Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Primary Objective
2.4. Quality Assessment
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics of Selected Studies
3.2. Temporal, Quantitative, and Qualitative Characteristics of PPIs Use
3.3. Impact of PPIs Use on PFS and OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-Driven Mechanisms at Different Stages of Cancer Development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.L.; Carson, T.L. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: A narrative review. Gut Pathog. 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Author Correction: Gut Microbiome Correlates of Response and Toxicity Following Anti-CD19 CAR T Cell Therapy. Nat. Med. 2023, 29, 2954. [Google Scholar] [CrossRef]
- Sitthideatphaiboon, P.; Somlaw, N.; Zungsontiporn, N.; Ouwongprayoon, P.; Sukswai, N.; Korphaisarn, K.; Poungvarin, N.; Aporntewan, C.; Hirankarn, N.; Vinayanuwattikun, C.; et al. Dietary Pattern and the Corresponding Gut Microbiome in Response to Immunotherapy in Thai Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). Sci. Rep. 2024, 14, 27791. [Google Scholar] [CrossRef]
- Lazzari, C.; Spagnolo, C.C.; Ciappina, G.; Di Pietro, M.; Squeri, A.; Passalacqua, M.I.; Marchesi, S.; Gregorc, V.; Santarpia, M. Immunotherapy in Early-Stage Non-Small Cell Lung Cancer (NSCLC): Current Evidence and Perspectives. Curr. Oncol. 2023, 30, 3684–3696. [Google Scholar] [CrossRef]
- Kim, P.; Joe, S.; Kim, H.; Jeong, H.; Park, S.; Song, J.; Kim, W.; Lee, Y.G. Hidden Partner of Immunity: Microbiome as an Innovative Companion in Immunotherapy. Int. J. Mol. Sci. 2025, 26, 856. [Google Scholar] [CrossRef]
- Fucà, G.; Galli, G.; Poggi, M.; Lo Russo, G.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of Peripheral Blood Immune Cells by Early Use of Steroids and Its Association with Clinical Outcomes in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. ESMO Open 2019, 4, e000457. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Sawaid, I.O.; Samson, A.O. Proton Pump Inhibitors and Cancer Risk: A Comprehensive Review of Epidemiological and Mechanistic Evidence. J. Clin. Med. 2024, 13, 1970. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Cardona, A.; Nagarkar, D.R.; Dhawahir Scala, A.; Gandara, D.R.; Rittmeyer, A.; Albert, M.L.; Powles, T.; Kok, M.; Herrera, F.G. Efficacy of Chemotherapy and Atezolizumab in Patients with Non-Small-Cell Lung Cancer Receiving Antibiotics and Proton Pump Inhibitors: Pooled Post Hoc Analyses of the OAK and POPLAR Trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef]
- Homicsko, K.; Dummer, R.; Hoeller, C.; Wolchok, J.D.; Hodi, F.S.; Larkin, J.; Ascierto, P.A.; Atkinson, V.; Robert, C.; Postow, M.A.; et al. Proton Pump Inhibitor Use and Efficacy of Nivolumab and Ipilimumab in Advanced Melanoma. Cancers 2022, 14, 2300. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lopes, S.; Pabst, L.; Dory, A.; Klotz, M.; Gourieux, B.; Michel, B.; Mascaux, C. Do Proton Pump Inhibitors Alter the Response to Immune Checkpoint Inhibitors in Cancer Patients? A Meta-Analysis. Front. Immunol. 2023, 14, 1070076. [Google Scholar] [CrossRef]
- Rizzo, A.; Cusmai, A.; Giovannelli, F.; Acquafredda, S.; Rinaldi, L.; Misino, A.; Montagna, E.S.; Ungaro, V.; Lorusso, M.; Palmiotti, G. Impact of Proton Pump Inhibitors and Histamine-2-Receptor Antagonists on Non-Small Cell Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1404. [Google Scholar] [CrossRef]
- Rizzo, A.; Santoni, M.; Mollica, V.; Ricci, A.D.; Calabrò, C.; Cusmai, A.; Gadaleta-Caldarola, G.; Palmiotti, G.; Massari, F. The Impact of Concomitant Proton Pump Inhibitors on Immunotherapy Efficacy among Patients with Urothelial Carcinoma: A Meta-Analysis. J. Pers. Med. 2022, 12, 842. [Google Scholar] [CrossRef]
- Chen, B.; Yang, C.; Dragomir, M.P.; Chi, D.; Chen, W.; Horst, D.; Calin, G.A.; Li, Q. Association of proton pump inhibitor use with survival outcomes in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221111703. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; Mao, H.; Tong, J.; Yang, M.; Yan, X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 753234. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, Y.; Huang, W.; Li, P.; Guo, C.; Li, Y. The Impact of Concomitant Proton Pump Inhibitors Therapy on Clinical Outcome of Cancer Patients Treated With Immune Checkpoint Inhibitors: A Meta-analysis. Am. J. Clin. Oncol. 2023, 46, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Li, X. A Meta-Analysis of the Impact of Proton Pump Inhibitors on Survival Outcomes in NSCLC Treated with Immunotherapy. J. Biol. Regul. Homeost. Agents 2025, 39, 3515. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-randomized Studies (MINORS): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Bowden, J.; Baker, R. How Does the DerSimonian and Laird Procedure for Random Effects Meta-Analysis Compare with Its More Efficient but Harder to Compute Counterparts? J. Stat. Plan. Inference 2010, 140, 961–970. [Google Scholar] [CrossRef]
- Altman, D.; Machin, D.; Bryant, T.; Gardner, M.J. Statistics with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Takada, K.; Takamori, S.; Shimokawa, M.; Pinato, D.J.; Cortellini, A. Prior Antibiotics, Proton Pump Inhibitors, and Probiotics in Patients with Extensive Stage Small Cell Lung Cancer Treated with Immune Checkpoint Blockade: A Post-hoc Analysis of the Phase I/III IMpower 133 Trial. Int. J. Cancer 2025, 156, 914–919. [Google Scholar] [CrossRef]
- Isono, T.; Furuno, H.; Onodera, Y.; Maruyama, T.; Takeuchi, Y.; Ayaka, K.; Nishida, T.; Kobayashi, Y.; Ishiguro, T.; Takaku, Y.; et al. Analysis of Immune Checkpoint Inhibitors for Advanced Non-Small Cell Lung Cancer in Patients Receiving Antacids. Respir. Investig. 2024, 62, 951–959. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.H.; Heo, J.Y.; Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, J.H. Impact of Concurrent Medications on Clinical Outcomes of Cancer Patients Treated with Immune Checkpoint Inhibitors: Analysis of Health Insurance Review and Assessment Data. J. Cancer Res. Clin. Oncol. 2024, 150, 186. [Google Scholar] [CrossRef]
- Lasagna, A.; Mascaro, F.; Figini, S.; Basile, S.; Gambini, G.; Klersy, C.; Lenti, M.V.; Di Sabatino, A.; Di Benedetto, A.; Calvi, M.; et al. Impact of Proton Pump Inhibitors on the Onset of Gastrointestinal Immune-related Adverse Events during Immunotherapy. Cancer Med. 2023, 12, 19530–19536. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Y.; Yang, G.; Chen, H.; Wang, X.; Fu, J.; Li, L.; Pan, X. The Impact of Proton Pump Inhibitors on the Efficacy of Immune Checkpoint Inhibitor Combinations in Patients with HBV-Associated Advanced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 1311–1321. [Google Scholar] [CrossRef]
- Kawachi, H.; Yamada, T.; Tamiya, M.; Negi, Y.; Goto, Y.; Nakao, A.; Shiotsu, S.; Tanimura, K.; Takeda, T.; Okada, A.; et al. Concomitant Proton Pump Inhibitor Use With Pembrolizumab Monotherapy vs. Immune Checkpoint Inhibitor Plus Chemotherapy in Patients With Non−Small Cell Lung Cancer. JAMA Netw. Open 2023, 6, e2322915. [Google Scholar] [CrossRef]
- Yamada, J.; Fukui, T.; Yatani, A.; Mimura, C.; Fukuda, K.; Hazama, D.; Katsurada, N.; Nagano, T.; Yamamoto, M.; Tachihara, M. Impact of Concurrent Medications on the Outcome of Immunotherapy in Non-small Cell Lung Carcinoma. Thorac. Cancer 2024, 15, 1228–1236. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Wang, J.; Liu, Y.; Yan, H.; Xing, X.; Yang, J. Proton Pump Inhibitors Alter Gut Microbiota by Promoting Oral Microbiota Translocation: A Prospective Interventional Study. Gut 2024, 73, 1098–1109. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Fossmark, R.; Olaisen, M. Changes in the Gastrointestinal Microbiota Induced by Proton Pump Inhibitors—A Review of Findings from Experimental Trials. Microorganisms 2024, 12, 1110. [Google Scholar] [CrossRef]
- Tian, L.; Huang, C.; Fu, W.; Gao, L.; Mi, N.; Bai, M.; Ma, H.; Zhang, C.; Lu, Y.; Zhao, J.; et al. Proton Pump Inhibitors May Enhance the Risk of Digestive Diseases by Regulating Intestinal Microbiota. Front. Pharmacol. 2023, 14, 1217306. [Google Scholar] [CrossRef]
- Moreels, N.; Boven, A.; Gressani, O.; Andersson, F.L.; Vlieghe, E.; Callens, S.; Engstrand, L.; Simin, J.; Brusselaers, N. The Combined Effect of Systemic Antibiotics and Proton Pump Inhibitors on Clostridioides Difficile Infection and Recurrence. J. Antimicrob. Chemother. 2024, 79, 608–616. [Google Scholar] [CrossRef]
- Lee, I.; Jo, J.-W.; Woo, H.-J.; Suk, K.T.; Lee, S.S.; Kim, B.-S. Proton Pump Inhibitors Increase the Risk of Carbapenem-Resistant Enterobacteriaceae Colonization by Facilitating the Transfer of Antibiotic Resistance Genes among Bacteria in the Gut Microbiome. Gut Microbes 2024, 16, 2341635. [Google Scholar] [CrossRef]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut Microbiota Influences the Efficiency of Immune Checkpoint Inhibitors by Modulating the Immune System (Review). Oncol. Lett. 2024, 27, 87. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Scolyer, R.A.; Long, G.V. Towards Modulating the Gut Microbiota to Enhance the Efficacy of Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2023, 20, 697–715. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar] [CrossRef]
- Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. [Google Scholar] [CrossRef]
- Yin, B.; Wang, X.; Yuan, F.; Li, Y.; Lu, P. Research Progress on the Effect of Gut and Tumor Microbiota on Antitumor Efficacy and Adverse Effects of Chemotherapy Drugs. Front. Microbiol. 2022, 13, 899111. [Google Scholar] [CrossRef]

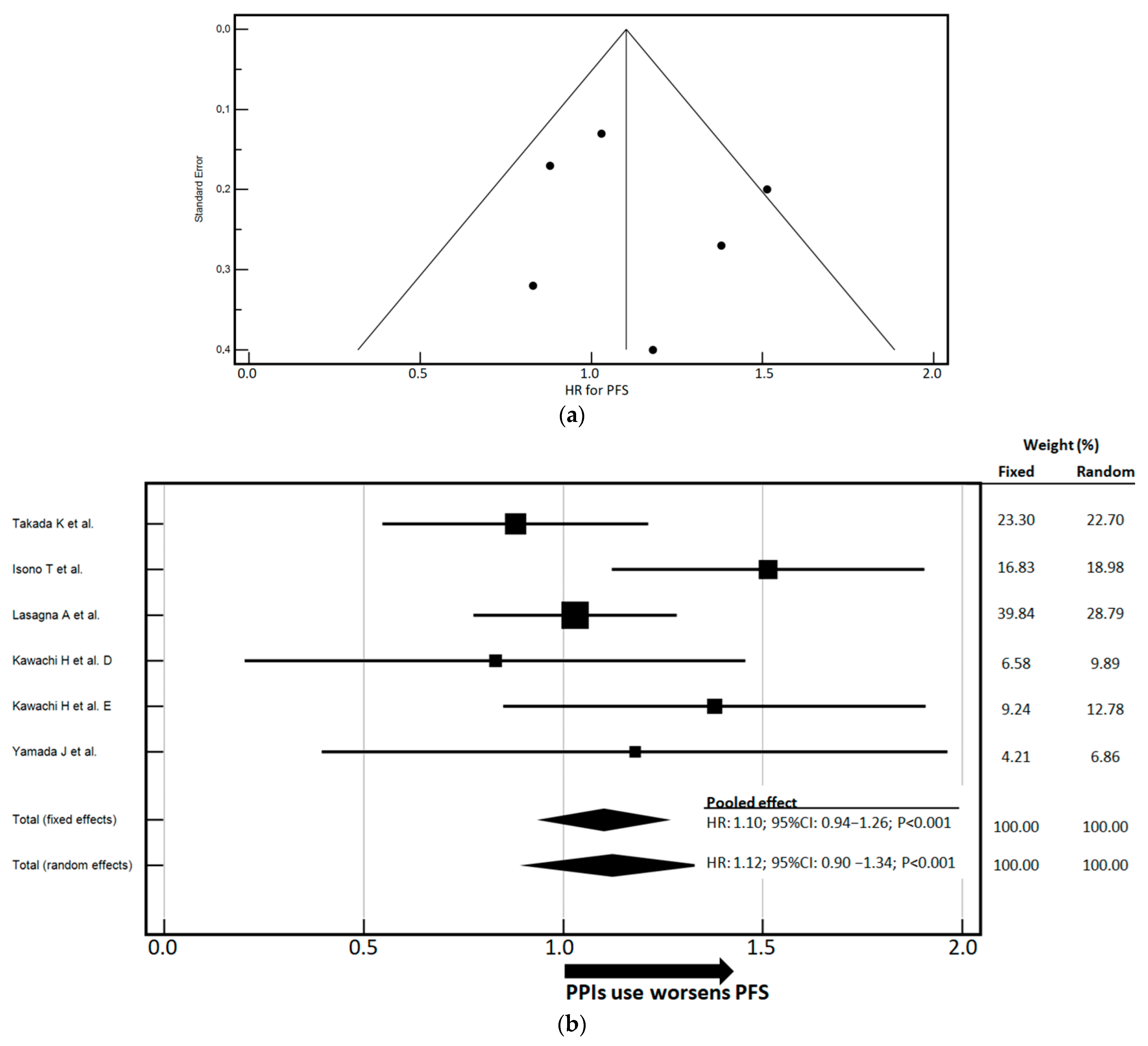
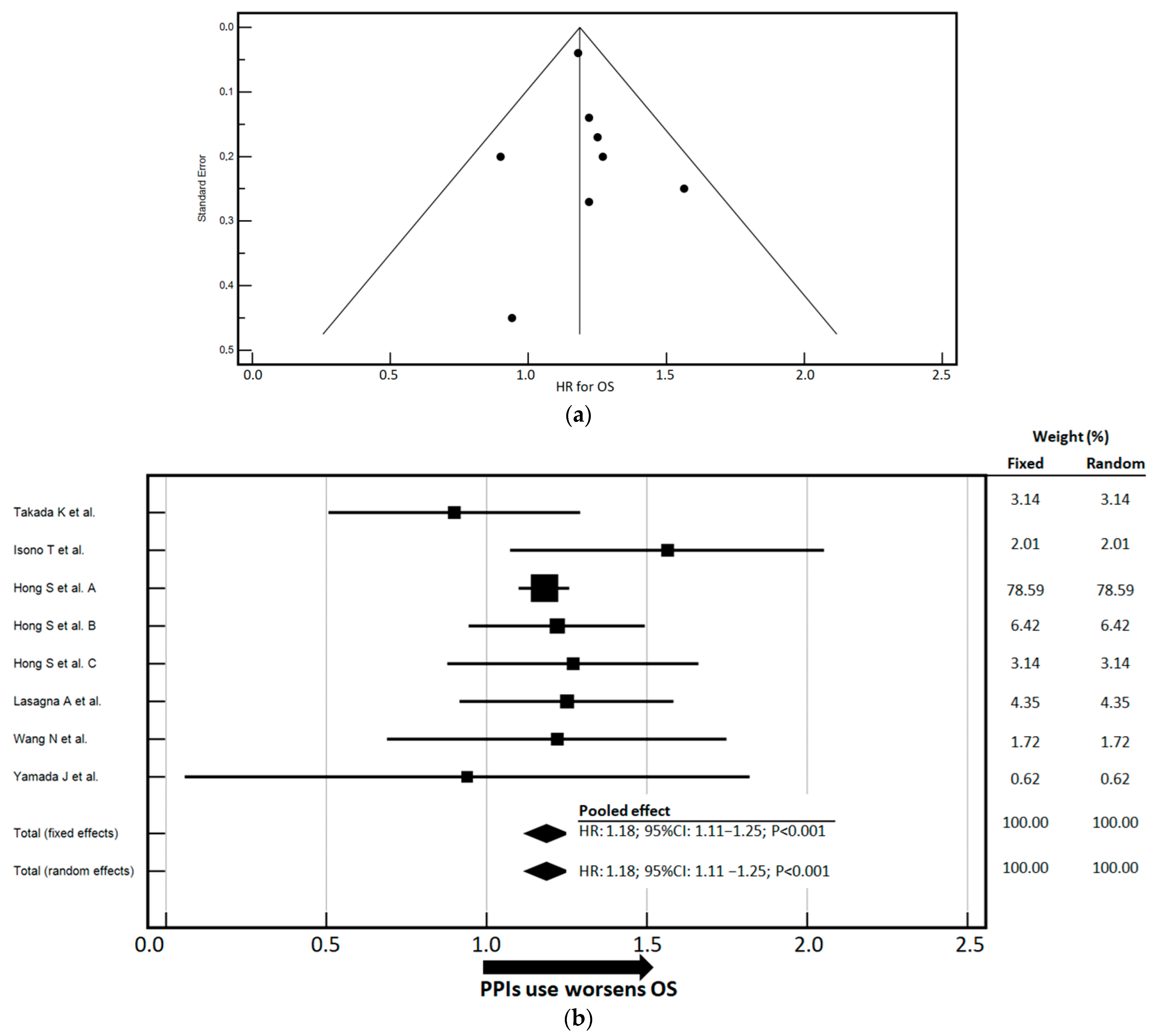
| Author | Study Design | Patients’ Number | Cancer Type | ICI Treatment | Treatment Line | Included in Association with Chemotherapy | Time-to-Outcome |
|---|---|---|---|---|---|---|---|
| Takada K et al. [29] | R | 198 | SCLC | Atezolizumab | First-line | No | OS, PFS |
| Isono T et al. [30] | R | 381 | NSCLC | Pembrolizumab, nivolumab, atezolizumab, nivolumab + ipilimumab, nivolumab + ipilimumab | All lines | Yes | OS, PFS |
| Hong S et al. [31] | R | 8870 | MM, NSCLC, UC | Pembrolizumab, nivolumab, atezolizumab | All lines | No | OS |
| Lasagna A et al. [32] | R | 363 | BC, EC, KC, HNC, MM, NSCLC, SCSC, UC | Nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, durvalumab | All lines | Yes | OS, PFS |
| Wang N et al. [33] | R | 183 | HC | Camrelizumab, simlizumab, tislizumab | All lines | Yes | OS |
| Kawachi H et al. [34] | R | 425 | NSCLC | Pembrolizumab, atezolizumab | First line | Yes | OS, PFS |
| Yamada J et al. [35] | R | 127 | NSCLC | Pembrolizumab, nivolumab, ipilimumab, atezolizumab | Up to third line | Yes | PFS |
| First Author, Year | Study Design | MINORS Score | NOS Score |
|---|---|---|---|
| Isono, 2024 [30] | R | 12 | 6 |
| Hong, 2024 [31] | R | 11 | 7 |
| Lasagna, 2023 [32] | R | 12 | 8 |
| Takada, 2024 [29] | R | 12 | 8 |
| Wang, 2024 [33] | R | 14 | 8 |
| Kawachi, 2023 [34] | R | 12 | 8 |
| Yamada, 2024 [35] | R | 12 | 8 |
| Author | PPIs Use Window | No. of Patients Treated with PPIs | Type of PPI | |
|---|---|---|---|---|
| Yes | No | |||
| Takada K et al. [29] | Within 30 days prior to ICI treatment | 43 | 155 | NS |
| Isono T et al. [30] | NS | 168 | 213 | Lansoprazole, rabeprazole, esomeprazole, vonoprazan, famotidine |
| Hong S et al. [31] | Within 30 days before ICI treatment | 2529 | 6341 | NS |
| Lasagna A et al. [32] | Concomitant | 189 | 174 | Pantoprazole plus others, NS |
| Wang N et al. [33] | Within 30 days before and after ICI treatment | 88 | 95 | Omeprazole, pantoprazole, rabeprazole, plus others, NS |
| Kawachi H et al. [34] | Concomitant | 134 | 291 | NS |
| Yamada J et al. [35] | Concomitant | 39 | 88 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciappina, G.; Ottaiano, A.; Santorsola, M.; Esposito, E.; De Luca, F.; Giorgi, C.; Zito, C.; Capra, A.P.; Carroccio, P.; Maurea, N.; et al. Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies. Cancers 2025, 17, 2228. https://doi.org/10.3390/cancers17132228
Ciappina G, Ottaiano A, Santorsola M, Esposito E, De Luca F, Giorgi C, Zito C, Capra AP, Carroccio P, Maurea N, et al. Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies. Cancers. 2025; 17(13):2228. https://doi.org/10.3390/cancers17132228
Chicago/Turabian StyleCiappina, Giuliana, Alessandro Ottaiano, Mariachiara Santorsola, Emanuela Esposito, Fabiola De Luca, Carlotta Giorgi, Concetta Zito, Anna Paola Capra, Patrizia Carroccio, Nicola Maurea, and et al. 2025. "Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies" Cancers 17, no. 13: 2228. https://doi.org/10.3390/cancers17132228
APA StyleCiappina, G., Ottaiano, A., Santorsola, M., Esposito, E., De Luca, F., Giorgi, C., Zito, C., Capra, A. P., Carroccio, P., Maurea, N., Quagliariello, V., Campo, I., Passalacqua, M. I., Incognito, D., Cacciola, I., Consolo, P., & Berretta, M. (2025). Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies. Cancers, 17(13), 2228. https://doi.org/10.3390/cancers17132228












