasis in patients with primary malignancy: A systematic review and meta-analysis. Eur. Radiol. 2019, 29, 3553–3563. [Google Scholar] [CrossRef]
- Choi, J.; Raghavan, M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Control 2012, 19, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Sachpekidis, C.; Sidiropoulou, P.; Hassel, J.C.; Drakoulis, N.; Dimitrakopoulou-Strauss, A. Positron Emission Tomography in Merkel Cell Carcinoma. Cancers 2020, 12, 2897. [Google Scholar] [CrossRef] [PubMed]
- Buder, K.; Lapa, C.; Kreissl, M.C.; Schirbel, A.; Herrmann, K.; Schnack, A.; Bröcker, E.B.; Goebeler, M.; Buck, A.K.; Becker, J.C. Somatostatin receptor expression in Merkel cell carcinoma as target for molecular imaging. BMC Cancer 2014, 14, 268. [Google Scholar] [CrossRef]
- Schneider, C.; Schlaak, M.; Bludau, M.; Markiefka, B.; Schmidt, M.C. 68Ga-DOTATATE-PET/CT positive metastatic lymph node in a 69-year-old woman with Merkel cell carcinoma. Clin. Nucl. Med. 2012, 37, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Epstude, M.; Tornquist, K.; Riklin, C.; di Lenardo, F.; Winterhalder, R.; Hug, U.; Strobel, K. Comparison of 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT imaging in metastasized Merkel cell carcinoma. Clin. Nucl. Med. 2013, 38, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Taralli, S.; Sollini, M.; Milella, M.; Perotti, G.; Filice, A.; Menga, M.; Versari, A.; Rufini, V. 18F-FDG and 68Ga-somatostatin analogs PET/CT in patients with Merkel cell carcinoma: A comparison study. EJNMMI Res. 2018, 8, 64. [Google Scholar] [CrossRef]
- Tai, P.; Yu, E.; Assouline, A.; Lian, J.D.; Joseph, K.; Miale, T.; Krzisch, C. Multimodality management for 145 cases of Merkel cell carcinoma. Med. Oncol. 2010, 27, 1260–1266. [Google Scholar] [CrossRef]
- Kacew, A.J.; Dharaneeswaran, H.; Starrett, G.J.; Thakuria, M.; LeBoeuf, N.R.; Silk, A.W.; DeCaprio, J.A.; Hanna, G.J. Predictors of immunotherapy benefit in Merkel cell carcinoma. Oncotarget 2020, 11, 4401–4410. [Google Scholar] [CrossRef]
- Iyer, J.G.; Blom, A.; Doumani, R.; Lewis, C.; Tarabadkar, E.S.; Anderson, A.; Ma, C.; Bestick, A.; Parvathaneni, U.; Bhatia, S.; et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016, 5, 2294–2301. [Google Scholar] [CrossRef]
- Cowey, C.L.; Mahnke, L.; Espirito, J.; Helwig, C.; Oksen, D.; Bharmal, M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017, 13, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.; Kaufman, H.L.; Bharmal, M.; Mahnke, L.; Phatak, H.; Becker, J.C. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017, 13, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.T.H.; Yu, E.; Winquist, E.; Hammond, A.; Stitt, L.; Tonita, J.; Gilchrist, J. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin (MCC): Case series and review of 204 cases. J. Clin. 2000, 18, 2493–2499. [Google Scholar] [CrossRef]
- Ramadoss, T.; Nichols, M.; Palacios, C.; Eroglu, Z.; Markowitz, J.; Karapetyan, L.; Tarhini, A.A.; Wuthrick, E.J.; Sondak, V.K.; Khushalani, N.I.; et al. Durability of response to immune checkpoint blockade following treatment discontinuation and efficacy of rechallenge in advanced Merkel cell carcinoma. J. Immunother. Cancer 2024, 12, e009816. [Google Scholar] [CrossRef] [PubMed]
- Weppler, A.M.; Da Meda, L.; da Silva, I.P.; Xu, W.; Grignani, G.; Menzies, A.M.; Carlino, M.S.; Long, G.V.; Lo, S.N.; Nordman, I.; et al. Durability of response to immune checkpoint inhibitors in metastatic Merkel cell carcinoma after treatment cessation. Eur. J. Cancer 2023, 183, 109–118. [Google Scholar] [CrossRef]
- Schadendorf, D.; Nghiem, P.; Bhatia, S.; Hauschild, A.; Saiag, P.; Mahnke, L.; Hariharan, S.; Kaufman, H.L. Immune evasion mechanisms and immune checkpoint inhibition in advanced merkel cell carcinoma. Oncoimmunology 2017, 6, e1338237. [Google Scholar] [CrossRef]
- Tai, P. A Practical Update of Surgical Management of Merkel Cell Carcinoma of the Skin. ISRN Surg. 2013, 2013, 850797. [Google Scholar] [CrossRef]
- Cornejo, C.; Miller, C.J. Merkel Cell Carcinoma: Updates on Staging and Management. Dermatol. Clin. 2019, 37, 269–277. [Google Scholar] [CrossRef]
- Singh, B.; Qureshi, M.M.; Truong, M.T.; Sahni, D. Demographics and outcomes of stage I and II Merkel cell carcinoma treated with Mohs micrographic surgery compared with wide local excision in the National Cancer Database. J. Am. Acad. Dermatol. 2018, 79, 126–134.e3. [Google Scholar] [CrossRef]
- Shaikh, W.R.; Sobanko, J.F.; Etzkorn, J.R.; Shin, T.M.; Miller, C.J. Utilization patterns and survival outcomes after wide local excision or Mohs micrographic surgery for Merkel cell carcinoma in the United States, 2004–2009. J. Am. Acad. Dermatol. 2018, 78, 175–177.e3. [Google Scholar] [CrossRef]
- Kline, L.; Coldiron, B. Mohs micrographic surgery for the treatment of merkel cell carcinoma. Dermatol. Surg. 2016, 42, 945–951. [Google Scholar] [CrossRef]
- Terushkin, V.; Brodland, D.G.; Sharon, D.J.; Zitelli, J.A. Mohs surgery for early-stage Merkel cell carcinoma (MCC) achieves local control better than wide local excision ± radiation therapy with no increase in MCC-specific death. Int. J. Dermatol. 2021, 60, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.C.; Ovits, C.; Wong, P.; Kim, R.H. Mohs micrographic surgery reduces the need for a repeat surgery for primary Merkel cell carcinoma when compared to wide local excision: A retrospective cohort study of a commercial insurance claims database. JAAD Int. 2022, 9, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.L.; Sharon, C.E.; Tortorello, G.N.; Keele, L.; Lukens, J.N.; Karakousis, G.C.; Miura, J.T. Delayed time to radiation and overall survival in Merkel cell carcinoma. J. Surg. Oncol. 2023, 128, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Moody, B. Postoperative radiation therapy for Merkel cell carcinoma: The sooner, the better. J. Am. Acad. Dermatol. 2024, 90, 449. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Torchio, M.; Prinzi, N.; Trevisan, F.; Dallera, P.; De Stefani, A.; Russo, A.; Vitali, E.; Bruschieri, L.; et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2019, 134, 211–219. [Google Scholar] [CrossRef]
- Wong, W.G.; Stahl, K.; Olecki, E.J.; Holguin, R.P.; Pameijer, C.; Shen, C. Survival Benefit of Guideline-Concordant Postoperative Radiation for Local Merkel Cell Carcinoma. J. Surg. Res. 2021, 266, 168–179. [Google Scholar] [CrossRef]
- Levy, S.; Blankenstein, S.A.; Grünhagen, D.J.; Jalving, M.; Hamming-Vrieze, O.; Been, L.B.; Tans, L.; van Akkooi, A.C.J.; Tesselaar, M.E.T. Postoperative radiotherapy in stage I–III Merkel cell carcinoma. Radiother. Oncol. 2022, 166, 203–211. [Google Scholar] [CrossRef]
- Harley, R.J.; Lyden, M.; Aribindi, S.; Socolovsky, L.; Harley, E.H., Jr. Head and Neck Merkel Cell Carcinoma: Therapeutic Benefit of Adjuvant Radiotherapy for Nodal Disease. Laryngoscope 2024, 134, 3587–3594. [Google Scholar] [CrossRef]
- Dinges, L.A.; Eichkorn, T.; Regnery, S.; Hörner-Rieber, J.; Debus, J.; Hassel, J.C.; Lang, K. Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis. Cancers 2022, 14, 6140. [Google Scholar] [CrossRef]
- Yusuf, M.B.; Gaskins, J.; Wall, W.; Tennant, P.; Bumpous, J.; Dunlap, N. Immune status and the efficacy of radiotherapy on overall survival for patients with localized Merkel cell carcinoma: An analysis of the National Cancer Database. J. Med. Imaging Radiat. Oncol. 2020, 64, 435–443. [Google Scholar] [CrossRef]
- Vordermark, D.; Höller, U. The role of radiotherapy in the updated German S2k guideline for management of Merkel cell carcinoma. Strahlenther. Onkol. 2023, 199, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Veness, M.; Prajapati, V.H.; Jones Thachuthara, A.; Lian, J.; Assouline, A.; Yu, E.; Joseph, K. Merkel-Cell Carcinoma: Local Recurrence Rate Versus Radiation Dose Study from a 949-Patient Database. Curr. Oncol. 2025, 32, 202. [Google Scholar] [CrossRef] [PubMed]
- Pairawan, S.S.; Dominguez, C.E.; Solomon, N.; Caba-Molina, D.; O’Leary, M.; Reeves, M.E.; Namm, J.P. Adjuvant Radiotherapy for Surgically Resected Stage III Merkel Cell Carcinoma. JAMA Surg. 2024, 159, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Sokil, M.M.; Warton, E.M.; Iyer, J.; Paulson, K.G.; Nghiem, P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with merkel cell carcinoma. JAMA Dermatol. 2014, 150, 716–723. [Google Scholar] [CrossRef]
- Broida, S.E.; Chen, X.T.; Baum, C.L.; Brewer, J.D.; Block, M.S.; Jakub, J.W.; Pockaj, B.A.; Foote, R.L.; Markovic, S.N.; Hieken, T.J.; et al. Merkel cell carcinoma of unknown primary: Clinical presentation and outcomes. J. Surg. Oncol. 2022, 126, 1080–1086. [Google Scholar] [CrossRef]
- Kim, S.; Wuthrick, E.; Blakaj, D.; Eroglu, Z.; Verschraegen, C.; Thapa, R.; Mills, M.; Dibs, K.; Liveringhouse, C.; Russell, J.; et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: A randomised, open label, phase 2 trial. Lancet 2022, 400, 1008–1019. [Google Scholar] [CrossRef]
- Becker, J.C.; Hassel, J.C.; Menzer, C.; Kähler, K.C.; Eigentler, T.K.; Meier, F.E.; Berking, C.; Gutzmer, R.; Mohr, P.; Kiecker, F.; et al. Adjuvant ipilimumab compared with observation in completely resected Merkel cell carcinoma (ADMEC): A randomized, multicenter DeCOG/ADO study. J. Clin. Oncol. 2018, 36, 9527. [Google Scholar] [CrossRef]
- Becker, J.C.; Ugurel, S.; Leiter, U.; Meier, F.; Gutzmer, R.; Haferkamp, S.; Zimmer, L.; Livingstone, E.; Eigentler, T.K.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): Disease-free survival results from a randomised, open-label, phase 2 trial. Lancet 2023, 402, 798–808. [Google Scholar] [CrossRef]

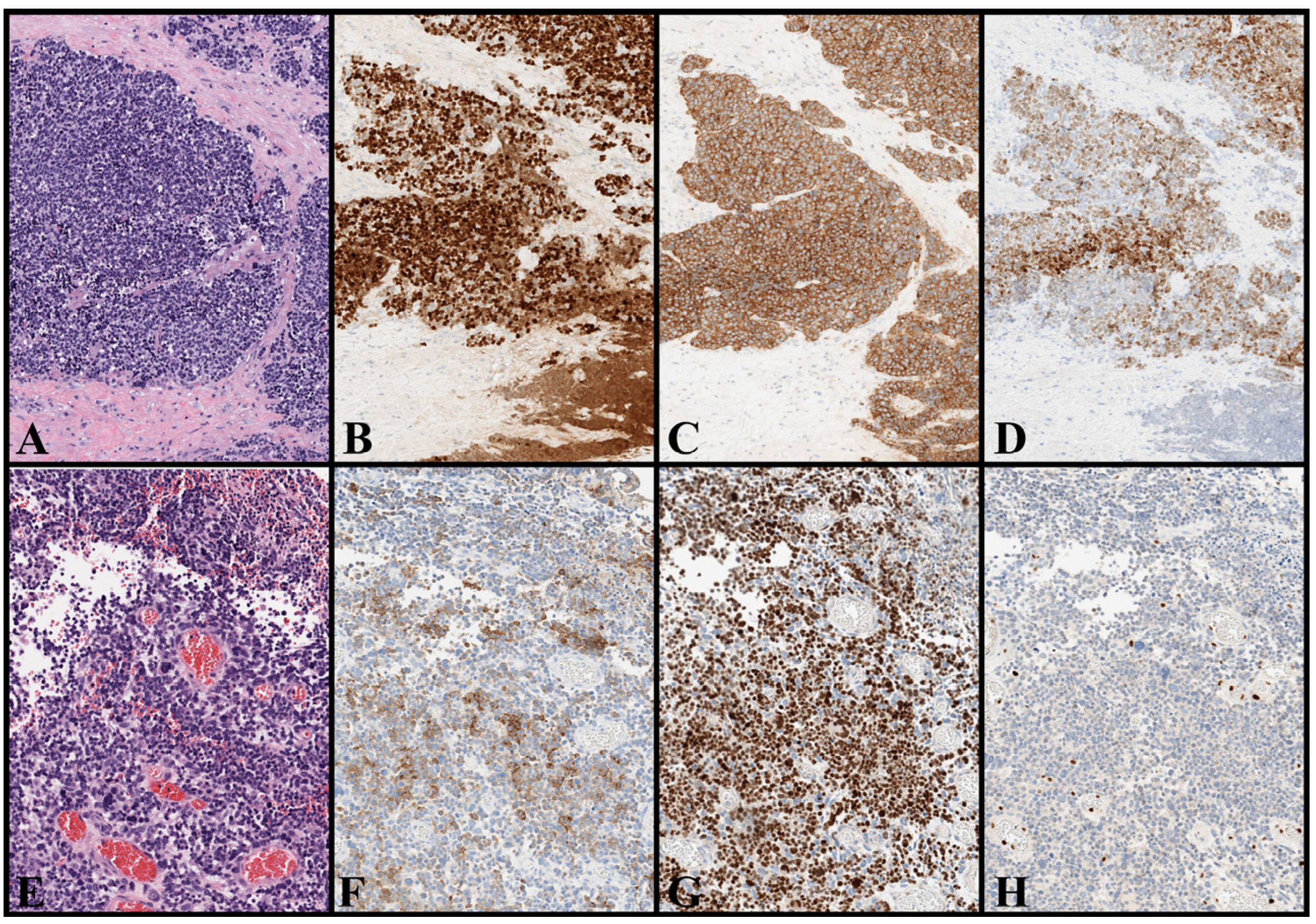

| Cell of Origin | Driving Mechanisms | Evidence Supporting the Origin of MCC from the Specific Candidate Cell | |
|---|---|---|---|
| Pros | Cons | ||
| Merkel cell | UV-related | Phenotypic similarities: CK 20, neuroendocrine markers (chromogranin A, SYA), Piezo2 and ATOH1. | No mitotic activity. No transformation/proliferation induced by MCPyV T antigens. Different anatomic localization between the candidate cell and MCC, with lack of connection between the tumor cells and epidermis. |
| Epithelial progenitor | UV-related | Presence of UV-signature (TP53, Rb inactivation). Ability to differentiate into Merkel cell and MCC. Shared mutations between in situ SCC and MCC in combined MCC/SCC tumors. Most likely origin of neuroendocrine carcinoma in other sites (SCLC). | Lack of connection between tumor cells and the epidermis. |
| Epithelial follicular progenitor | MCPyV- related | Similar DNA methylation profiles genes of UV-related MCC cell lines from epithelial origin. Common somatic mutations in combined trichoblastoma and MCPyV-MCC tumor. Expression of human MCC markers (dot-like KRT8 staining) and dermal localization of the tumors without connection to the epidermis/hair follicles in murine model. | Lack of UV signature. Genomic-level p53 inactivation in murine models, with no evidence of causation by MCPyV. |
| Fibroblast | MCPyV- related | Ability of MCPyV antigens to induce transformation in these cell types. Explain the exclusive dermal/hypodermal localization of MCC. | Lack of UV signature. No evidence of fibroblasts acquiring a Merkel cell-like phenotype. Unpredicted origin for neuroendocrine carcinoma. |
| Pre/Pro B-cell | MCPyV- related | Epidemiologic data on the association between MCC and B-cell neoplasia. Co-expression of B-cell markers (PAX5, TdT, Ig). Detection of MCPyV integration in B-cell neoplasia. | Lack of UV signature. No evidence of B-cells acquiring a Merkel cell-like phenotype. Unpredicted origin for neuroendocrine carcinoma. |
| Stain | MCC | SCLC | Other-Site SC Carcinoma | Neuroblastoma | Ewing Sarcoma | Small-Cell Melanoma | Lymphoma | Germ Cell Tumors |
|---|---|---|---|---|---|---|---|---|
| Neurofilament (NF) | + | − | − | + | + | − | − | − |
| Cytokeratin (CK) 20 | + | − | Depending on the site | − | − | − | − | − |
| Cytokeratin (CK) 7 | − * | +/− | Depending on the site | − | − | − | − | − |
| Thyroid transcription factor-1 (TTF-1) | +/− | + | − | − | − | − | − | − |
| Neuron-specific enolase (NSE) | + | + | + | + | +/− | − | − | − |
| Insulinoma-associated protein 1 (INSM1) | + | + | + | +/− | − | − | − | − |
| Chromogranin A | + | + | + | + | − | − | − | − |
| Synaptophysin (SYP) | + | + | + | + | +/− | − | − | − |
| Neural cell adhesion molecule (NCAM)/CD56 | + | + | + | + | +/− | + | +/− | − |
| S100, SOC10, and other melanocytic markers | − | − | − | − | − | + | − | − |
| Leukocyte common antigen (LCA)/CD45 and other lymphocytic/lymphoblastic markers | − | − | − | − | − | − | + | − |
| Sal-like protein 4 (SALL4) | − | − | − | − | − | − | − | + |
| PRAME | +/− | − | − | − | +/− | + | − | +/− |
| SATB2 | + | − | − | − | − | − | − | − |
| ALK | +/− | − | − | +/− | +/− | − | +/− | − |
| Rb | Loss in MCPyV-negative cases | Frequently loss | Frequently loss | Not loss | Not loss | Rarely loss | Not loss | Not loss |
| SOX2 | + | − | − | + | +/− | − | − | It depends on the histotype |
| MCPyV large T-antigen (LTAg) | + in MCPyV-positive cases | − | − | − | − | − | − | − |
| AJCC Stage | TNM Staging | Primary Tumor | Lymph Node | Metastasis | |
|---|---|---|---|---|---|
| 0 | Tis, N0, M0 | In situ (within the epidermis only) | No regional lymph node metastasis | No distant metastasis | |
| I | Clinical * | T1, N0, M0 | ≤2 cm maximum tumor dimension | Nodes negative by clinical exam (no pathological exam performed) | No distant metastasis |
| Pathologic ** | T1, pN0, M0 | ≤2 cm maximum tumor dimension | Nodes negative by pathologic exam | No distant metastasis | |
| IIA | Clinical * | T2-3, N0, M0 | >2 cm tumor dimension | Nodes negative by clinical exam (no pathological exam performed) | No distant metastasis |
| Pathologic ** | T2-3, pN0, M0 | >2 cm tumor dimension | Nodes negative by pathological exam | No distant metastasis | |
| IIB | Clinical * | T4, N0, M0 | Primary tumor invades bone, muscle, fascia, or cartilage | Nodes negative by clinical exam (no pathological exam performed) | No distant metastasis |
| Pathologic ** | T4, pN0, M0 | Primary tumor invades bone, muscle, fascia, or cartilage | Nodes negative by pathologic exam | No distant metastasis | |
| III | Clinical * | T0-4, N1-3 *****, M0 | Any size/depth tumor | Nodes positive by clinical exam (no pathological exam performed) | No distant metastasis |
| IIIA | Pathologic ** | T1-4, pN1a(sn) *** or pN1a, M0 | Any size/depth tumor | Nodes positive by pathological exam only (nodal disease not apparent on clinical exam) | No distant metastasis |
| T0, pN1b, M0 | Not detected (“unknown primary”) | Nodes positive by clinical exam, and confirmed via pathological exam | No distant metastasis | ||
| IIIB | Pathologic ** | T1-4, pN1b-3, M0 | Any size/depth tumor | Nodes positive by clinical exam, and confirmed via pathological exam OR in-transit metastasis **** | No distant metastasis |
| IV | Clinical * | T0-4, any N, M1 | Any | +/− Regional nodal involvement | Distant metastasis detected via clinical exam |
| Pathologic ** | T0-4, any pN, M1 | Any | +/− Regional nodal involvement | Distant metastasis confirmed via pathological exam | |
| Author | Study’ Type | Patient/s | Merkel Cell Carcinoma | Other Site/s of Distant Metastasis (n., %) * | OS for Bone/BM Metastatic Patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (n.) | Age (Years) | Sex | Primary MCC | Bone/BM Metastases | |||||||
| Known | Unknown | ||||||||||
| Male n (%) | Female n (%) | n. (%) | n. (%) | n. (%) | Therapy | ||||||
| Lewis et al. [6] | Original article | 215 | / | 176 (82) | 39 (18) | 173 (80) | 42 (20) | 64 (21) | CHT, RT | Non-regional LNs (88, 41%) | / |
| Kim et al. [7] ***** | Original article | 151 | 76 (62) *** | 101 (66.9) | 50 (23.1) | 134 (88.8) | 17 (11.2) | 40 (26.5) | IT, RT, surgery | LNs (94, 62.3), skin/soft tissue (40, 26.5) | 15.1 months (median) |
| Maloney et al. [125] | Original article | 331 | 74.6 (15.5) *** | 241 (72.8) | 90 (27.2) | **** | **** | 6 (1.9) | / | Liver (89, 28.7), lung (51, 16.4), brain (6, 1.9) | 5-year median OS rate of 11.2% |
| Xia et al. [126] | Original article | 273 | **** | 200 (73.3) | 73 (26.7) | 184 (67.4) | 89 (32.6) | 31 (11.3) ** | CHT, RT, surgery | Liver (37, 13.5) | 1-year median OS rate of 38.7% ** |
| Wang et al. [127] | Case report # | 1 | 79 | / | 1 (100) | / | 1 | 1 BM | / | / | / |
| Keow et al. [128] ***** | Case report # | 1 | 71 | 1 (100) | / | 1 | / | 1 BM | / | / | / |
| Goepfert et al. [129] | Original article | 41 | 66 (55) *** | **** | **** | **** | **** | 4 (9.8) | CHT | Skin (5, 12.1%), LNs (4, 9.8%) | / |
| Haykal et al. [130] | Case report # | 1 | 49 | / | 1 (100) | 1 Vulva | / | 1 Intradural intramedullary C4-C5 | CHT, RT | Regional and non-regional LNs, liver | - |
| Barkdull et al. [131] | Case report # | 1 | 55 | 1 (100) | / | 1 Scalp | / | 1 Sternum | CHT | Regional LNs, subcutaneous tissue, pancreas | 9 months |
| Khaddour et al. [132] ***** | Original article | 34 |
70.2 (51.4) *** | 20 (58.8) | 14 (41.2) | 14 (41.2) | 20 (58.8) | 10 (29.4) | CHT, IT | Regional LNs (28, 82.4) | 8.2 months (median) |
| Payne et al. [133] | Case report # | 1 | 77 | / | 1 (100) | 1 Buttock | / | 1 T4 vertebra | RT | Bone, lung | 12 months |
| Abul-Kasim et al. [134] | Case report # | 1 | 65 | 1 (100) | / | / | 1 | 1 Epidural and intradural L1, L5 | RT, surgery | Non-regional LNs, brain, retroperitoneum, lung | 8 months |
| Pennisi et al. [135] ***** | Case report # | 1 | 73 | / | 1 (100) | 1 Face | / | 1 Intradural extramedullary C6-C7 | IT (Avelumab), RT | Skin, subcutaneous tissue | 5 months |
| Leão et al. [136] ***** | Case report # | 1 | 61 | 1 (100) | / | 1 Buttock | / | 1 Sacrum | CHT, IT (Avelumab) | In-transit metastasis | 30 months |
| Lentz et al. [137] | Case report # | 1 | 55 | 1 (100) | / | 1 Scalp | / | 1 BM | CHT | Regional LNs, parotid gland | 12 months |
| Khan et al. [138] ***** | Case report # | 1 | 80 | / | 1 (100) | 1 Trunk | / | 1 BM | CHT, RT | Regional LNs | 1 month |
| Morris et al. [139] | Case report # | 1 | 72 | 1 (100) | / | 1 Shoulder | / | 1 BM | Death before starting CHT | Regional LNs | 4 months |
| Kressin et al. [140] | Case report # | 1 | 64 | 1 (100) | / | 1 Forehead | / | 1 BM | Death before starting CHT | Regional LNs | 3 months |
| Durmus et al. [141] ***** | Case report # | 1 | 60 | 1 (100) | / | 1 Thigh | / | 1 BM | Death before starting IT | Regional LNs, liver | 7 months |
| Nemoto et al. [142] | Case report # | 1 | 73 | / | 1 (100) | 1 Cheek | / | 1 BM | Death before starting therapy | Regional LNs | 8 months |
| Highland et al. [143] ***** | Case report # | 1 | 74 | 1 (100) | / | 1 Lip | / | 1 BM | CHT | Regional LNs | 13 months |
| Smadja et al. [144] | Case report # | 1 | 34 | / | 1 (100) | 1 Shoulder | / | 1 BM | CHT | Lung, brain | 4 months |
| Le Gall-Ianotto et al. [145] | Case report # | 1 | 65 | 1 (100) | / | / | 1 | 1 BM | CHT, RT | / | 3 months |
| Kobrinski et al. [146] | Case report # | 1 | 86 | 1 (100) | / | 1 Trunk | / | 1 BM | RT | Regional LNs | 12 months |
| Folyovich et al. [147] ***** | Case report # | 1 | 62 | / | 1 (100) | 1 Arm | / | 1 skull | CHT, RT | Non-regional LNs | 24 months |
| Vlad et al. [148] | Case report # | 1 | 72 | 1 (100) | / | 1 Arm | / | 1 BM | CHT | Regional LNs | 8 months |
| Goodwin et al. [149] | Case report # | 1 | 76 | 1 (100) | / | 1 Back | / | 1 Epidural T5 | RT, surgery | Bone | 15 months |
| Madden et al. [150] | Case report # | 1 | 55 | 1 (100) | / | 1 Neck | / | 1 Epidural T6-T8 | RT, surgery | Bone | 4 months |
| Moayed et al. [151] | Case report # | 1 | 70 | 1 (100) | / | / | 1 | 1 Lumbosacral spine, epidural S1, hip | CHT, RT | Regional LNs | 9 moths |
| Nguyen et al. [152] | Case report # | 1 | 69 | 1 (100) | / | 1 Cheek | / | 1 Tibia | Surgery | / | 19 months |
| Kamijo et al. [153] | Case report # | 1 | 75 | / | 1 (100) | 1 Cheek | / | 1 Femur | RT, surgery | Subcutaneous tissue | 16 months |
| Pectasides et al. [154] | Case report # | 1 | 48 | 1 (100) | / | 1 Buttock | / | 1 T11, L2 vertebra | CHT, RT | Regional LNs | 5 months |
| Pilotti et al. [155] | Original article | 50 | 62 (45) *** | 22 (44) | 28 (56) | 40 (80) | 10 (20) | 1 (2) | CHT | Skin (4, 8), liver (2, 4), pancreas (2, 4), lung (1, 2) | 12 months |
| Principe et al. [156] ***** | Case report # | 1 | 79 | 1 (100) | / | 1 Ear | / | 1 T2, T7, T10-11, L3 vertebra | IT (Avelumab), RT | Regional LNs, parotid gland | 18 months |
| Vijay et al. [157] | Case report # | 1 | 57 | / | 1 (100) | / | 1 | 1 Extra-dural T8, L4, S1 | CHT, RT | Non-regional LNs | 1 month |
| Ng et al. [158] | Case report # | 1 | 73 | 1 (100) | / | 1 Arm | / | 1 Extra-dural T5-T7 | Surgery, death before starting CHT/RT | / | 1 month |
| Turgut et al. [159] | Case report # | 1 | 63 | 1 (100) | / | 1 Abdomen | / | 1 Extradural L5–S1 | CHT | “Massive” **** | 2 months |
| Zhao et al. [160] | Case report # | 1 | 54 | 1 (100) | / | / | 1 | 1 T6, T12, L2 vertebra | CHT, RT, surgery | Regional LNs, liver | 21 moths |
| Maugeri et al. [161] | Case report # | 1 | 59 | / | 1(100) | 1 Scalp | / | 1 T7-T8 vertebra | CHT, RT | Liver, lung | 8 months |
| Chao et al. [162] | Case report # | 1 | 23 | / | 1 (100) | 1 Back | / | 1 Extradural T3-T4 | CHT, RT | Lung, heart | 23 months |
| Turgut et al. [163] | - | - | - | - | - | - | - | - | - | - | - |
| Tam et al. [164] | Case report # | 1 | 66 | 1 (100) | / | 1 Forearm | / | 1 BM | Death before therapy | / | 6 months |
| - | - | 1 | 55 | 1 (100) | / | / | 1 | 1 BM | CHT | / | 1.5 month |
| Gooptu et al. [165] | Case report # | 1 | 68 | / | 1 (100) | 1 Leg | / | 1 BM | CHT | Non-regional LNs | 2 months |
| - | - | 1 | 55 | 1 (100) | / | 1 Neck | / | 1 Vertebra | RT | Non-regional LNs, brain | 6 months |
| Park et al. [166] | Case report # | 1 | 30 | 1 (100) | / | 1 Hand | / | 1 C6 vertebra | Death before starting CH | / | 1 month |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, B.; Broseghini, E.; Ricci, C.; Corti, B.; Viola, C.; Misciali, C.; Baraldi, C.; Vaccari, S.; Lambertini, M.; Venturi, F.; et al. Merkel Cell Carcinoma: An Updated Review Focused on Bone and Bone Marrow Metastases. Cancers 2025, 17, 2253. https://doi.org/10.3390/cancers17132253
Scotti B, Broseghini E, Ricci C, Corti B, Viola C, Misciali C, Baraldi C, Vaccari S, Lambertini M, Venturi F, et al. Merkel Cell Carcinoma: An Updated Review Focused on Bone and Bone Marrow Metastases. Cancers. 2025; 17(13):2253. https://doi.org/10.3390/cancers17132253
Chicago/Turabian StyleScotti, Biagio, Elisabetta Broseghini, Costantino Ricci, Barbara Corti, Costanza Viola, Cosimo Misciali, Carlotta Baraldi, Sabina Vaccari, Martina Lambertini, Federico Venturi, and et al. 2025. "Merkel Cell Carcinoma: An Updated Review Focused on Bone and Bone Marrow Metastases" Cancers 17, no. 13: 2253. https://doi.org/10.3390/cancers17132253
APA StyleScotti, B., Broseghini, E., Ricci, C., Corti, B., Viola, C., Misciali, C., Baraldi, C., Vaccari, S., Lambertini, M., Venturi, F., Magnaterra, E., Alessandrini, A., Ferrari, T., Lepri, M., Argenziano, G., Melotti, B., Campione, E., Campana, D., Ferracin, M., & Dika, E. (2025). Merkel Cell Carcinoma: An Updated Review Focused on Bone and Bone Marrow Metastases. Cancers, 17(13), 2253. https://doi.org/10.3390/cancers17132253









