Clinical and Pathological Features and Survival Outcomes of Breast Cancers with Intermediate ER Expression
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef]
- Zhao, X.; Rødland, E.A.; Tibshirani, R.; Plevritis, S. Molecular subtyping for clinically defined breast cancer subgroups. Breast Cancer Res. 2015, 17, 29. [Google Scholar] [CrossRef]
- Rios-Hoyo, A.; Shan, N.L.; Karn, P.L.; Pusztai, L. Clinical Implications of Breast Cancer Intrinsic Subtypes. Adv. Exp. Med. Biol. 2025, 1464, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Li, Q.; Aushev, V.N.; Neugut, A.I.; Santella, R.M.; Teitelbaum, S.; Chen, J. PAM50- and immunohistochemistry-based subtypes of breast cancer and their relationship with breast cancer mortality in a population-based study. Breast Cancer 2021, 28, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Canberk, S.; Schmitt, F.; Vale, N. Molecular Subtypes and Mechanisms of Breast Cancer: Precision Medicine Approaches for Targeted Therapies. Cancers 2025, 17, 1102. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voutsadakis, I.A. Breast Cancers with Intermediate Estrogen Receptor Expression: Characteristics, Prognosis and Treatment. Clin. Breast Cancer 2024, 25, 214–222. [Google Scholar] [CrossRef]
- Yoder, R.; Kimler, B.F.; Staley, J.M.; Schwensen, K.; Wang, Y.Y.; Finke, K.; O’dea, A.; Nye, L.; Elia, M.; Crane, G.; et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer 2022, 8, 80. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch. Pathol. Lab. Med. 2023, 147, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837, Erratum in J. Clin. Oncol. 2022, 40, 2514. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, W.; Waliszczak, G.; Jach, R.; Dulińska-Litewka, J. Steroid Receptors in Breast Cancer: Understanding of Molecular Function as a Basis for Effective Therapy Development. Cancers 2021, 13, 4779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voutsadakis, I.A. Epithelial-Mesenchymal Transition (EMT) and Regulation of EMT Factors by Steroid Nuclear Receptors in Breast Cancer: A Review and in Silico Investigation. J. Clin. Med. 2016, 5, 11. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Makhlouf, S.; Quinn, C.; Toss, M.; Alsaleem, M.; Atallah, N.M.; Ibrahim, A.; Rutland, C.S.; Mongan, N.P.; Rakha, E.A. Quantitative expression of oestrogen receptor in breast cancer: Clinical and molecular significance. Eur. J. Cancer 2023, 197, 113473. [Google Scholar] [CrossRef]
- Muftah, A.A.; Aleskandarany, M.; Sonbul, S.N.; Nolan, C.C.; Rodriguez, M.D.; Caldas, C.; Ellis, I.O.; Green, A.R.; Rakha, E.A. Further evidence to support bimodality of oestrogen receptor expression in breast cancer. Histopathology 2016, 70, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Cejalvo, J.M.; Pascual, T.; Fernández-Martínez, A.; Brasó-Maristany, F.; Gomis, R.R.; Perou, C.M.; Muñoz, M.; Prat, A. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat. Rev. 2018, 67, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, A.; Pascual, T.; Perrone, G.; Morales, S.; de la Haba, J.; González-Rivera, M.; Galván, P.; Zalfa, F.; Amato, M.; Gonzalez, L.; et al. Limitations in predicting PAM50 intrinsic subtype and risk of relapse score with Ki67 in estrogen receptor-positive HER2-negative breast cancer. Oncotarget 2017, 8, 21930–21937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, T.; Kantor, O.; Harrison, B.; Giordano, J.; McGrath, M.; Burstein, H.J.; Schnitt, S.J.; Rahman, T.; Vora, H.; Garrido-Castro, A.; et al. Defining the Biology of Estrogen Receptor-Low-Positive Breast Cancer. Ann. Surg. Oncol. 2024, 31, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Stravodimou, A.; Voutsadakis, I.A. The Future of ER+/HER2- Metastatic Breast Cancer Therapy: Beyond PI3K Inhibitors. Anticancer Res. 2020, 40, 4829–4841. [Google Scholar] [CrossRef]
- Morgan, D.A.; Refalo, N.A.; Cheung, K.L. Strength of ER-positivity in relation to survival in ER-positive breast cancer treated by adjuvant tamoxifen as sole systemic therapy. Breast 2011, 20, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Fohlin, H.; Nordenskjöld, A.; Rosell, J.; Fernö, M.; Fornander, T.; Rydén, L.; Skoog, L.; Nordenskjöld, B.; Stål, O. Breast cancer hormone receptor levels and benefit from adjuvant tamoxifen in a randomized trial with long-term follow-up. Acta Oncol. 2024, 63, 535–541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stravodimou, A.; Voutsadakis, I.A. The level of estrogen receptor (ER) expression and the length of adjuvant hormonal therapy in ER positive breast cancer. Gland. Surg. 2025, 14, 246–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lam, C.; Varghese, D.; Collins, J.; Nordstrom, B.; Murphy, B.; Mehta, S.; Faherty, E. Treatment patterns and associated outcomes among patients with HER2+ metastatic breast cancer in the United States: An observational cohort study. Oncologist 2025, 30, oyae280. [Google Scholar] [CrossRef]
- André, F.; Cortés, J.; Curigliano, G.; Modi, S.; Li, W.; Park, Y.H.; Chung, W.P.; Kim, S.B.; Yamashita, T.; Pedrini, J.L.; et al. A pooled analysis of trastuzumab deruxtecan in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer with brain metastases. Ann. Oncol. 2024, 35, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Q.; Surgeoner, B.; Manna, M.; Boileau, J.F.; Gelmon, K.A.; Brackstone, M.; Brezden-Masley, C.; Jerzak, K.J.; Prakash, I.; Sehdev, S.; et al. Guidance for Canadian Breast Cancer Practice: National Consensus Recommendations for Clinical Staging of Patients Newly Diagnosed with Breast Cancer. Curr. Oncol. 2024, 31, 7226–7243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
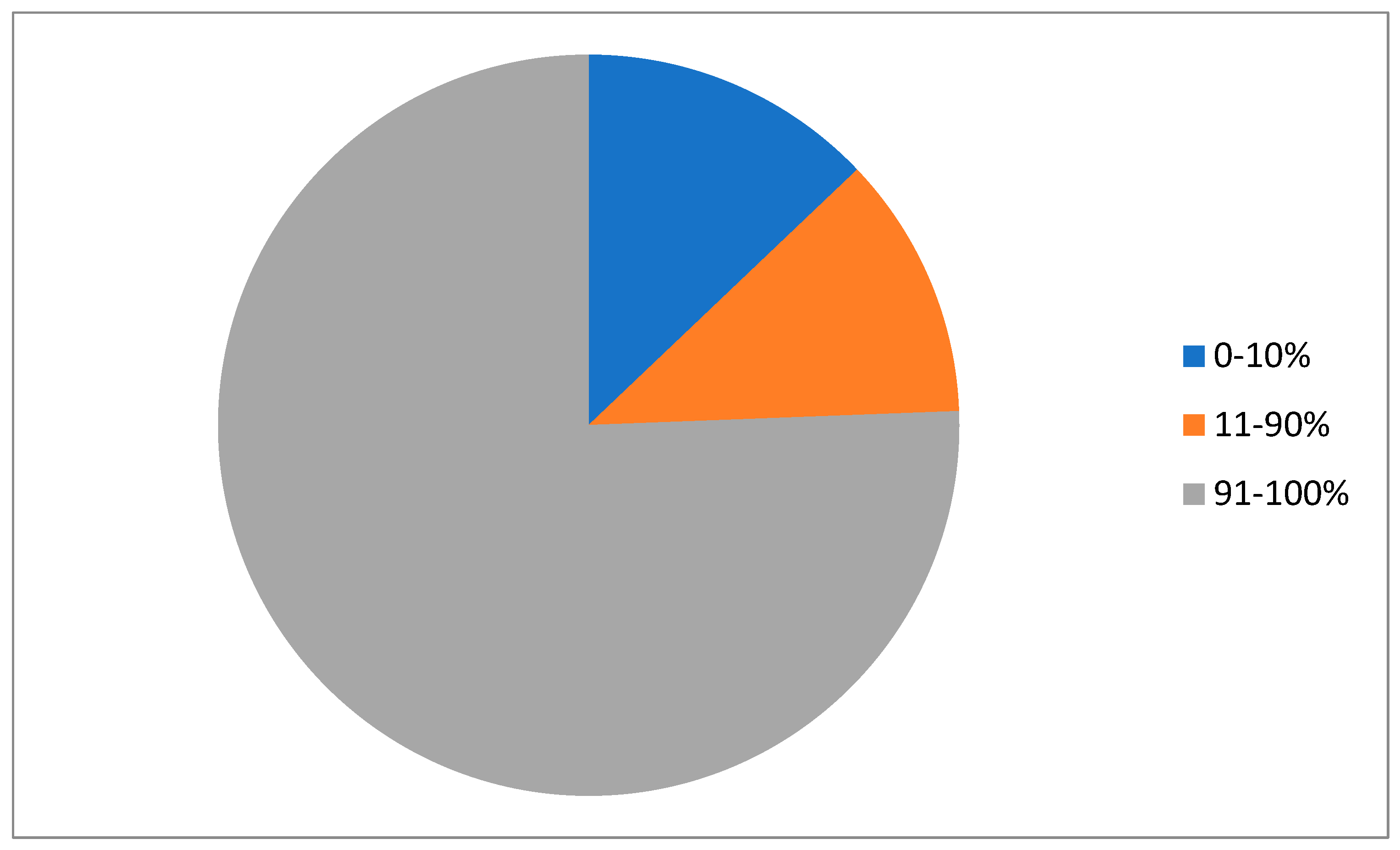
| Parameter | Entire Series (n = 794) | ER-Negative/Low (0–10%) (n = 103) | ER-Intermediate (11–90%) (n = 91) | ER-High (91–100%) (n = 600) | p |
|---|---|---|---|---|---|
| Mean age (n = 793) | 65.15 (±12.8) | 61.6 (±14.9) | 59.8 (±14.9) | 66.5 (±11.72) | <0.00001 |
| Age | |||||
| >65 years old | 396 (49.9%) | 41 (39.8%) | 33 (36.3%) | 322 (53.7%) | 0.0007 |
| ≤65 years old | 397 (50.1%) | 62 (60.2%) | 58 (63.7%) | 277 (46.3%) | |
| Menopause status (n = 793) | |||||
| Post-menopausal | 701 (88.4%) | 87 (84.5%) | 69 (75.8%) | 545 (90.9%) | <0.00001 |
| Pre-menopausal | 92 (11.6%) | 16 (15.5%) | 22 (24.2%) | 54 (9.01%) | |
| Mode of detection (n = 710) | |||||
| Clinical/Self | 337 (47.5%) | 60 (67%) | 57 (64%) | 220 (41.4%) | <0.00001 |
| Screening | 373 (52.5%) | 30 (33%) | 32 (36%) | 311 (58.6%) | |
| Obesity (n = 752) | |||||
| Obese (BMI > 30) | 323 (43%) | 36 (37.5%) | 39 (47.6%) | 248 (43.2%) | 0.38 |
| Non-obese (BMI ≤ 30) | 429 (57%) | 60 (62.5%) | 43 (52.4%) | 326 (56.8%) | |
| Diabetes (n = 784) | |||||
| Yes | 106 (13.5%) | 9 (8.7%) | 10 (11.6%) | 87 (14.6%) | 0.23 |
| No | 678 (86.5%) | 94 (92.3%) | 76 (88.4%) | 508 (85.4%) | |
| Hypertension (n = 785) | |||||
| Yes | 284 (36.2%) | 29 (28.2%) | 24 (27.6%) | 231 (38.8%) | 0.02 |
| No | 501 (63.8%) | 74 (71.8%) | 63 (72.4%) | 364 (61.2%) | |
| Dyslipidemia (n = 785) | |||||
| Yes | 147 (18.7%) | 15 (14.6%) | 14 (16.1%) | 118 (19.8%) | 0.35 |
| No | 638 (81.3%) | 88 (85.4%) | 73 (83.9%) | 477 (80.2%) | |
| Parameter | Entire Series (n = 794) | ER-Negative/Low (0–10%) (n = 103) | ER-Intermediate (11–90%) (n = 91) | ER-High (91–100%) (n = 600) | p |
|---|---|---|---|---|---|
| Stage (n = 777) | |||||
| I | 400 (51.5%) | 32 (31%) | 39 (44.3%) | 329 (55.9%) | <0.00001 |
| II | 240 (30.9%) | 35 (34%) | 23 (26.1%) | 182 (30.9%) | |
| III | 50 (6.4%) | 7 (6.8%) | 4 (4.6%) | 39 (6.6%) | |
| IV | 87 (11.2%) | 26 (25.2%) | 22 (25%) | 39 (6.6%) | |
| Tumor size (n = 700) | |||||
| >2 cm | 279 (39.9%) | 42 (71.2%) | 37 (46.2%) | 200 (35.6%) | <0.00001 |
| ≤2 cm | 421 (60.1%) | 17 (28.8%) | 43 (53.8%) | 361 (64.4%) | |
| LN status (n = 708) | |||||
| Positive | 183 (25.8%) | 22 (25%) | 25 (30.9%) | 136 (25.2%) | 0.54 |
| Negative | 525 (74.2%) | 66 (75%) | 56 (69.1%) | 403 (74.8%) | |
| Histology (n = 794) | |||||
| IDC | 591 (74.4) | 89 (86.4) | 62 (68.1) | 440 (73.3) | 0.001 |
| ILC/Mixed | 104 (13.1) | 2 (1.9) | 12 (13.2) | 90 (15) | |
| Other | 99 (12.5) | 12 (11.7) | 17 (18.7) | 70 (11.7) | |
| Grade (n = 709) | |||||
| 1–2 | 463 (65.3%) | 9 (10.6%) | 27 (37.5%) | 427 (77.4%) | <0.00001 |
| 3 | 246 (34.7%) | 76 (89.4%) | 45 (62.5%) | 125 (22.6%) | |
| LVI (n = 722) | |||||
| Present | 49 (6.8%) | 7 (7.7%) | 11 (14.3%) | 31 (5.6%) | 0.01 |
| Absent | 673 (93.2%) | 84 (92.3%) | 66 (85.7%) | 523 (94.4%) | |
| PNI (n = 725) | |||||
| Present | 12 (1.7%) | 0 (0.00%) | 3 (3.9%) | 9 (1.6%) | 0.27 |
| Absent | 713 (98.3%) | 91 (100%) | 74 (96.1%) | 548 (98.4%) | |
| ER histoscore (n = 794) | |||||
| >240 | 606 (76.3%) | 0 (0%) | 20 (22%) | 586 (97.7%) | <0.00001 |
| ≤240 | 188 (23.7%) | 103 (100%) | 71 (78%) | 14 (2.3%) | |
| PR histoscore (n = 751) | |||||
| >60 | 440 (58.6%) | 0 (0%) | 30 (33.3%) | 410 (73.5%) | <0.00001 |
| ≤60 | 311 (41.4%) | 103 (100%) | 60 (66.7%) | 148 (26.5%) | |
| HER2 status (n = 794) | |||||
| Positive | 97 (12.2%) | 25 (24.3%) | 33 (36.3%) | 39 (6.5%) | <0.00001 |
| Negative | 697 (87.8%) | 78 (75.7%) | 58 (63.7%) | 561 (93.5%) | |
| Oncotype Dx RS (n = 284) | |||||
| ≤18 | 203 (71.5%) | 0 | 8 (38.1%) | 195 (75.3%) | <0.00001 |
| 19–24 | 42 (14.8%) | 0 | 2 (9.5%) | 40 (15.4%) | |
| >24 | 39 (13.7%) | 4 (100%) | 11 (52.4%) | 24 (9.3%) | |
| Parameter | Entire Series | ER-Negative/Low (0–10%) | ER-Intermediate (11–90%) | ER-High (91–100%) | p |
|---|---|---|---|---|---|
| Surgery type (n = 729) | |||||
| Lumpectomy | 513 (70%) | 42 (46.7) | 49 (62%) | 422 (75.4%) | <0.00001 |
| Mastectomy | 216 (30%) | 48 (53.3%) | 30 (38%) | 138 (24.6%) | |
| Adjuvant hormonal therapy (n = 790) | |||||
| Yes | 617 (78.1%) | 13 (12.6%) | 70 (80.5%) | 534 (89%) | <0.00001 |
| No | 173 (21.9%) | 90 (87.4%) | 17 (19.5%) | 66 (11%) | |
| Adjuvant radiotherapy (n = 790) | |||||
| Yes | 548 (69.4%) | 61 (59.2%) | 56 (64.37%) | 431 (71.8%) | 0.02 |
| No | 242 (30.6%) | 42 (40.8%) | 31 (35.63%) | 169 (28.2%) | |
| Adjuvant chemotherapy (n = 790) | |||||
| Yes | 269 (34.1) | 76 (73.8%) | 45 (52%) | 148 (24.7%) | <0.00001 |
| No | 521 (65.9%) | 27 (26.2%) | 42 (48%) | 452 (75.3%) | |
| Parameter | Entire Series | ER-Negative/Low (0–10%) | ER-Intermediate (11–90%) | ER-High (91–100%) | p |
|---|---|---|---|---|---|
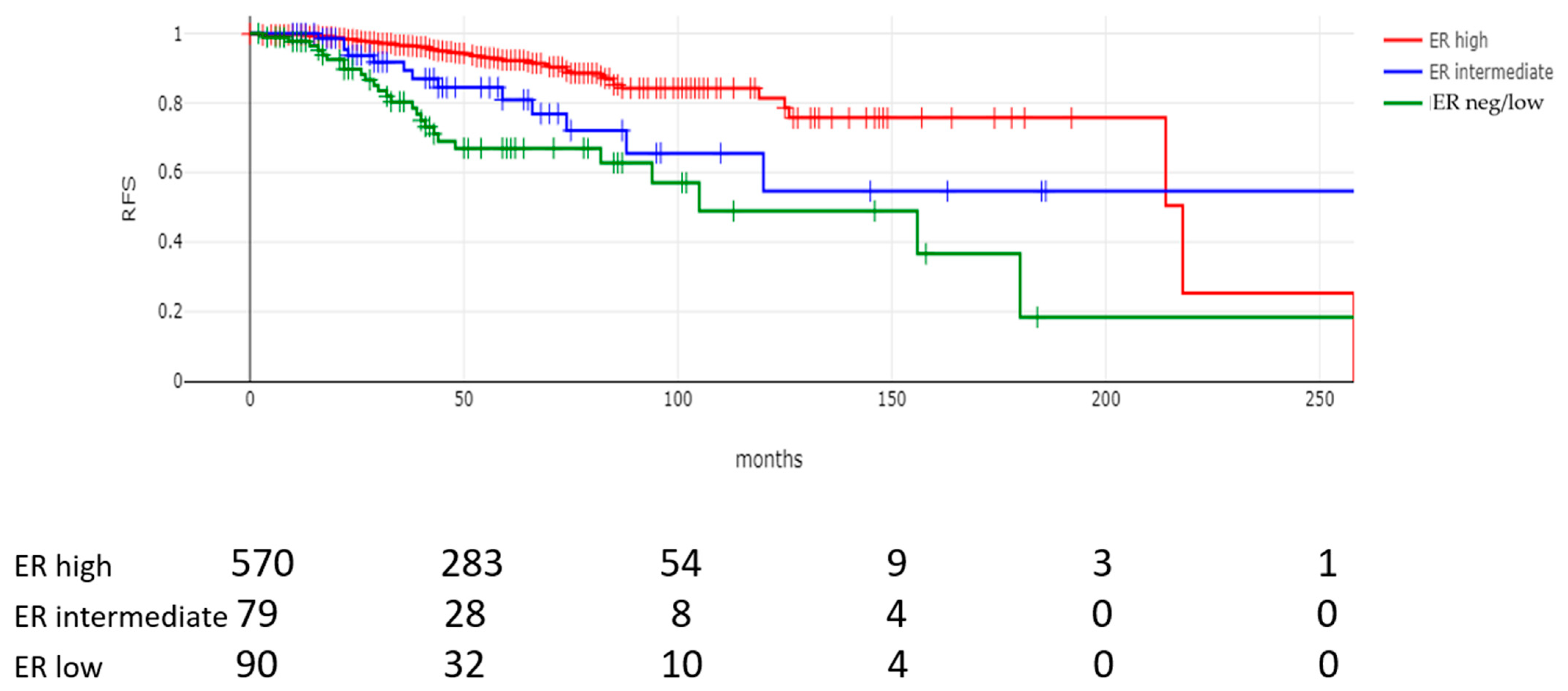
| Relapse rate n = 739 | 87 (11.8%) | 26 (28.9%) | 13 (16.5%) | 48 (8.4%) | <0.00001 |
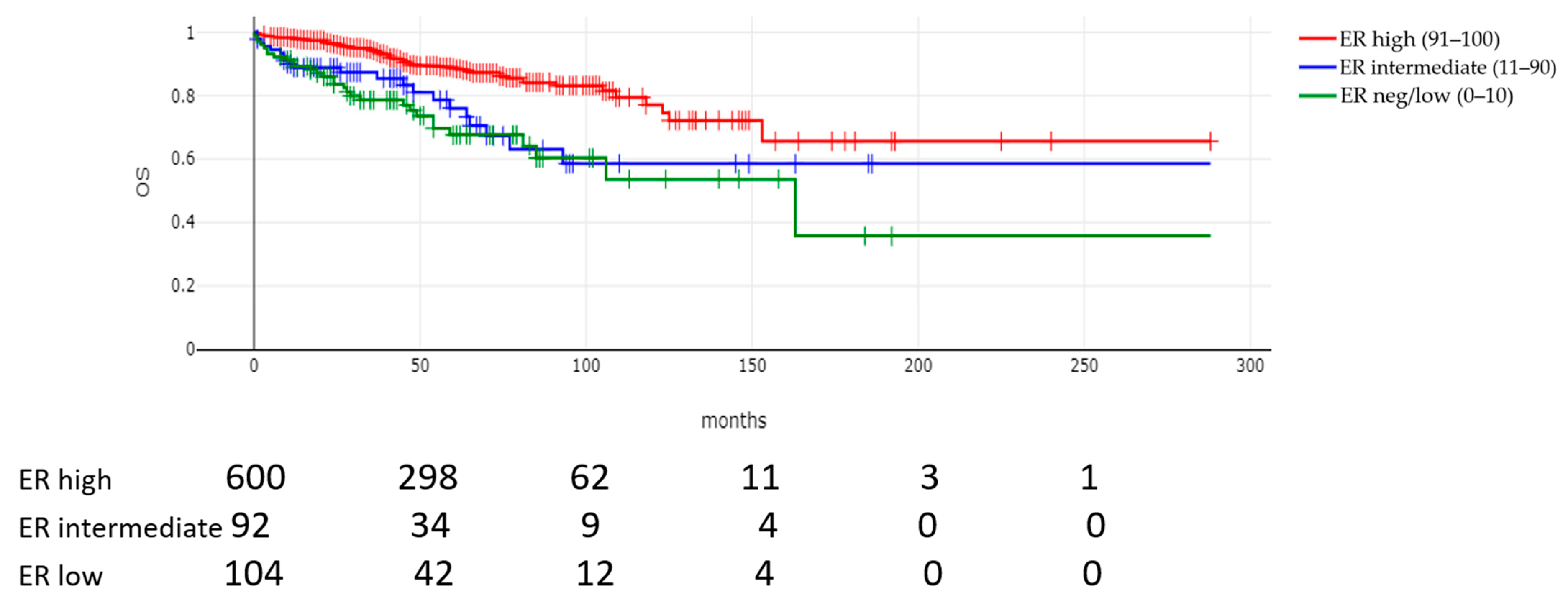
| Mortality rate n = 794 | 118 (14.9%) | 30 (29.1%) | 21 (23.1%) | 67 (11.7%) | <0.00001 |
| Median time to relapse (months, IQR) n = 739 | 46 (26–74) | 38 (21.2–61) | 32 (22–65.5) | 49 (30–75) | 0.001 |
| Median survival time (months, IQR) n = 794 | 47(27–74.5) | 41 (21.5–64) | 37 (18.5–67.5) | 49 (30–76) | 0.002 |
| Variable | Coefficient | Hazard Ratio | 95% CI | p |
|---|---|---|---|---|
| Age | 0.046 | 1.04 | 1.02–1.06 | 0.0001 |
| ER | −0.001 | 0.99 | 0.98–1.00 | 0.76 |
| PR | −0.001 | 0.99 | 0.99–1.00 | 0.78 |
| Stage | 0.597 | 1.81 | 1.33–2.47 | 0.0001 |
| Grade | 0.592 | 1.80 | 1.14–2.86 | 0.01 |
| Histology | 0.375 | 1.45 | 0.98–2.16 | 0.06 |
| Hormonal treatment | −0.906 | 0.40 | 0.19–0.84 | 0.01 |
| Variable | Coefficient | Hazard Ratio | 95% CI | p |
|---|---|---|---|---|
| Age | 0.0005 | 1.00 | 0.98–1.01 | 0.95 |
| ER | −0.011 | 0.98 | 0.97–0.99 | 0.03 |
| PR | 0.003 | 0.99 | 0.98–1.00 | 0.4 |
| Stage | 0.704 | 2.02 | 1.46–2.79 | 0.00001 |
| Grade | 0.279 | 1.32 | 0.84–2.07 | 0.22 |
| Histology | 0.357 | 1.42 | 0.97–2.09 | 0.06 |
| Hormonal treatment | −0.561 | 0.57 | 0.26–1.23 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, J.; Lambert, N.; Voutsadakis, I.A. Clinical and Pathological Features and Survival Outcomes of Breast Cancers with Intermediate ER Expression. Cancers 2025, 17, 2252. https://doi.org/10.3390/cancers17132252
Hammond J, Lambert N, Voutsadakis IA. Clinical and Pathological Features and Survival Outcomes of Breast Cancers with Intermediate ER Expression. Cancers. 2025; 17(13):2252. https://doi.org/10.3390/cancers17132252
Chicago/Turabian StyleHammond, Jonathan, Nicholas Lambert, and Ioannis A. Voutsadakis. 2025. "Clinical and Pathological Features and Survival Outcomes of Breast Cancers with Intermediate ER Expression" Cancers 17, no. 13: 2252. https://doi.org/10.3390/cancers17132252
APA StyleHammond, J., Lambert, N., & Voutsadakis, I. A. (2025). Clinical and Pathological Features and Survival Outcomes of Breast Cancers with Intermediate ER Expression. Cancers, 17(13), 2252. https://doi.org/10.3390/cancers17132252






