Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. HER2 Status Definition
2.3. Patient Population
2.4. Study Aims and Endpoints
2.5. Statistical Analyses
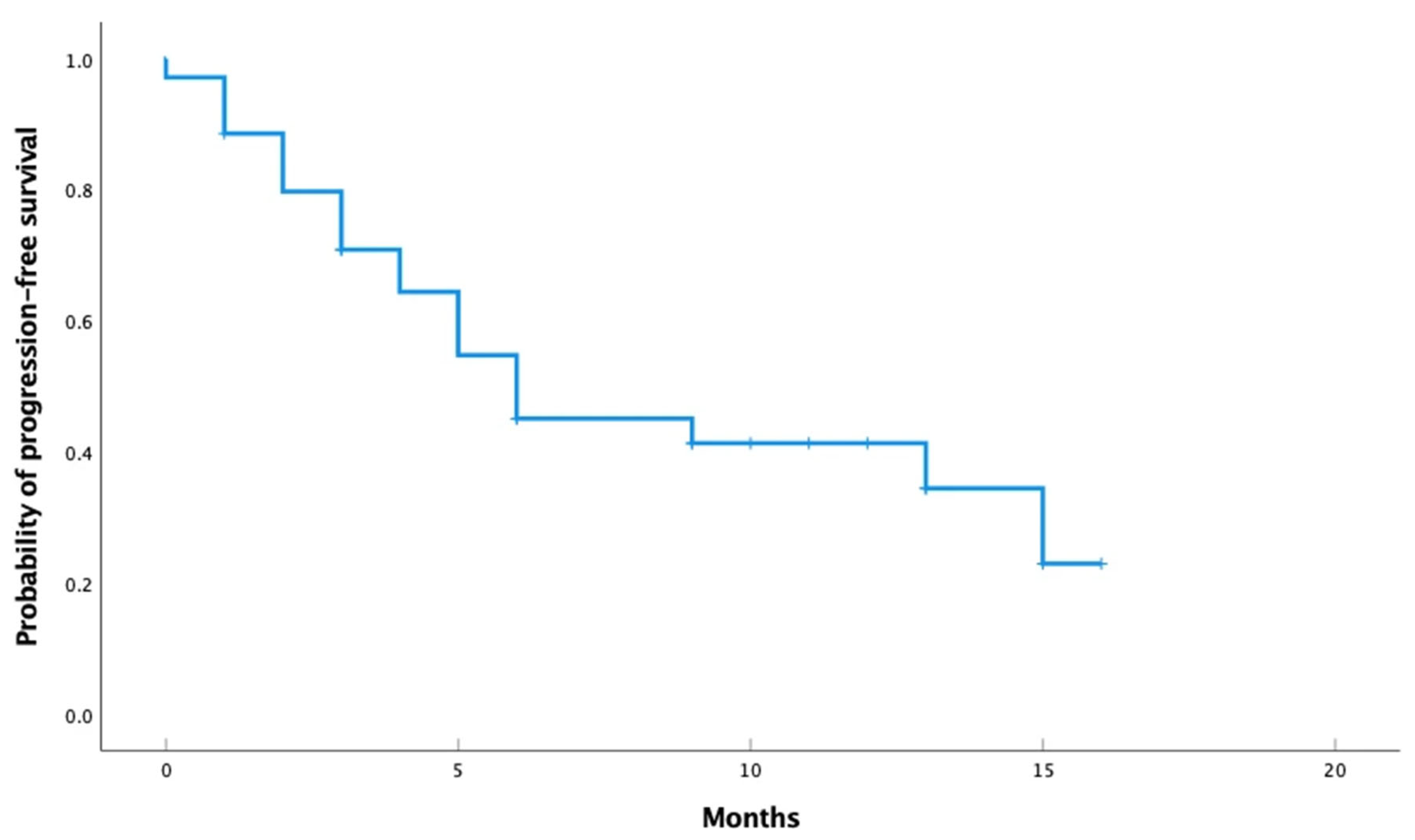
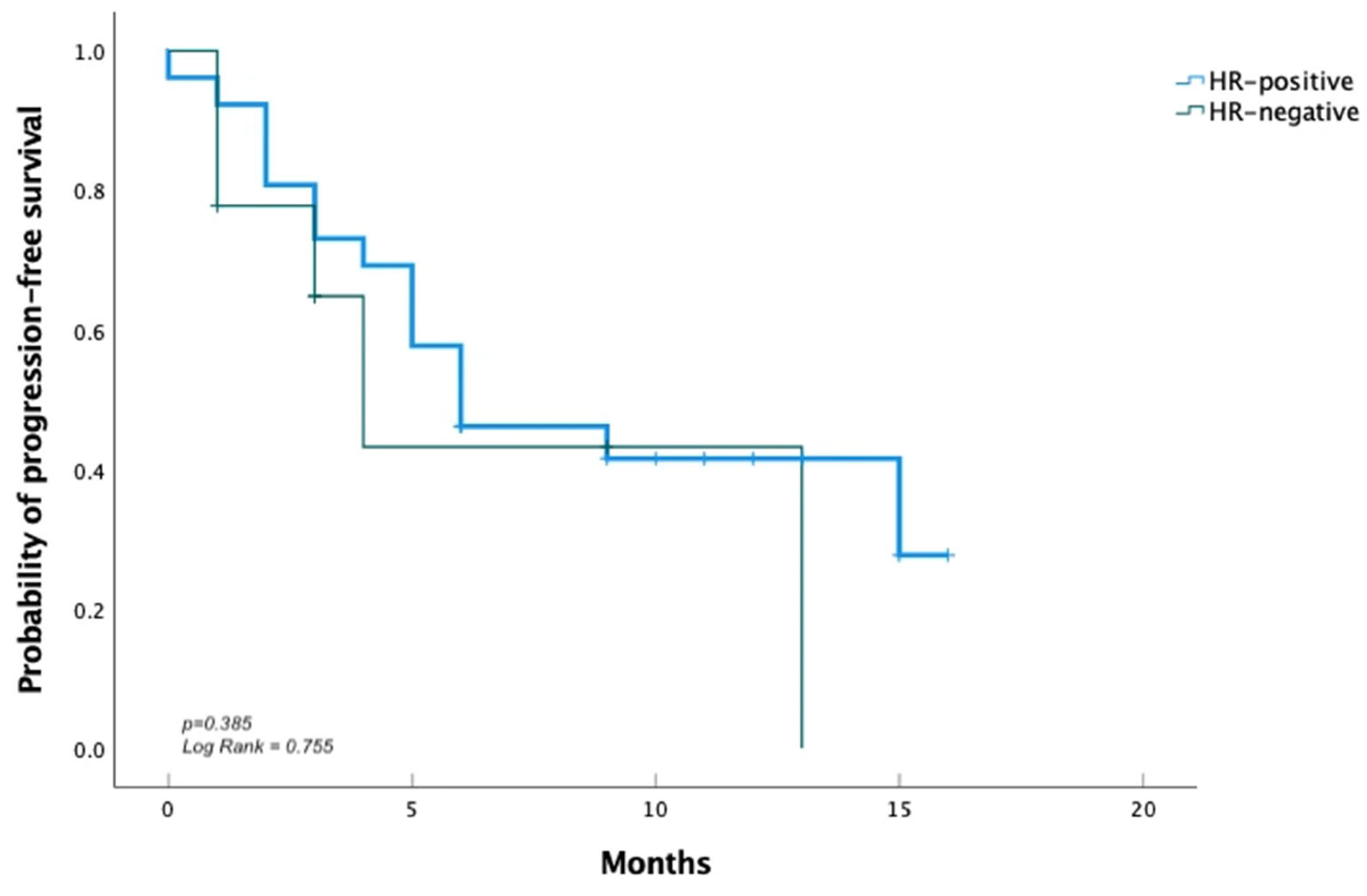
3. Results
3.1. Efficacy
3.2. Safety
4. Discussion
Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.; Ding, Y.; Chen, Y.; Cai, Y.; Wang, L.; Huang, L.; Liu, C.; Shao, Z.; Yu, K. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Woo, J.W.; Lee, K.; Park, S.Y. HER2 status in breast cancer: Changes in guidelines and complicating factors for interpretation. J. Pathol. Transl. Med. 2020, 54, 34–44. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Marty, M.; Cognetti, F.; Maraninchi, D.; Snyder, R.; Mauriac, L.; Tubiana-Hulin, M.; Chan, S.; Grimes, D.; Antón, A.; Lluch, A.; et al. Randomized Phase II Trial of the Efficacy and Safety of Trastuzumab Combined With Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer Administered As First-Line Treatment: The M77001 Study Group. J. Clin. Oncol. 2005, 23, 4265–4274. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Roy, A.M.; Kumarasamy, V.M.; Dhakal, A.; O’regan, R.; Gandhi, S. A review of treatment options in HER2-low breast cancer and proposed treatment sequencing algorithm. Cancer 2023, 129, 2773–2788. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody–Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Lee, D.; Foldi, J.; Soulos, P.R.; Gross, C.P.; Grinda, T.; Winer, E.P.; Lin, N.U.; Krop, I.E.; Tolaney, S.M.; et al. Outcomes with trastuzumab deruxtecan (T-DXd) by HER2 status and line of treatment in a large real-world database of patients with metastatic breast cancer. J. Clin. Oncol. 2024, 42, 1077. [Google Scholar] [CrossRef]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’shaughnessy, J.A.; Wildiers, H.; Pierga, J.-Y.; Zhang, Q.; Saura, C.; et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Al Sukhun, S.; Koczwara, B.; Temin, S.; Arun, B.K. Systemic Treatment of Patients With Metastatic Breast Cancer: ASCO Resource–Stratified Guideline Q and A. JCO Glob. Oncol. 2024, 10, e2300411. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, P.; Li, F.; Lai, H.; Qi, T.; Wang, Y. Advances in the study of marketed antibody-drug Conjugates (ADCs) for the treatment of breast cancer. Front. Pharmacol. 2024, 14, 1332539. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Géraud, A.; Gougis, P.; de Nonneville, A.; Beaufils, M.; Bertucci, F.; Billon, E.; Brisou, G.; Gravis, G.; Greillier, L.; Guerin, M.; et al. Pharmacology and pharmacokinetics of antibody-drug conjugates, where do we stand? Cancer Treat. Rev. 2025, 135, 102922. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Tarantino, P.; Pestana, R.C.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.; Curigliano, G. Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA A Cancer J. Clin. 2021, 72, 165–182. [Google Scholar] [CrossRef]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: Current landscape and future perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef]
| Characteristics | |
|---|---|
| Median age-yr (range) | 54 (40–76) |
| Female sex-no (%) | 34 (97.1) |
| Race-no (%) | |
| White | 34 (97.1) |
| Black | 1 (2.9) |
| HER2-low status-no (%) ‡ | |
| IHC 1+ | 26 (74.3) |
| IHC 2+ and ISH-negative | 9 (25.7) |
| ECOG performance status score-no (%) § | |
| 0 | 13 (37.1) |
| 1 | 22 (62.9) |
| Hormone receptor-positive-no (%) | 26 (74.3) |
| Metastasis-no (%) | |
| Brain | 6 (17.1) |
| Bone | 26 (74.3) |
| Visceral | 29 (82.9) |
| Median no. of lines (range) | 4 (1–7) |
| No of lines (%) | |
| 1 | 1 (2.9) |
| 2 | 4 (11.4) |
| ≥3 | 30 (85.7) |
| Events | All Grade | Grade 1–2 | Grade ≥3 |
|---|---|---|---|
| Number of patients (percent %) | |||
| Blood and lymphatic system disorders | |||
| Neutropenia | 5 (14.3%) | 4 (11.4%) | 1 (2.9%) |
| Anemia | 12 (34.3%) | 12 (34.3%) | 0 |
| Thrombocytopenia | 3 (8.6%) | 3 (8.6%) | 0 |
| Gastrointestinal disorders | |||
| Nausea | 12 (34.3%) | 12 (34.3%) | 0 |
| Vomiting | 7 (20.0%) | 7 (20.0%) | 0 |
| Diarrhea | 6 (17.1%) | 6 (17.1%) | 0 |
| Constipation | 4 (11.4%) | 4 (11.4%) | 0 |
| General disorders | |||
| Fatigue | 22 (62.9%) | 21(57.0%) | 1 (2.9%) |
| Anorexia | 13 (37.1%) | 13 (37.1%) | 0 |
| Skin and subcutaneous tissue disorders | |||
| Alopecia | 11 (31.4%) | 11(31.4%) | 0 |
| Cardiac toxicity (LVEF reduction) | 2 (5.7%) | 2 (5.7%) | 0 |
| Hepatic toxicity (increased aminotransferase levels) | 9 (25.7%) | 9 (25.7%) | 0 |
| Pulmonary toxicity (interstitial lung disease or pneumonitis) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares Miranda, L.; Sousa, M.J.; Martins Braga, M.; Couto, M.; Vieira Fernandes, I.; Abreu, F.; Eiriz, I.; Lopes Fernandes, C.; Fonseca Marques, A.; Marques, M.T.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population. Cancers 2025, 17, 1911. https://doi.org/10.3390/cancers17121911
Soares Miranda L, Sousa MJ, Martins Braga M, Couto M, Vieira Fernandes I, Abreu F, Eiriz I, Lopes Fernandes C, Fonseca Marques A, Marques MT, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population. Cancers. 2025; 17(12):1911. https://doi.org/10.3390/cancers17121911
Chicago/Turabian StyleSoares Miranda, Luísa, Maria João Sousa, Miguel Martins Braga, Marisa Couto, Isabel Vieira Fernandes, Francisca Abreu, Inês Eiriz, Catarina Lopes Fernandes, Alice Fonseca Marques, Maria Teresa Marques, and et al. 2025. "Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population" Cancers 17, no. 12: 1911. https://doi.org/10.3390/cancers17121911
APA StyleSoares Miranda, L., Sousa, M. J., Martins Braga, M., Couto, M., Vieira Fernandes, I., Abreu, F., Eiriz, I., Lopes Fernandes, C., Fonseca Marques, A., Marques, M. T., Romão, R., Gonçalves, F., Simões, J., & Araújo, A. (2025). Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population. Cancers, 17(12), 1911. https://doi.org/10.3390/cancers17121911







