Cancer- and Chemotherapy-Induced Changes in Cerebral Metabolism in Patients with Diffuse Large B-Cell Lymphoma: A Serial [18F]FDG PET Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
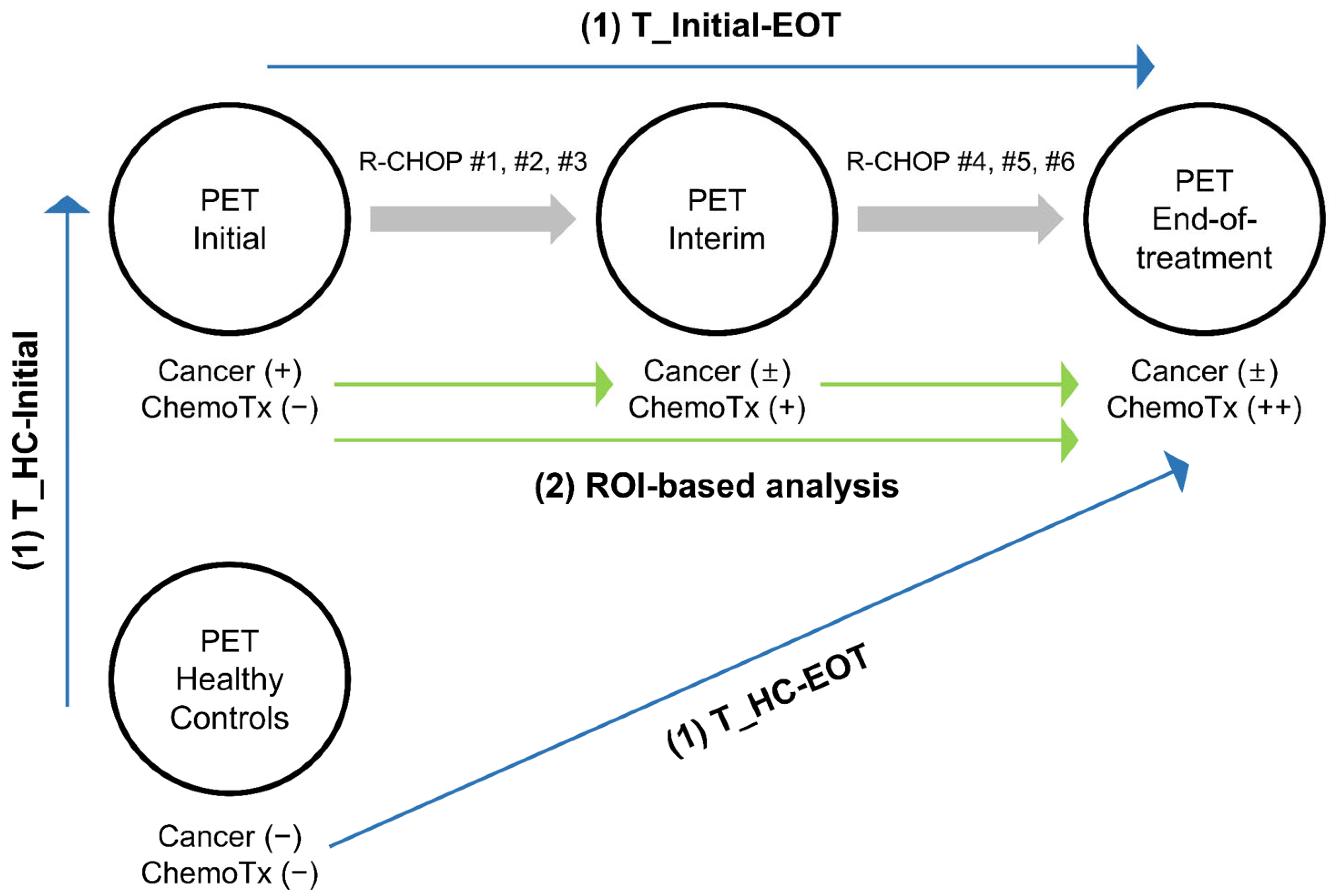
- (1)
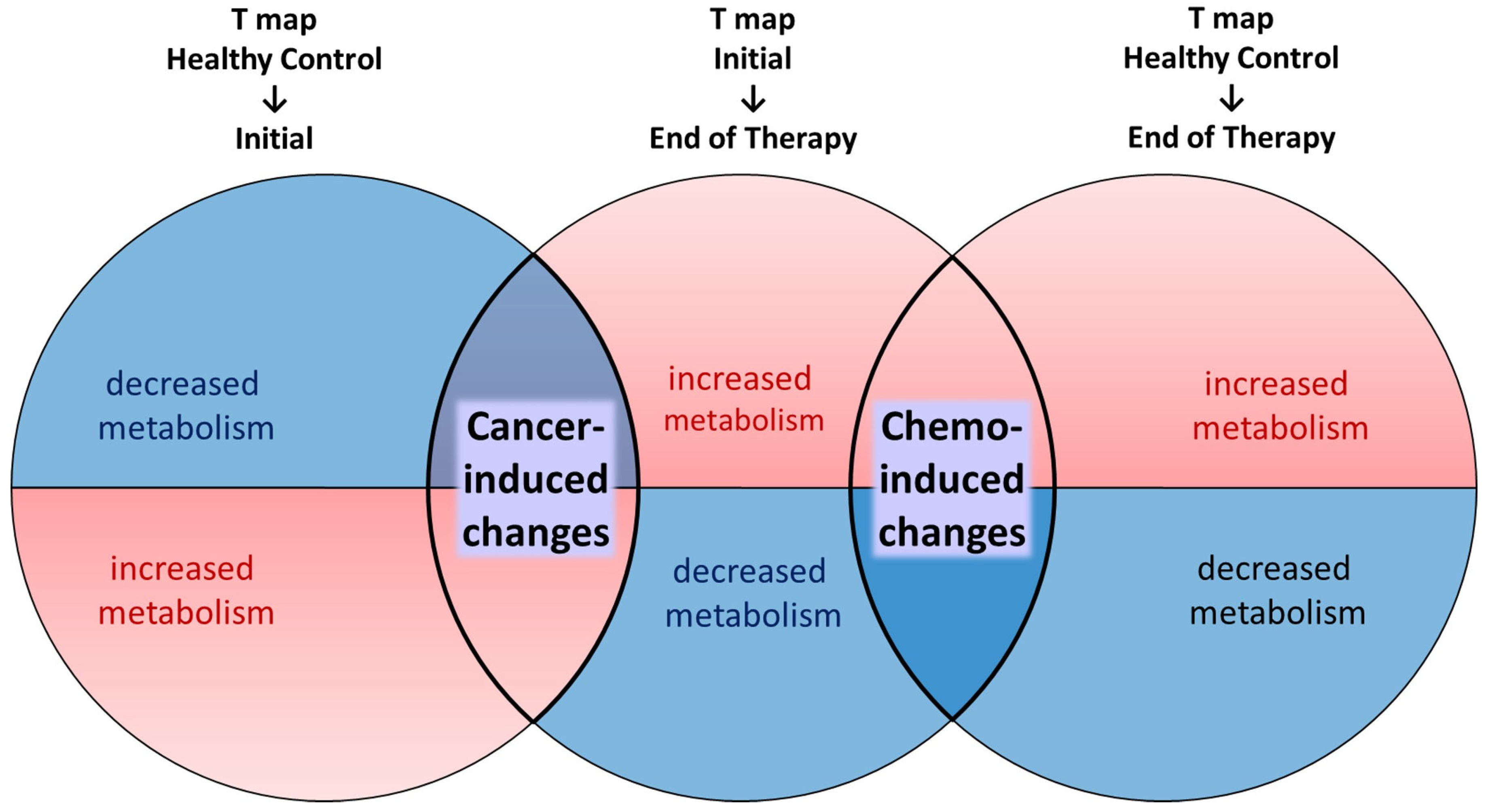
- Voxel-based analysis: Three t-statistics maps were generated using three sets of PET scans. T_Initial-EOT demonstrates significant changes from PET_Initial to PET_EOT using a voxel-wise paired t-test. T_HC-Initial and T_HC-EOT demonstrate significant changes from PET_HC to PET_Initial and PET_EOT, respectively, using voxel-wise two-sample t-tests. By comparing these three t-statistics maps, clusters of changes in cerebral metabolism attributable mainly to cancer and others attributable mainly to chemotherapy were identified.
- (2)
- Region of interest (ROI)-based analysis: The clusters identified by the voxel-based analysis were employed as ROIs, and we examined whether there were significant differences in cerebral metabolism within the ROIs at the three time points (PET_Initial, PET_Interim, and PET_EOT).
2.1. Participants
2.2. PET Image Acquisition & Reconstruction
2.3. Image Preprocessing
2.4. Voxel-Based Analysis
2.5. ROI-Based Analysis
2.6. Statistical Analysis
2.7. Use of Artificial Intelligence
3. Results
3.1. Demographics and Clinical Characteristics
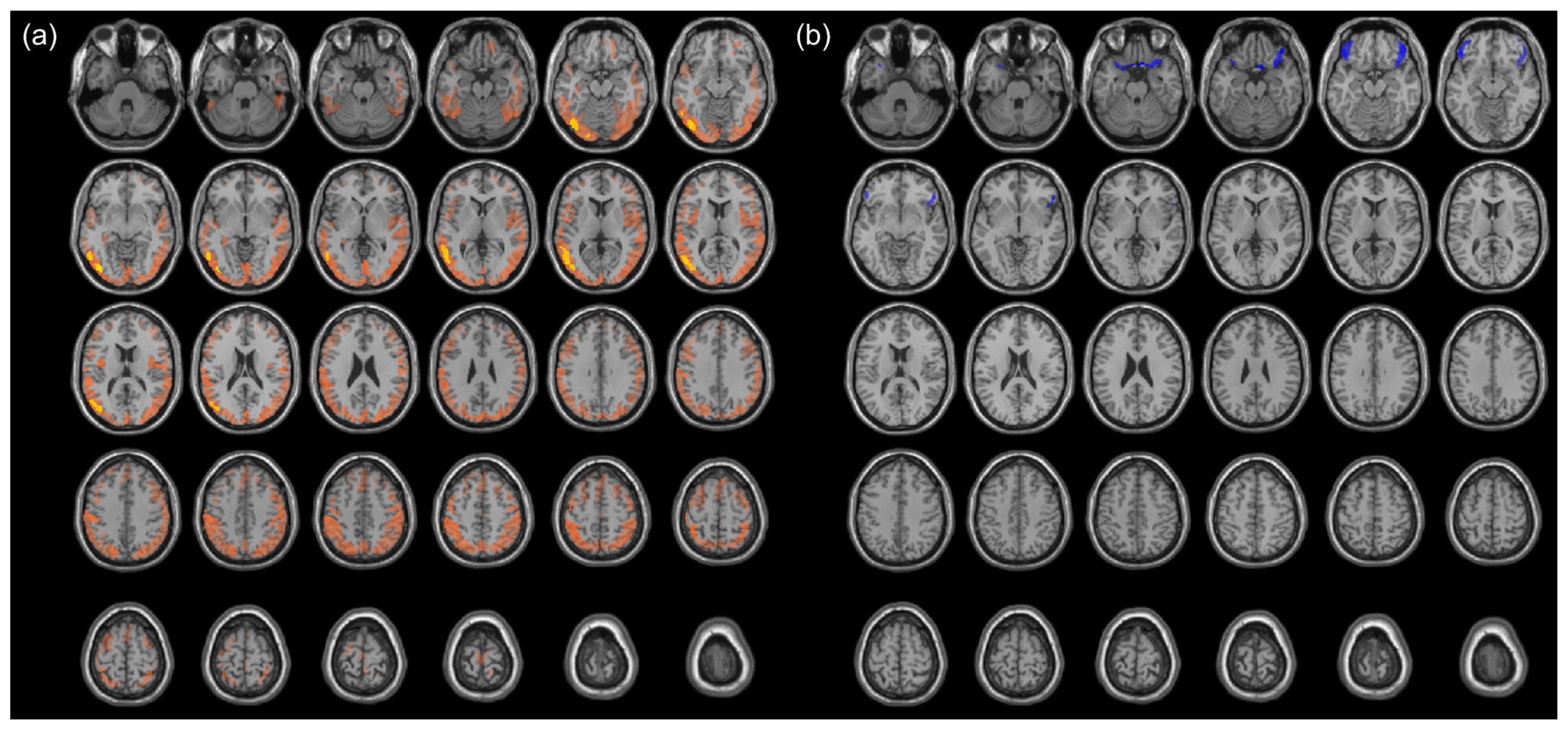
3.2. Cancer-Induced Changes in Cerebral Metabolism
3.3. Chemotherapy-Induced Change in Cerebral Metabolism
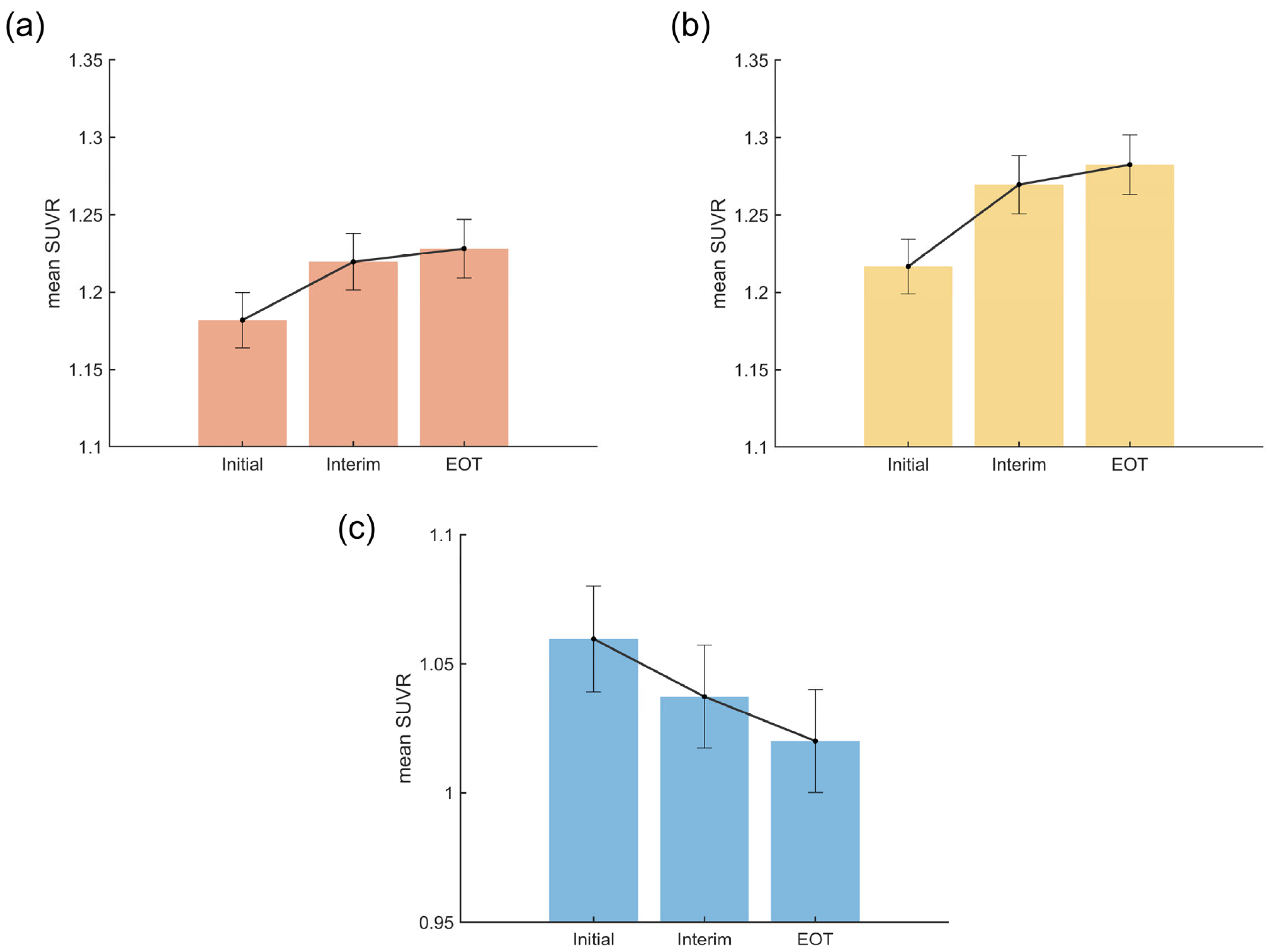
3.4. Temporal Patterns of Cancer- and Chemotherapy-Induced Metabolic Changes
4. Discussion
4.1. Limitations
4.2. Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Vannorsdall, T.D. Cognitive Changes Related to Cancer Therapy. Med. Clin. N. Am. 2017, 101, 1115–1134. [Google Scholar] [CrossRef]
- Haywood, D.; Dauer, E.; Baughman, F.D.; Lawrence, B.J.; Rossell, S.L.; Hart, N.H.; O’Connor, M. “Is My Brain Ever Going to Work Fully Again?”: Challenges and Needs of Cancer Survivors with Persistent Cancer-Related Cognitive Impairment. Cancers 2023, 15, 5331. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; George, S.; Ahles, T. Renaming “Chemobrain”. Cancer Investig. 2007, 25, 373–377. [Google Scholar] [CrossRef]
- Meyers, C.A.; Byrne, K.S.; Komaki, R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer 1995, 12, 231–235. [Google Scholar] [CrossRef]
- Meyers, C.A.; Albitar, M.; Estey, E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005, 104, 788–793. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Bekele, T.; Renton, C.; Dodd, A.; Zhang, H.; Beale, P.; Clarke, S.; et al. Cognitive Function in Patients with Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. J. Clin. Oncol. 2015, 33, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Baekelandt, B.M.; Hjermstad, M.J.; Nordby, T.; Fagerland, M.W.; Kure, E.H.; Heiberg, T.; Buanes, T.; Labori, K.J. Preoperative cognitive function predicts survival in patients with resectable pancreatic ductal adenocarcinoma. HPB 2016, 18, 247–254. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Jung, W.F.; Grande, L.J.; Chen, J.; Stone, R.M.; Soiffer, R.J.; Driver, J.A.; Abel, G.A. Prevalence of Cognitive Impairment and Association with Survival Among Older Patients with Hematologic Cancers. JAMA Oncol. 2018, 4, 686–693. [Google Scholar] [CrossRef]
- Olson, B.; Marks, D.L. Pretreatment Cancer-Related Cognitive Impairment-Mechanisms and Outlook. Cancers 2019, 11, 687. [Google Scholar] [CrossRef]
- Simo, M.; Rifa-Ros, X.; Rodriguez-Fornells, A.; Bruna, J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 1311–1321. [Google Scholar] [CrossRef]
- Rolls, E.T.; Joliot, M.; Tzourio-Mazoyer, N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 2015, 122, 1–5. [Google Scholar] [CrossRef]
- Nonokuma, M.; Kuwabara, Y.; Takano, K.; Tamura, K.; Ishitsuka, K.; Yoshimitsu, K. Evaluation of regional cerebral glucose metabolism in patients with malignant lymphoma of the body using statistical image analysis. Ann. Nucl. Med. 2014, 28, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Otomi, Y.; Otsuka, H.; Shono, N.; Onishi, H.; Mitsuhashi, R.; Matsuzaki, S.; Takaoka, Y.; Enomoto, H.; Sakamoto, Y.; Sasahara, M.; et al. A reduced physiological (18)F-fluorodeoxyglucose uptake in the brain and liver caused by malignant lymphoma being deprived of the tracer. J. Med. Investig. 2021, 68, 181–185. [Google Scholar] [CrossRef]
- Yi, H.K.; Yoo, J.; Kim, S.J.; Choi, J.Y.; Lee, K.H. Lymphoma total lesion glycolysis leads to hyperlactatemia and reduction of brain glucose utilization. Sci. Rep. 2022, 12, 12639. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Cui, C.; Zhang, Y. Altered Regional Brain Glucose Metabolism in Diffuse Large B-Cell Lymphoma Patients Treated with Cyclophosphamide, Epirubicin, Vincristine, and Prednisone: An Fluorodeoxyglucose Positron Emission Tomography Study of 205 Cases. Front. Neurosci. 2022, 16, 914556. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Pagani, M.; Cantonetti, M.; Pietro, B.D.; Tavolozza, M.; Travascio, L.; Biagio, D.D.; Danieli, R.; Schillaci, O. Brain metabolic changes in Hodgkin disease patients following diagnosis and during the disease course: An (18)F-FDG PET/CT study. Oncol. Lett. 2015, 9, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ponto, L.L.; Menda, Y.; Magnotta, V.A.; Yamada, T.H.; Denburg, N.L.; Schultz, S.K. Frontal hypometabolism in elderly breast cancer survivors determined by [(18)F]fluorodeoxyglucose (FDG) positron emission tomography (PET): A pilot study. Int. J. Geriatr. Psychiatry 2015, 30, 587–594. [Google Scholar] [CrossRef] [PubMed]
- López Zunini, R.A.; Scherling, C.; Wallis, N.; Collins, B.; MacKenzie, J.; Bielajew, C.; Smith, A.M. Differences in verbal memory retrieval in breast cancer chemotherapy patients compared to healthy controls: A prospective fMRI study. Brain Imaging Behav. 2013, 7, 460–477. [Google Scholar] [CrossRef]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Boogerd, W.; Oldenburg, H.S.; Reneman, L.; Schagen, S.B. Changes in brain activation in breast cancer patients depend on cognitive domain and treatment type. PLoS ONE 2017, 12, e0171724. [Google Scholar] [CrossRef]
- Haber, S.N.; Behrens, T.E. The neural network underlying incentive-based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron 2014, 83, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.D. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007, 30, 31–56. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef] [PubMed]
- Pullens, M.J.; De Vries, J.; Roukema, J.A. Subjective cognitive dysfunction in breast cancer patients: A systematic review. Psychooncology 2010, 19, 1127–1138. [Google Scholar] [CrossRef]
- Hanaoka, K.; Hosono, M.; Shimono, T.; Usami, K.; Komeya, Y.; Tsuchiya, N.; Yamazoe, Y.; Ishii, K.; Tatsumi, Y.; Sumita, M. Decreased brain FDG uptake in patients with extensive non-Hodgkin’s lymphoma lesions. Ann. Nucl. Med. 2010, 24, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Han, E.J.; O, J.H.; Choi, B.O.; Park, G.; Jung, S.E.; Yahng, S.A.; Eom, K.S.; Cho, S.G.; on behalf of Catholic University Lymphoma Group. Early Interim Chemotherapy Response Evaluation by F-18 FDG PET/CT in Diffuse Large B Cell Lymphoma. Diagnostics 2020, 10, 1002. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, Y.; Guo, L.; Zhao, H.; Shi, Y.; Li, S.; Wang, A.; Zhang, X.; Shi, J.; Zhu, J.; et al. Predictive value of baseline (18)F-FDG PET/CT and interim treatment response for the prognosis of patients with diffuse large B-cell lymphoma receiving R-CHOP chemotherapy. Oncol. Lett. 2021, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Itti, E.; Haioun, C.; Petegnief, Y.; Luciani, A.; Dupuis, J.; Paone, G.; Talbot, J.N.; Rahmouni, A.; Meignan, M. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J. Nucl. Med. 2007, 48, 1626–1632. [Google Scholar] [CrossRef]
- Malek, E.; Sendilnathan, A.; Yellu, M.; Petersen, A.; Fernandez-Ulloa, M.; Driscoll, J.J. Metabolic tumor volume on interim PET is a better predictor of outcome in diffuse large B-cell lymphoma than semiquantitative methods. Blood Cancer J. 2015, 5, e326. [Google Scholar] [CrossRef]
- Apple, A.C.; Schroeder, M.P.; Ryals, A.J.; Wagner, L.I.; Cella, D.; Shih, P.A.; Reilly, J.; Penedo, F.J.; Voss, J.L.; Wang, L. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clin. 2018, 20, 110–118. [Google Scholar] [CrossRef]
- Kardan, O.; Reuter-Lorenz, P.A.; Peltier, S.; Churchill, N.W.; Misic, B.; Askren, M.K.; Jung, M.S.; Cimprich, B.; Berman, M.G. Brain connectivity tracks effects of chemotherapy separately from behavioral measures. NeuroImage Clin. 2019, 21, 101654. [Google Scholar] [CrossRef] [PubMed]
| HC (n = 88) | DLBCL (n = 88) | Test Statistics | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 58.8 ± 9.9 | 58.8 ± 15.5 | 0.02 † | 0.982 |
| Sex, Male, Female | 58, 30 | 58, 30 | - | - |
| Ann-Arbor stage, I–II, III–IV | 49, 39 | |||
| Time Intervals | ||||
| Initial to Interim (days) | - | 73.5 ± 11.7 | −1.26 ‡ | 0.210 |
| Interim to EOT (days) | - | 76.4 ± 19.4 | ||
| Treatment Cycles | ||||
| Initial to Interim (cycles) | - | 3.1 ± 0.2 | 5 § | 0.453 |
| Interim to EOT (cycles) | - | 3.0 ± 0.4 | ||
| Response at EOT, CR, non-CR | - | 81, 7 |
| Regional SUVR | Initial a (n = 88) | Interim b (n = 88) | EOT c (n = 88) | F | p-Valueadj | η2 | Pairwise Comparison |
|---|---|---|---|---|---|---|---|
| Cancer-induced (FDR) | 1.18 ± 0.08 | 1.22 ± 0.09 | 1.23 ± 0.09 | 28.58 | 2.78 × 10−11 | 0.25 | a < b ***, a < c *** |
| Cancer-induced (FWE) | 1.22 ± 0.08 | 1.27 ± 0.09 | 1.28 ± 0.09 | 36.35 | 1.93 × 10−13 | 0.30 | a < b ***, a < c *** |
| Chemotherapy-induced (FDR) | 1.06 ± 0.10 | 1.04 ± 0.09 | 1.02 ± 0.09 | 27.75 | 3.47 × 10−11 | 0.24 | a > b ***, a > c ***, b > c ** |
| Regional SUVR | Contrast | Estimate | SE | t | p Valueadj |
|---|---|---|---|---|---|
| Cancer-induced (FDR) | Linear *** | 0.046 | 0.007 | 6.31 | 1.70 × 10−8 |
| Quadratic ** | −0.029 | 0.010 | −3.03 | 0.005 | |
| Cancer-induced (FWE) | Linear *** | 0.066 | 0.009 | 7.28 | 4.21 × 10−10 |
| Quadratic ** | −0.040 | 0.012 | −3.20 | 0.006 | |
| Chemotherapy-induced (FDR) | Linear *** | −0.040 | 0.006 | −6.67 | 2.88 × 10−9 |
| Quadratic | 0.005 | 0.008 | 0.66 | 0.514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, I.; Kang, Y.-k.; Min, J.W.; Ha, S.; O, J.H. Cancer- and Chemotherapy-Induced Changes in Cerebral Metabolism in Patients with Diffuse Large B-Cell Lymphoma: A Serial [18F]FDG PET Study. Cancers 2025, 17, 2222. https://doi.org/10.3390/cancers17132222
Chung I, Kang Y-k, Min JW, Ha S, O JH. Cancer- and Chemotherapy-Induced Changes in Cerebral Metabolism in Patients with Diffuse Large B-Cell Lymphoma: A Serial [18F]FDG PET Study. Cancers. 2025; 17(13):2222. https://doi.org/10.3390/cancers17132222
Chicago/Turabian StyleChung, Insung, Yeon-koo Kang, Jae Won Min, Seunggyun Ha, and Joo Hyun O. 2025. "Cancer- and Chemotherapy-Induced Changes in Cerebral Metabolism in Patients with Diffuse Large B-Cell Lymphoma: A Serial [18F]FDG PET Study" Cancers 17, no. 13: 2222. https://doi.org/10.3390/cancers17132222
APA StyleChung, I., Kang, Y.-k., Min, J. W., Ha, S., & O, J. H. (2025). Cancer- and Chemotherapy-Induced Changes in Cerebral Metabolism in Patients with Diffuse Large B-Cell Lymphoma: A Serial [18F]FDG PET Study. Cancers, 17(13), 2222. https://doi.org/10.3390/cancers17132222





