Simple Summary
Locally advanced rectal cancer treatment consists of neoadjuvant treatment (NAT) followed by surgery. Neoadjuvant treatments can include chemoradiotherapy (CRT), short-course radiotherapy (SCRT), radiotherapy (RT) or total neoadjuvant treatment (TNT). Each of these treatment modalities impact a patient’s quality of life. Finding predictive markers of tumor response enabling a personalized treatment approach is of high interest. Most of the research on biomarkers has focused on tumor response following CRT. This review aims at gathering current knowledge of biomarkers predicting response to neoadjuvant treatments other than CRT, such as SCRT, RT and TNT regiments.
Abstract
Background/Objectives: Treatment of locally advanced rectal cancer (LARC) very often requires a neoadjuvant multimodal approach. Neoadjuvant treatment (NAT) encompasses treatments like chemoradiotherapy (CRT), short-course radiotherapy (SCRT), radiotherapy (RT) or a combination of either of these two with additional induction or consolidation chemotherapy, namely total neoadjuvant treatment (TNT). In case of complete radiological and clinical response, the non-operative watch-and-wait strategy can be adopted in selected patients. This strategy is impacted by a regrowth rate of approximately 30%. Predicting biomarkers of tumor response to NAT could improve guidance of clinicians during clinical decision making, improving treatment outcomes and decreasing unnecessary treatment exposure. To this day, there is no validated biomarker to predict tumor response to any NAT strategies in clinical use. Most research focused on CRT neglects the study of other regimens. Methods: We conducted a narrative literature review which aimed at summarizing the status of biomarkers predicting tumor response to NAT other than CRT in LARC. Results: Two hundred and fourteen articles were identified. After screening, twenty-one full-text articles were included. Statistically significant markers associated with improved tumor response pre-treatment were as follows: low circulating CEA levels; BCL-2 expression; high cellular expression of Ku70, MIB-1(Ki-67) and EGFR; low cellular expression of VEGF, hPEBP4 and nuclear β-catenin; the absence of TP53, SMAD4, KRAS and LRP1B mutations; the presence of the G-allel of LCS-6; and MRI features such as the conventional biexponential fitting pseudodiffusion (Dp) mean value and standard deviation (SD), the variable projection Dp mean value and lymph node characteristics (short axis, smooth contour, homogeneity and Zhang et al. radiomic score). In the interval post-treatment and before surgery, significant markers were as follows: a reduction in the median value of circulating free DNA, higher presence of monocytic myeloid-derived suppressor cells, lower presence of CTLA4+ or PD1+ regulatory T cells and standardized index of shape changes on MRI. Conclusions: Responders to neoadjuvant SCRT and RT tended to have a tumor microenvironment with an immune–active phenotype, whereas responders to TNT tended to have a less active tumor profile. Although some biomarkers hold great promise, scarce publications, inconsistent results, low statistical power, and low reproducibility prevent them from reliably predicting tumor response following NAT.
1. Introduction
Rectal cancer (RC) represents one third of all colorectal cancers with 125’000 incidental cases in 2017 and a mortality of 4–10 deaths/100’000 individuals [1]. In Europe, locally advanced RC (LARC), defined as stage II and III RC, is the most prevalent stage at diagnosis, representing 42–44% of patients at diagnosis according to the EUROCARE-5 study [1,2]. According to European and American medical oncology guidelines, LARC should receive neoadjuvant treatment (NAT) with either chemoradiotherapy (CRT) or short-course radiotherapy (SCRT) followed by curative surgery for the least advanced cases [1,3]. More advanced disease, typically mrT3 threatening or invading the mesorectal fascia, mrT4 and/or mrN2, are offered intensified treatments with additional induction and/or consolidations chemotherapies—called total neoadjuvant treatment (TNT) [4,5,6,7].
In LARC, current neoadjuvant treatments lead to pathological complete response (pCR) in 6 to 39% of patients [8,9]. Being able to predict tumor response to NAT is of utmost importance, as it could determine which patients would benefit from it and spare the side effects for those who would not [9,10]. Furthermore, clinical complete response (cCR) or near cCR used as a surrogate of pCR can lead to a watch-and-wait strategy, allowing organ preservation and avoiding surgical complications [7,11].
To this day, no biomarkers are available in clinics to predict the tumor response to NAT in LARC patients [1,12,13,14,15]. Furthermore, research has focused on the tumor response to CRT; while the treatment landscape has evolved in the past years, only a scarce number of studies have searched for predictive biomarkers in patients treated with SCRT or TNT. The aim of our review is to clarify whether there are predictive biomarkers of tumor response in LARC patients treated with SCRT, TNT or other radiotherapy (RT) alternatives to CRT.
2. Materials and Methods
We performed a literature search in EMBASE and MEDLINE on the 28 March 2025 using the following keywords: “locally advanced rectal cancer”, “rectal cancer”, “LARC”, “short-course radiotherapy”, “SCRT”, “total neoadjuvant treatment”, “TNT”, “neoadjuvant radiotherapy”, “biomarker”, “liquid biopsy”, “gene expression”, “protein marker”, “tumor response”, “tumor regression”, “tumor regression grade”, “pathological complete response”, “tumor remission”, “predictive value” and “prediction”, with the Boolean AND/OR.
Included full-text articles involved LARC patients (stage II and III RC) that were treated with neoadjuvant SCRT (5 × 5 Gy), TNT or other neoadjuvant RT schemes. Studies on patients undergoing standard CRT or chemotherapy alone were excluded. In cases where a study had multiple subgroups based on treatment, only the subgroups meeting the inclusion criteria were considered.
All types of biomarkers (circulating, proteomic, genomic or radiomic) were considered.
All the articles needed to have the tumor response as the main or secondary outcome. PCR, tumor regression grade (TRG), the Response Evaluation Criteria in Solid Tumors (RECIST) or tumor downstaging were accepted assessment methods of tumor response. Studies evaluating only overall survival (OS) and/or disease-free survival (DFS) were not included.
3. Results
3.1. Literature Searches
The literature search identified two hundred and fourteen articles. Six additional records meeting the inclusion criteria were identified through reviews and citations. After removing duplicates (n = 5), two hundred and fifteen articles were screened. One hundred and seventy-three articles were excluded based on title and abstract. Forty-two full-text articles were assessed for eligibility and twenty-one articles were excluded based on the inclusion and exclusion criteria. In total, twenty-one studies were included (Figure 1).
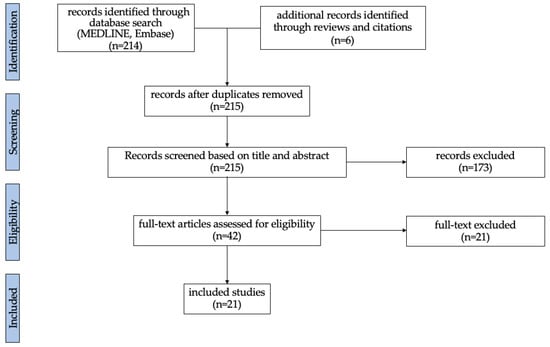
Figure 1.
Flow chart illustrating the screening and selection process.
Seven studies investigated biomarkers in association with SCRT [16,17,18,19,20,21,22]. Eight studies investigated biomarkers in association with other RT regimens [23,24,25,26,27,28,29,30]. Six studies investigated biomarkers in association with TNT [31,32,33,34,35,36]. An overview of the selected articles is available in Table 1.

Table 1.
Overview of selected articles.
3.2. Circulating Markers
Circulating markers were investigated in seven articles [17,20,22,28,31,34].
3.2.1. CEA
Carcinoembryonic antigen (CEA) is a glycoprotein expressed on epithelial cell membrane [37]. It loses polarization in tumor cells and may be measured in the blood circulation. It is measured in RC to assess prognosis and detect tumor relapse or progression after surgery [1].
Two studies considered CEA as a predictive biomarker of tumor response in LARC patients [28,31]. Chapman et al. found that low pre-treatment levels of CEA (<3 ng/L) were associated with pCR in LARC patients receiving TNT (p = 0.003) [31]. In patients receiving RT, Wang et al. found that lower pre-treatment levels of CEA (<5 ng/L and <10 ng/L) were associated with downstaging (p = 0.001) and particularly with T downstaging (p = 0.00014) [28].
3.2.2. Liquid Biopsy
Liquid biopsies consist of the analysis of non-solid tissues for tumor early diagnosis, detection of tumor relapse after surgery, or identification of treatment targets with the measure of circulating tumor cells (CTC) or nucleic acids, usually circulating free DNA (cfDNA), circulating tumor DNA (ctDNA) or microRNA (miRNA) [38,39].
cfDNA can be measured in circulation in oncologic, autoimmune or inflammatory conditions following tissue damage or after being actively secreted [40,41]. One study considered the cfDNA predictive value [20]. Truelsen et al. showed that, for patients treated with SCRT, a reduction in the median cfDNA value below the 75th percentile following SCRT was associated with pCR (p = 0.001) [20].
ctDNA is a fragmented part of tumor DNA found in the blood circulation [42]. Two studies investigated ctDNA levels in LARC patients undergoing TNT but could not show any association between ctDNA levels and pCR [34,36].
3.2.3. Flow Cytometry
Flow Cytometry (FC) characterizes cells at a single-cell level from blood, bone marrow or tissue [43]. Immunophenotyping is one of the main applications of FC but it can also be used for cell cycle, protein or antigen response analysis.
Two articles studied FC for patients treated with SCRT [17,22].
Gasinska et al. performed FC on LARC biopsies and surgical specimens of 122 LARC patients [17]. Only the relative MIB-1 LI (ratio between post- and pre-treatment MIB-1 LI) was associated with the tumor response (p = 0.005). Furthermore, when separating the results by gender, it only remained statistically significant in the female subgroup (p = 0.041).
Napolitano et al. performed FC on fresh blood samples pre-treatment (T0) at week 2 (T2) and week 5 (T5) post-treatment, pre-surgery (T8) and 6–12 months post-surgery (T9/T10) to characterize peripheral Granulocytic myeloid-derived suppressor cells (G-MDSC)(LIN−/HLA-DR−/CD11b+/CD14−/CD15+/CD33+), monocytic myeloid-derived suppressor cells (M-MDSC) (CD14+/HLA-DR−/lowCD11b+/CD33+) and regulatory T cells (Treg) (CD4+/CD25+/FOXP3+/CTLA4+ and CD4+/CD25+/FOXP3+/PD1+). Good responders had higher M-MDSC levels at T5 (p = 0.045) and T8 (p = 0.012), lower CTLA4+ Treg at T0 (p = 0.045) and T5 (p = 0.032) and lower PD1+ Treg level at T8 (p = 0.043) and T9 (p = 0.027) [22].
3.3. Tissue Markers
3.3.1. Immunohistochemistry
Immunohistochemistry (IHC) is a testing method used on pathological samples such as biopsies or surgical specimens [44]. The tissue is usually formalin-fixed, paraffin-embedded (FFPE). It allows us to assess specific protein expression in the selected tissue. Eleven studies used IHC to investigate the following markers in LARC: APAF-1, BCL2, CD34, DCC, EGFR, GLUT-1, HER2, hPEBP4, Ki-67, Ku70, mismatch repair status (MMR), MRE11/RAD50/NBS1, nuclear ß-catenin, p21, p53, PD-L1, tumor-infiltrating lymphocytes (TILS) (CD3-CD8+), TS and VEGF [18,19,21,22,23,24,25,27,29,30,32].
BCL-2 is an anti-apoptotic protein, and its overexpression may induce radioresistance in tumor cells [45]. Two studies considered BCL-2 expression [18,30].
Gasinska et al. showed that expression of cytoplasmic BCL-2 in pre-SCRT biopsies was associated with an improved TRG, but only in patients having short breaks before surgery (being defined as has having surgery less than 15 days after SCRT) [18].
Zlobec et al. did not find an association between cytoplasmic BCL-2 expression and tumor response using pCR in pre-treatment samples of patients treated with RT (26 Gy in 4 fractions) [30].
The Epidermal Growth Factor Receptor (EGFR) harbors a tyrosine kinase function, triggering cell growth and survival, a pro-inflammatory reaction and actin remodeling. Mutated EGFR is associated with tumorigenesis and represents a therapeutic target [46].
Zlobec et al. found that higher cytoplasmic and membranous expression of EGFR (>20%) in pre-treatment samples underdoing RT (26 Gy in 4 fractions) were associated with higher pCR rates in univariate (p = 0.003) and multivariate analysis (p = 0.01) [30].
Human phosphatidylethanolamine-binding protein 4 (hPEBP4) is an anti-apoptotic molecule overexpressed in several solid tumors and is believed to generate radioresistance [47,48]. Qiu et al. found that lower cytoplasmic expression of hPEBP4 (<70%) in biopsy specimens was associated with an improved TRG in univariate (p = 0.001) and multivariate analysis (p = 0.001) for patients treated with alternative RT (4 × 5 Gy) [25].
Ki-67 is a protein mainly present in the perinucleolar region in the G1 phase and is a marker of cell proliferation that may be highly expressed in tumor cells [49]. Gasinska et al. found that higher nuclear Ki-67 expression (cut-off not specified) using the MIB-1 labeling index in pre-treatment biopsies was associated with an improved tumor response according to RECIST criteria (p = 0.023) after SCRT with a short break (<15 days) before surgery [18].
Ku70 is a protein that plays a role in DNA repair by binding to double-stranded DNA breaks. Its downregulation has been associated with colitis and the development of colorectal cancer [50].
In LARC patients, Gasisnka et al. found that higher pre-treatment nuclear Ku70 expression (cut-off not specified) was associated with higher tumor downstaging in their female subgroup undergoing SCRT with delayed surgery (>15 days) (p = 0.035) [18]. No association with tumor response was found in the whole group.
Nuclear β-catenin is part of the Wnt signaling pathway. It is found in the cytoplasm and cell membrane binded to E-cadherin and plays a role in maintaining normal cell structure. In the presence of the Wnt signal, it translocates to the nucleus and initiates a signaling cascade that leads to cell proliferation [51]. It has also been associated with immune invasion in cancer by inhibiting the differentiation of naïve CD8+ T cells into effector cells [52].
Wang et al. found that higher nuclear expression (>50%) of β-catenin in LARC patients undergoing SCRT was associated with radioresistance and a diminished tumor downstaging in univariate (p < 0.001) and multivariate analysis (p < 0.001) [21]. On the other hand, having a high expression of membrane nuclear β-catenin was associated with better TRG, although this association was not statistically significant.
Vascular Endothelial Growth Factor (VEGF) is essential in angiogenesis to allow cells to meet their oxygen supply [53]. It is overexpressed in numerous solid tumors and plays a role in their growth, and is therefore a therapeutic target [53]. Zlobec et al. found that low expression of cytoplasmic VEGF (<20%) in pre-treatment biopsies underdoing RT (26 Gy in 4 fractions) was associated with an improved pCR rate in univariate (p = 0.004) and multivariate analysis (p = 0.009) [30].
No significant associations with tumor response treated with RT, SCRT or TNT were found for APAF-1, CD-34, DCC, GLUT-1, HER-2, MMR status, MRN complex, p21, p53, PD-L1, TILS and TS [18,19,23,24,27,29,30,31,32].
3.3.2. Genomic Markers
The tumors cell genome undergoes point-mutations or alterations such as inversions and translocations that are involved in cell growth, proliferation, metastasis or immune system evasion [54]. Genomic markers are identified by sequencing methods that can be targeted using polymerase chain reaction (PCR) or untargeted using sequencing panels that can include whole genome sequencing [55,56]. It allows tumor profiling and can unveil treatment targets guiding clinical decisions [56].
Six studies looked at genomic markers for possible association with tumor response [23,26,27,31,32,33].
p53 is a tumor suppressor protein that is active after DNA damage and able to induce its repair, cell cycle arrest or cell apoptosis [57]. Mutations in p53 can induce the proliferation of cells with unstable genomes as seen in tumors [57].
Kandioler et al. and Rebischung et al. investigated p53 mutation in LARC patients undergoing RT (25 Gy in 10 fractions and 39 Gy in 13 fractions, respectively) [23,26]. Both found that the absence of mutation was associated with a better tumor T downstaging (p < 0.001 and p < 0.04, respectively).
Chapman et al. used next-generation sequencing (NGS) to record mutation of KRAS, NRAS, BRAF, PIK3CA, APC, FBXW7, SMAD4 and p53 in biopsies from LARC patients undergoing TNT with CAPOX or FOLFOX followed by CRT [31]. They found that the absence of mutation in p53 (p = 0.023) and SMAD4 (p = 0.040) was associated with pCR. SMAD4 is a tumor suppressor involved in the TGF- β signaling pathway [58].
Iseas et al. used DNA sequencing with a panel of 72 cancer driver genes in biopsies from LARC patients that underwent TNT with CAPOX followed by CRT [32]. TP53, APC, KRAS, ATM and PIK3CA were the most frequently mutated genes, however only KRAS mutations were associated with worse TRG in univariate (p = 0.013) and multivariate analysis (p = 0.042). KRAS is an oncogene involved in cell proliferation when mutated in several solid tumors [59].
Sclafani et al. analyzed the LCS-6 variant using PCR in biopsies from patients involved in the expert-T clinic trial, a randomized phase II trial that evaluated the efficacy of neoadjuvant CAPOX followed by CRT with or without cetuximab in LARC [33,60]. LCS-6 is a microRNA associated with KRAS expression [61]. They found that carriers of the G allele of LCS-6 had a higher pCR rate (28.1% versus 10.6%; p = 0.020) and a better 5-year progression free survival (PFS) rate. Furthermore, this increase in pCR rate was independent of the use of cetuximab.
Lit et al. performed NGS on pre-treatment samples from patients that underwent TNT with CAPOX and camrelizumab followed by CRT [36]. Patients without further disease progression also received consolidation regiment with CAPOX. They found that mutation of the low-density lipoprotein receptor-related protein (LRP1B) was associated with cCR (p = 0.03) and a tumor shrinkage of more than 50% (p = 0.04). LRP1B is a gene that codes for a cell-surface receptor with diverse biological functions such as receptor-mediated endocytosis and cell signaling [62]. Its role in tumors remains unclear, however its inactivation has been associated with several tumor types.
3.4. Radiomic Markers
Radiomic markers represent a non-invasive approach to assess patient’s characteristics through imaging data extracted mainly from CT scans, MRI or PET-CT [63]. Radiomics could constitute surrogate biomarkers of interest for tumor characterization, molecular tumor phenotyping, tumor response or prognosis assessment [63,64]. Several articles have already described the predictive value of radiomics in LARC patients undergoing CRT [65]. Two studies using alternatives neoadjuvant therapies were identified [16,35].
Fusco et al. explored parameters derived from MRI to assess tumor response in LARC patients undergoing SCRT [16]. They showed that the pre-treatment mean value and standard deviation (SD) of conventional biexponential fitting pseudo diffusion (CBFM Dp) (p = 0.05 and p = 0.03, respectively) as well deviation of the mean value of variable projection pseudo diffusion (VARPRO Dp) (p = 0.008) were associated with pCR. They also found that a change in pre- and post-treatment standardized index of shape (SIS) was associated with pCR (p < 0.001).
Zhang et al. assessed the predictive value of MRI pre-treatment lymph node (LN) characteristics in patients undergoing TNT with CRT followed by mFOLFOX6 or CapeOX [35]. They found that short-axis LN (<8 mm) (p = 0.024), smooth LN borders (p < 0.001), homogeneous signal intensity (p < 0.01) and their radiomic score based on five radiomic features (contrast, long run high gray level emphasis, Idn. wavelet, Imc1.wavelet, large dependence high gray level emphasis.wavelet) (p < 0.001) were associated with LN regression. These results remained statistically significant in their validation cohort.
4. Discussion
This review explores the potential value of several markers to predict LARC response following neoadjuvant SCRT, TNT and RT. The 21 selected studies explored circulating biomarkers, tissue molecular biomarkers, tissue genomic biomarkers and MRI-derived radiomic markers. Statistically significant markers are summarized in Figure 2.
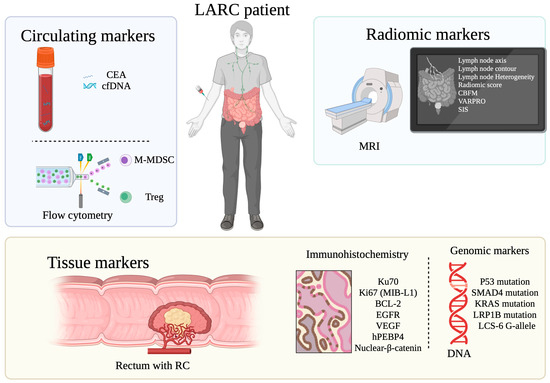
Figure 2.
Overview of statistically significant biomarkers for tumor response prediction to SCRT, TNT and RT in LARC patients. Created in BioRender. Machado Carvalho, J. (2025) https://www.BioRender.com/seftlv5 (assessed on 18 April 2025).
Markers of response to SCRT were studied in seven studies gathering four hundred and eighty three patients, showing significant results. In the pre-treatment setting, biomarkers associated with an improved tumor response were as follows: lower levels of CTLA4+ Treg, BCL-2 expression, high expression of Ku70 and MIB-1(Ki-67), low expression of nuclear β-catenin and MRI modifications (CBPFM Dp mean value and SD and VARPRO Dp mean value). In the time post-treatment but pre-surgery, reduction in the median value of cfDNA, higher levels of M-MDSC, lower levels of CTLA4+ Treg or PD1+ Treg and SIS changes on MRI were associated with an increased tumor response.
In TNT, six studies gathering four hundred and sixty eight patients found that pre-treatment markers associated with tumor response were as follows: low CEA levels, wild type TP53, SMAD4, KRAS and LRP1B, presence of the G-allele of LCS-6 and MRI features including short LN axis, smooth contour LN, homogeneous LN and Zhang et al. Rad score [35].
Finally, in alternative neoadjuvant RT regiments (eight studies gathering seven hundred and eighty five patients), significant pre-treatment predictive markers were as follows: low CEA circulating values, low hPEBP4 expression, high EGFR expression, low VEGF expression and wild type TP53.
Overall, the biomarker profile of responders to SCRT and RT supported an immune-permissive or immune-active tumor microenvironment. First, statistically significant markers included notably lower CTLA4+ Treg, which are known immunosuppressive cells that can repress the antitumor activity of the immune system [66]. Second, nuclear β-catenin plays a role in cell proliferation and immune evasion by inducing poor CD8+ T cells activity [51,52]. Third, hPEPB4 is associated with radioresistance [47,48]. Fourth, high expression of Ki67 and EGFR is associated with cell proliferation and high expression of BCL-2 is associated with cell survival [45,49]. These results correlate with the literature findings, where the immunosuppressive tumor microenvironment (TME) favorizes tumor growth, immune evasion and treatment resistance [67,68,69].
In TNT, however, responders had a less active tumor profile with lower CEA levels and without mutation in TP53, KRAS, LRP1B and SMAD4, which are usually associated with tumor proliferation [57,58,59]. This difference in the responder profile may reflect the potential effect of TNT on the TME. In fact, the impact of NAT may induce a modification of the TME, inducing immune activation, which can be correlated with improved tumor response and patient survival in RC and other solid tumor types [69,70,71,72].
These results should be interpreted with caution, as several limiting factors may impede their generalization. First, most studies had limited sample sizes (n = 12 to 240), lowering statistical power. Second, low reproducibility of the results may hinder their generalization. Most markers studied were assessed in single studies; only TP53 expression and mutations (nine articles), MMR status (four articles) and circulating CEA (three articles) have been replicated. Third, heterogeneity in the treatment options can be observed, especially in the TNT and in the alternative RT articles. Fourth, the lack of standardized assessment methods for tumors can lead to discrepancies in the interpretation of the results [13]. Some of the articles used the tumor regression grade with different scales, others used pCR and some used the TNM downstaging, sometimes considering only the T or the N downstaging. Fifth, the lack of standardized methods for the pre-analytic setting (sample type, sampling method, sample conservation, sampling timing, etc.) may influence studies results.
This review highlights the lack of translational research focusing on discovering predictive biomarkers to neoadjuvant SCRT, TNT or RT compared to the large number of studies focusing on predictive biomarkers for neoadjuvant CRT [14,15,65].
For instance, in the context of CRT, the mismatch repair (MMR) system and tumor-infiltrating lymphocytes (TILs) have emerged as biomarkers of interest for predicting tumor response and guiding treatment decisions [73,74,75]. Cercek and colleagues showed that MSI rectal tumors up to 25% are resistant to chemotherapy alone but tended to be sensitive to CRT [76]. Recently, several studies have shown that MSI LARC patients can probably be cured with anti-PD-1 immunotherapy alone [73,75].
The immune system plays a central role in tumor development, exhibiting both pro-tumor and anti-tumor activities [77]. In patients treated with neoadjuvant CRT, a high density of CD8+ TILs has been associated with an increased likelihood of achieving pathological complete response (pCR) [74,78]. Despite these promising findings, only four studies have investigated the predictive value of MMR status in non-CRT neoadjuvant settings [19,24,31,32], and only one study considered TILS in TNT without being able to reach statistical significance [32].
This limited evidence can be partially attributed to the relatively recent adoption of these alternative neoadjuvant regimens compared to CRT [79,80]. Additionally, the growing number of treatment protocols within NAT strategies poses challenges for conducting studies with sufficient sample sizes to yield meaningful conclusions.
5. Conclusions
LARC patient responders to neoadjuvant SCRT and RT tended to have a more immune-active tumor microenvironment, whereas responders to TNT tended to have a less active tumor profile. Although some biomarkers hold great promises, scarce publications, inconsistent results, low statistical power, and low reproducibility prevent them from reliably predicting tumor response following neoadjuvant SCRT, TNT or RT. This review highlights the urgent need for translational research with larger cohorts and standardized methods to tackle the challenge of discovering predictive markers of response to neoadjuvant treatments in patients with LARC treated with neoadjuvant SCRT, TNT or RT.
Author Contributions
Conceptualization, J.V.M.C.; methodology, J.V.M.C.; data curation, J.V.M.C.; writing—original draft preparation, J.V.M.C.; writing—review and editing, J.V.M.C., J.M., F.R., A.D., A.B., A.R., C.C., and T.K.; supervision, C.C. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-FU | 5-Fluorouracil |
| APAF-1 | Apoptotic protease activating factor-1 |
| BrdUrdLI | Bromodeoxyuridine labeling index |
| CAPOX | Capecitabin and oxaliplatin |
| CBFM | Conventional biexponential fitting |
| cCR | Clinical complete response |
| CD | Cluster of differentiation |
| CEA | Carcinoembryonic antigen |
| cfDNA | Circulating free DNA |
| CRT | Chemoradiotherapy |
| CTC | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| DCC | Deleted in colon cancer |
| Dp | Pseudo diffusion |
| dUMP | Deoxyuridine monophosphate |
| DFS | Disease-free survival |
| dTMP | Deoxythymidine monophosphate |
| EGFR | Epidermal growth factor receptor |
| ESMO | European Society for Medical Oncology |
| FC | Flow cytometry |
| FFPE | Fixed paraffin embedded |
| FOLFOX | Folinic acid, fluorouracil and oxaliplatin |
| Glut-1 | Glucose transporter 1 |
| G-MDSC | Granulocytic-myeloid derived suppressor cells |
| HER-2 | Human epidermal growth factor receptor-2 |
| hPEBP4 | Human phosphatidylethanolamine-binding protein 4 |
| IHC | Immunohistochemistry |
| LARC | Locally advanced rectal cancer |
| LN | Lymph node |
| LRP1B | Low-density lipoprotein receptor-related protein 1b |
| mFOLFOX | Modified FOLFOX |
| MIB-1 LI | MIB-1 labeling index |
| M-MDSC | Monocytic-myeloid derived suppressor cells |
| MMR | Microsatellite mismatch repair system |
| MRN | MRE11/RAD50/NBS1 |
| MSI | Microsatellite instability |
| MSS | Microsatellite stable |
| N | Lymph node stage according to the TNM classification |
| NAT | Neoadjuvant treatment |
| NGS | Next-generation sequencing |
| NPV | Negative predictive value |
| OS | Overall survival |
| PCR | Polymerase chain reaction |
| pCR | Pathological complete response |
| PPV | Positive predictive value |
| RC | Rectal cancer |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RT | Radiotherapy |
| SCRT | Short-course radiotherapy |
| SD | Standard deviation |
| SIS | Standardized index of shape |
| Sn | Sensitivity |
| Sp | Specificity |
| SPF | S-phase fraction |
| T | Tumor size according to the TNM classification |
| TILs | Tumor-infiltrating lymphocytes |
| TME | Tumor microenvironment |
| TNT | Total neoadjuvant treatment |
| Treg | Regulatory T cells |
| TRG | Tumor regression grade |
| TS | Thymidilate synthase |
| TTT | Treatment |
| VARPRO | Variable projection |
| VEGF | Vascular growth factor |
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Minicozzi, P.; Innos, K.; Sanchez, M.J.; Trama, A.; Walsh, P.M.; Marcos-Gragera, R.; Dimitrova, N.; Botta, L.; Visser, O.; Rossi, S.; et al. Quality analysis of population-based information on cancer stage at diagnosis across Europe, with presentation of stage-specific cancer survival estimates: A EUROCARE-5 study. Eur. J. Cancer 2017, 84, 335–353. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Li, L.; Lee, K.C.; Lam, K.O.; Wong, K.H.; Ho, W.M.; Ma, B. Total Neoadjuvant Therapy for High Risk Rectal Cancer in Western and Asian Populations—Current Evidence and Clinical Applications. Clin. Color. Cancer 2022, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Kardosh, A.; Nabavizadeh, N.; Lopez, C.D. Evolving Treatment Options and Future Directions for Locally Advanced Rectal Cancer. Clin. Color. Cancer 2019, 18, 231–237. [Google Scholar] [CrossRef]
- He, W.; Li, Q.; Li, X. Changing patterns of neoadjuvant therapy for locally advanced rectal cancer: A narrative review. Crit. Rev. Oncol. Hematol. 2023, 181, 103885. [Google Scholar] [CrossRef]
- Timmerman, C.; Taveras, L.R.; Huerta, S. Clinical and molecular diagnosis of pathologic complete response in rectal cancer: An update. Expert Rev. Mol. Diagn. 2018, 18, 887–896. [Google Scholar] [CrossRef]
- Gollins, S.; Sebag-Montefiore, D. Neoadjuvant Treatment Strategies for Locally Advanced Rectal Cancer. Clin. Oncol. (R Coll. Radiol.) 2016, 28, 146–151. [Google Scholar] [CrossRef]
- Bach, S.P. STAR-TREC: An International Three-arm Multicentre, Partially Randomised Controlled Trial Incorporating an External Pilot. Clin. Oncol. (R Coll. Radiol.) 2023, 35, e107–e109. [Google Scholar] [CrossRef]
- Smolskas, E.; Mikulskyte, G.; Sileika, E.; Suziedelis, K.; Dulskas, A. Tissue-Based Markers as a Tool to Assess Response to Neoadjuvant Radiotherapy in Rectal Cancer-Systematic Review. Int. J. Mol. Sci. 2022, 23, 6040. [Google Scholar] [CrossRef] [PubMed]
- Amintas, S.; Giraud, N.; Fernandez, B.; Dupin, C.; Denost, Q.; Garant, A.; Frulio, N.; Smith, D.; Rullier, A.; Rullier, E.; et al. The Crying Need for a Better Response Assessment in Rectal Cancer. Curr. Treat. Options Oncol. 2023, 24, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Machado Carvalho, J.V.; Dutoit, V.; Corro, C.; Koessler, T. Promises and Challenges of Predictive Blood Biomarkers for Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Cells 2023, 12, 413. [Google Scholar] [CrossRef]
- Slipsager, A.; Henrichsen, S.N.; Falkmer, U.G.; Dybkaer, K.; Belting, M.; Poulsen, L.O. Predictive biomarkers in radioresistant rectal cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2023, 186, 103991. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Sansone, M.; Granata, V.; Grimm, R.; Pace, U.; Delrio, P.; Tatangelo, F.; Botti, G.; Avallone, A.; Pecori, B.; et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: A comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom. Radiol. 2019, 44, 3683–3700. [Google Scholar] [CrossRef]
- Gasinska, A.; Richter, P.; Darasz, Z.; Niemiec, J.; Bucki, K.; Malecki, K.; Sokolowski, A. Gender-related differences in repopulation and early tumor response to preoperative radiotherapy in rectal cancer patients. J. Gastrointest. Surg. 2011, 15, 1568–1576. [Google Scholar] [CrossRef]
- Gasinska, A.; Adamczyk, A.; Niemiec, J.; Biesaga, B.; Darasz, Z.; Skolyszewski, J. Gender-related differences in pathological and clinical tumor response based on immunohistochemical proteins expression in rectal cancer patients treated with short course of preoperative radiotherapy. J. Gastrointest. Surg. 2014, 18, 1306–1318. [Google Scholar] [CrossRef]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Ng, W.; Lee, M.; de Souza, P.; Shin, J.S.; et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 2018, 18, 869. [Google Scholar] [CrossRef]
- Truelsen, C.G.; Kronborg, C.S.; Sorensen, B.S.; Callesen, L.B.; Spindler, K.G. Circulating cell-free DNA as predictor of pathological complete response in locally advanced rectal cancer patients undergoing preoperative chemoradiotherapy. Clin. Transl. Radiat. Oncol. 2022, 36, 9–15. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.M.; Li, Z.; Liu, X.J.; Chai, J.; Zhang, G.Y.; Cheng, Y.F. Overexpression of nuclear beta-catenin in rectal adenocarcinoma is associated with radioresistance. World J. Gastroenterol. 2013, 19, 6876–6882. [Google Scholar] [CrossRef]
- Napolitano, M.; D’Alterio, C.; Cardone, E.; Trotta, A.M.; Pecori, B.; Rega, D.; Pace, U.; Scala, D.; Scognamiglio, G.; Tatangelo, F.; et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 2015, 6, 8261–8270. [Google Scholar] [CrossRef]
- Kandioler, D.; Zwrtek, R.; Ludwig, C.; Janschek, E.; Ploner, M.; Hofbauer, F.; Kuhrer, I.; Kappel, S.; Wrba, F.; Horvath, M.; et al. TP53 genotype but not p53 immunohistochemical result predicts response to preoperative short-term radiotherapy in rectal cancer. Ann. Surg. 2002, 235, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Negri, F.V.; Campanini, N.; Camisa, R.; Pucci, F.; Bui, S.; Ceccon, G.; Martinelli, R.; Fumagalli, M.; Losardo, P.L.; Crafa, P.; et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br. J. Cancer 2008, 98, 143–147. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, G.; Shen, Z.; Xie, Y.; Wang, L. hPEBP4 as a predictive marker for the pathological response of rectal cancer to preoperative radiotherapy. Int. J. Color. Dis. 2013, 28, 241–246. [Google Scholar] [CrossRef]
- Rebischung, C.; Gerard, J.P.; Gayet, J.; Thomas, G.; Hamelin, R.; Laurent-Puig, P. Prognostic value of P53 mutations in rectal carcinoma. Int. J. Cancer 2002, 100, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Saw, R.P.; Morgan, M.; Koorey, D.; Painter, D.; Findlay, M.; Stevens, G.; Clarke, S.; Chapuis, P.; Solomon, M.J. p53, deleted in colorectal cancer gene, and thymidylate synthase as predictors of histopathologic response and survival in low, locally advanced rectal cancer treated with preoperative adjuvant therapy. Dis. Colon Rectum 2003, 46, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhong, X.G.; Peng, Y.F.; Li, Z.W.; Gu, J. Prognostic value of pretreatment level of carcinoembryonic antigen on tumour downstaging and early occurring metastasis in locally advanced rectal cancer following neoadjuvant radiotherapy (30 Gy in 10 fractions). Color. Dis. 2014, 16, 33–39. [Google Scholar] [CrossRef]
- Yao, Y.F.; Du, C.Z.; Chen, N.; Chen, P.; Gu, J. Expression of HER-2 in rectal cancers treated with preoperative radiotherapy: A potential biomarker predictive of metastasis. Dis. Colon Rectum 2014, 57, 602–607. [Google Scholar] [CrossRef]
- Zlobec, I.; Vuong, T.; Compton, C.C.; Lugli, A.; Michel, R.P.; Hayashi, S.; Jass, J.R. Combined analysis of VEGF and EGFR predicts complete tumour response in rectal cancer treated with preoperative radiotherapy. Br. J. Cancer 2008, 98, 450–456. [Google Scholar] [CrossRef]
- Chapman, B.C.; Lai, S.H.; Friedrich, T.; Lieu, C.H.; Moskalenko, M.; Olsen, J.R.; Herter, W.; Birnbaum, E.H.; McCarter, M.D.; Vogel, J.D. Rectal Cancer: Clinical and Molecular Predictors of a Complete Response to Total Neoadjuvant Therapy. Dis. Colon Rectum 2023, 66, 521–530. [Google Scholar] [CrossRef]
- Iseas, S.; Sendoya, J.M.; Robbio, J.; Coraglio, M.; Kujaruk, M.; Mikolaitis, V.; Rizzolo, M.; Cabanne, A.; Ruiz, G.; Salanova, R.; et al. Prognostic Impact of An Integrative Landscape of Clinical, Immune, and Molecular Features in Non-Metastatic Rectal Cancer. Front. Oncol. 2021, 11, 801880. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, F.; Chau, I.; Cunningham, D.; Peckitt, C.; Lampis, A.; Hahne, J.C.; Braconi, C.; Tabernero, J.; Glimelius, B.; Cervantes, A.; et al. Prognostic role of the LCS6 KRAS variant in locally advanced rectal cancer: Results of the EXPERT-C trial. Ann. Oncol. 2015, 26, 1936–1941. [Google Scholar] [CrossRef]
- Vidal, J.; Casadevall, D.; Bellosillo, B.; Pericay, C.; Garcia-Carbonero, R.; Losa, F.; Layos, L.; Alonso, V.; Capdevila, J.; Gallego, J.; et al. Clinical Impact of Presurgery Circulating Tumor DNA after Total Neoadjuvant Treatment in Locally Advanced Rectal Cancer: A Biomarker Study from the GEMCAD 1402 Trial. Clin. Cancer Res. 2021, 27, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, B.; Yu, M.; He, L.; Zheng, P.; Yan, C.; Li, J.; Peng, Q. Development and Validation of a Radiomics Model Based on Lymph-Node Regression Grading After Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 821–833. [Google Scholar] [CrossRef]
- Li, Y.; Pan, C.; Gao, Y.; Zhang, L.; Ji, D.; Cui, X.; Zhang, X.; Cai, Y.; Zhang, Y.; Yao, Y.; et al. Total Neoadjuvant Therapy With PD-1 Blockade for High-Risk Proficient Mismatch Repair Rectal Cancer. JAMA Surg. 2024, 159, 529–537. [Google Scholar] [CrossRef]
- Hammarstrom, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef]
- Reimers, N.; Pantel, K. Liquid biopsy: Novel technologies and clinical applications. Clin. Chem. Lab. Med. 2019, 57, 312–316. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Wentzel, J.F.; Aucamp, J.; van Dyk, E.; du Plessis, L.; Pretorius, P.J. Characterization of the cell-free DNA released by cultured cancer cells. Biochim. Biophys. Acta 2016, 1863, 157–165. [Google Scholar] [CrossRef]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer--a survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped By Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Shi, S.R.; Key, M.E.; Kalra, K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: An enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991, 39, 741–748. [Google Scholar] [CrossRef]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Li, H.; Liu, B.; Qiu, J.; Chen, T.; Cao, X. Silencing of human phosphatidylethanolamine-binding protein 4 sensitizes breast cancer cells to tumor necrosis factor-alpha-induced apoptosis and cell growth arrest. Clin. Cancer Res. 2005, 11, 7545–7553. [Google Scholar] [CrossRef]
- Qiu, J.; Tao, Y.; Yang, G.; Xu, K.; Lin, A.L.; Li, L. Effect of a chemical inhibitor of human phosphatidylethanolamine-binding protein 4 on radiosensitivity of rectal cancer cells. World J. Surg. Oncol. 2016, 14, 221. [Google Scholar] [CrossRef]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef]
- Pandey, A.; Shen, C.; Feng, S.; Enosi Tuipulotu, D.; Ngo, C.; Liu, C.; Kurera, M.; Mathur, A.; Venkataraman, S.; Zhang, J.; et al. Ku70 senses cytosolic DNA and assembles a tumor-suppressive signalosome. Sci. Adv. 2024, 10, eadh3409. [Google Scholar] [CrossRef]
- Sanchez-Canteli, M.; Juesas, L.; Garmendia, I.; Otero-Rosales, M.; Calvo, A.; Alvarez-Fernandez, M.; Astudillo, A.; Montuenga, L.M.; Garcia-Pedrero, J.M.; Rodrigo, J.P. Tumor-Intrinsic Nuclear beta-Catenin Associates with an Immune Ignorance Phenotype and a Poorer Prognosis in Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2022, 23, 11559. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/beta-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Dienstmann, R.; Jang, I.S.; Bot, B.; Friend, S.; Guinney, J. Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Cancer Discov. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Hernandez Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Wan, R.; Feng, J.; Tang, L. Consequences of Mutations and Abnormal Expression of SMAD4 in Tumors and T Cells. Onco Targets Ther. 2021, 14, 2531–2540. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Dewdney, A.; Cunningham, D.; Tabernero, J.; Capdevila, J.; Glimelius, B.; Cervantes, A.; Tait, D.; Brown, G.; Wotherspoon, A.; Gonzalez de Castro, D.; et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J. Clin. Oncol. 2012, 30, 1620–1627. [Google Scholar] [CrossRef]
- Sha, D.; Lee, A.M.; Shi, Q.; Alberts, S.R.; Sargent, D.J.; Sinicrope, F.A.; Diasio, R.B. Association study of the let-7 miRNA-complementary site variant in the 3′ untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial). Clin. Cancer Res. 2014, 20, 3319–3327. [Google Scholar] [CrossRef]
- Principe, C.; Dionisio de Sousa, I.J.; Prazeres, H.; Soares, P.; Lima, R.T. LRP1B: A Giant Lost in Cancer Translation. Pharmaceuticals 2021, 14, 836. [Google Scholar] [CrossRef]
- Wang, J.H.; Wahid, K.A.; van Dijk, L.V.; Farahani, K.; Thompson, R.F.; Fuller, C.D. Radiomic biomarkers of tumor immune biology and immunotherapy response. Clin. Transl. Radiat. Oncol. 2021, 28, 97–115. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Bourbonne, V.; Schick, U.; Pradier, O.; Visvikis, D.; Metges, J.P.; Badic, B. Radiomics Approaches for the Prediction of Pathological Complete Response after Neoadjuvant Treatment in Locally Advanced Rectal Cancer: Ready for Prime Time? Cancers 2023, 15, 432. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef]
- Su, W.; Ling, Y.; Yang, X.; Wu, Y.; Xing, C. Tumor microenvironment remodeling after neoadjuvant chemoradiotherapy in local advanced rectal cancer revealed by single-cell RNA sequencing. J. Transl. Med. 2024, 22, 1037. [Google Scholar] [CrossRef]
- Dias Costa, A.; Vayrynen, S.A.; Chawla, A.; Zhang, J.; Vayrynen, J.P.; Lau, M.C.; Williams, H.L.; Yuan, C.; Morales-Oyarvide, V.; Elganainy, D.; et al. Neoadjuvant Chemotherapy Is Associated with Altered Immune Cell Infiltration and an Anti-Tumorigenic Microenvironment in Resected Pancreatic Cancer. Clin. Cancer Res. 2022, 28, 5167–5179. [Google Scholar] [CrossRef]
- Feng, X.; Meng, X.; Tang, D.; Guo, S.; Liao, Q.; Chen, J.; Xie, Q.; Liu, F.; Fang, Y.; Sun, C.; et al. Reversal of the immunosuppressive tumor microenvironment via platinum-based neoadjuvant chemotherapy in cervical cancer. Cancer Pathog. Ther. 2024, 2, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wu, M.L.; Huang, K.C.; Huang, I.P.; Chung, Y.L. The Effects of Neoadjuvant Treatment on the Tumor Microenvironment in Rectal Cancer: Implications for Immune Activation and Therapy Response. Clin. Color. Cancer 2020, 19, e164–e180. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Orhan, A.; Khesrawi, F.; Tvilling Madsen, M.; Peuliche Vogelsang, R.; Dohrn, N.; Kanstrup Fiehn, A.M.; Gogenur, I. Tumor-Infiltrating Lymphocytes as Biomarkers of Treatment Response and Long-Term Survival in Patients with Rectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 636. [Google Scholar] [CrossRef]
- Piringer, G. ASCO highlights: Neoadjuvant immunotherapy in mismatch repair deficient colorectal cancer. Mag. Eur. Med. Oncol. 2025, 18, 26–29. [Google Scholar] [CrossRef]
- Cercek, A.; Dos Santos Fernandes, G.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- El Sissy, C.; Kirilovsky, A.; Van den Eynde, M.; Musina, A.M.; Anitei, M.G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef]
- Mowery, Y.M.; Salama, J.K.; Zafar, S.Y.; Moore, H.G.; Willett, C.G.; Czito, B.G.; Hopkins, M.B.; Palta, M. Neoadjuvant long-course chemoradiation remains strongly favored over short-course radiotherapy by radiation oncologists in the United States. Cancer 2017, 123, 1434–1441. [Google Scholar] [CrossRef]
- Krook, J.E.; Moertel, C.G.; Gunderson, L.L.; Wieand, H.S.; Collins, R.T.; Beart, R.W.; Kubista, T.P.; Poon, M.A.; Meyers, W.C.; Mailliard, J.A.; et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N. Engl. J. Med. 1991, 324, 709–715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).