Breaking Dogmas in Axillary Lymphadenectomy and Quality of Life
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Ambulatory Surgery and Early Discharge After ALND
3.1. Benefits of Ambulatory Surgery
3.2. The Role of ERAS Protocols in Facilitating Early Discharge
3.3. Early Discharge Feasibility and Alternatives to Drains
3.4. Economic Impact of Ambulatory Surgery
4. Omission of Surgical Drains in Lymphadenectomy
4.1. Types of Drainage Protocols
4.2. Use of Drains
4.3. Alternative Techniques to Reduce Seroma
4.4. Recent Scientific Evidence
5. Use of Tissue Sealants in ALDN
5.1. Types of Sealants
5.1.1. Fibrin-Based Sealants
5.1.2. Polyethylene Glycol (PEG)-Based Sealants
5.1.3. Cyanoacrylate-Based Sealants
5.1.4. Thrombin and Fibrinogen Combipatches
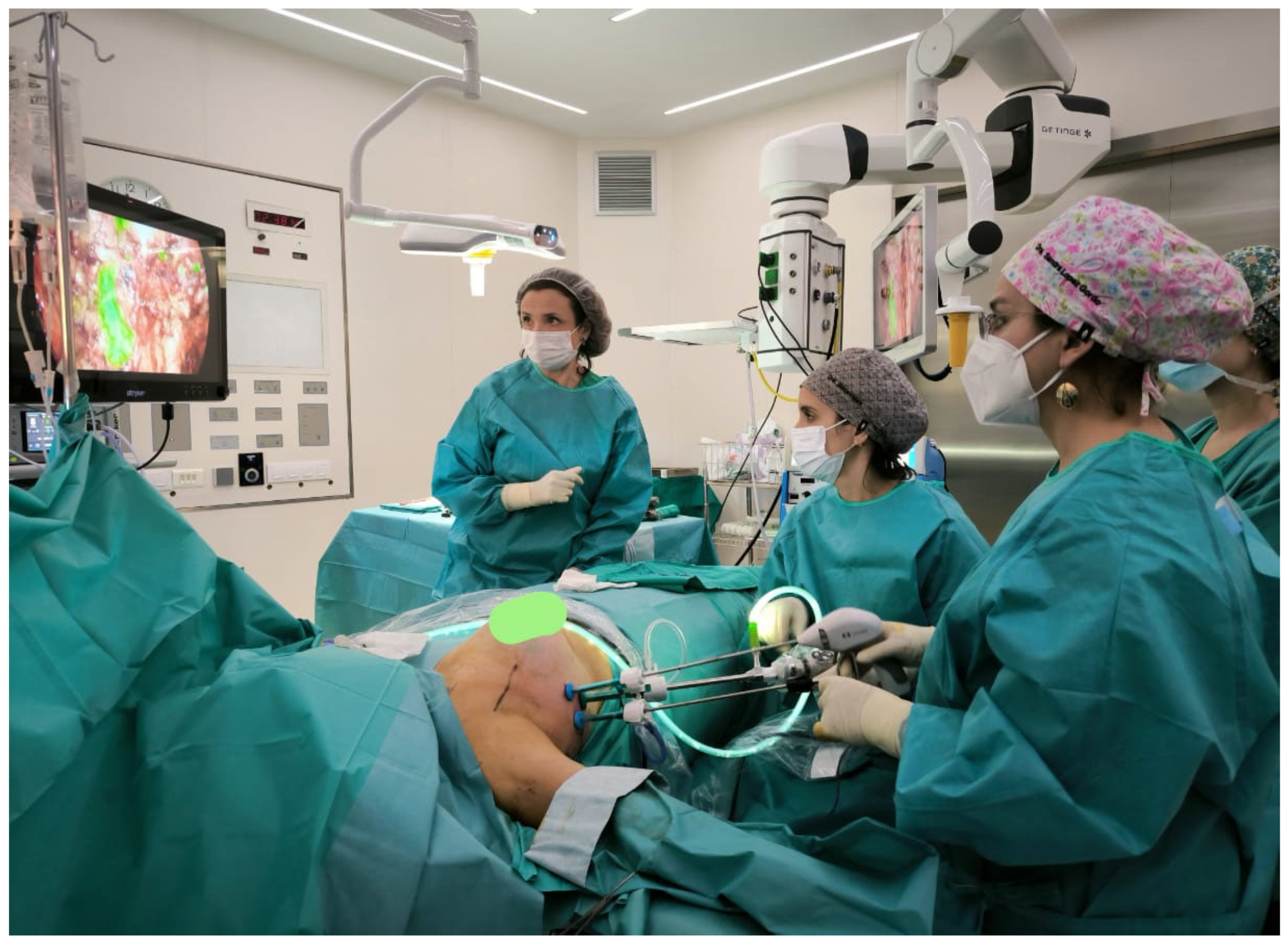
6. Minimally Invasive and Endoscopic Surgical Approaches
6.1. Endoscopic Axillary Lymphadenectomy
6.2. Robotics and the New Frontier of Axillary Surgery
7. Breast Cancer Axillary Lymphadenectomy and Quality of Life
7.1. Introduction
7.2. Conservative Surgery of the Axilla
7.3. Quality of Life and Breast Cancer Surgery
8. Discussion
8.1. Ambulatory ALDN
8.2. Use of Drains
8.3. Use of Sealants
8.4. Minimally Invasive ALDN
8.5. Quality of Life and ALDN
8.6. Clinical Considerations
9. Limitations
10. Future Directions
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Bemmel, A.J.M.; Van De Velde, C.J.H.; Schmitz, R.F.; Liefers, G.J. Prevention of seroma formation after axillary dissection in breast cancer: A systematic review. Eur. J. Surg. Oncol. 2011, 37, 829–835. [Google Scholar] [CrossRef]
- Jain, P.K.; Sowdi, R.; Anderson, A.D.G.; MacFie, J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br. J. Surg. 2004, 91, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Rai, S.; Hoar, F.; Brown, H.; Vishwanath, L. Breast cancer surgery without suction drainage: The impact of adopting a ‘no drains’ policy on symptomatic seroma formation rates. Eur. J. Surg. Oncol. 2013, 39, 334–338. [Google Scholar] [CrossRef]
- Gunn, J.; Gibson, T.; Li, Z.; Diehl, N.; Bagaria, S.; McLaughlin, S. Symptomatic Axillary Seroma after Sentinel Lymph Node Biopsy: Incidence and Treatment. Ann. Surg. Oncol. 2016, 23, 3347–3353. [Google Scholar] [CrossRef]
- Tamminen, A.; Meretoja, T.; Koskivuo, I. Same-day mastectomy and axillary lymph node dissection is safe for most patients with breast cancer. J. Surg. Oncol. 2022, 125, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Karakousis, C.P. Axillary Padding as an Alternative to Closed Suction Drain for Ambulatory Axillary Lymphadenectomy—Invited Critique. Arch. Surg. 2002, 137, 173. [Google Scholar] [CrossRef]
- Al-Hilli, Z.; Thomsen, K.M.; Habermann, E.B.; Jakub, J.W.; Boughey, J.C. Reoperation for Complications after Lumpectomy and Mastectomy for Breast Cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP). Ann. Surg. Oncol. 2015, 22, 459–469. [Google Scholar] [CrossRef]
- Medarde-Ferrer, M.; Garriga, X.G.; Rodriguez, Ó.A.; Barraca, I.V.; Gago, A.P.; Soto, S.N.; López, L.M. Aplicación de un protocolo ERAS (enhanced recovery after surgery) para la práctica cirugía oncológica compleja de la mama en régimen ambulatorio. Rev. Senol. Y Patol. Mamar. 2024, 37, 100614. [Google Scholar] [CrossRef]
- Barry, M.; Weber, W.P.; Lee, S.; Mazzella, A.; Sclafani, L.M. Enhancing the clinical pathway for patients undergoing axillary lymph node dissection. Breast 2012, 21, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Siegel, B.M.; Mayzel, K.A.; Love, S.M. Level I and II Axillary Dissection in the Treatment of Early-Stage Breast Cancer. Arch. Surg. 1990, 125, 1144. [Google Scholar] [CrossRef]
- Bonnema J, van Wersch AM, van Geel AN, Pruyn JF, Schmitz PI, Uyl-de Groot CA, Wiggers TCost of care in a randomised trial of early hospital discharge after surgery for breast cancer. Eur. J. Cancer 1998, 34, 2015–2020. [CrossRef] [PubMed]
- Bundred, N.; Maguire, P.; Reynolds, J.; Grimshaw, J.; Morris, J.; Thomson, L.; Barr, L.; Baildam, A. Randomised controlled trial of effects of early discharge after surgery for breast cancer. BMJ 1998, 317, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, C.; West, N.; Mansel, R.E.; Horgan, K. The satisfaction and savings of early discharge with drain in situ following axillary lymphadenectomy in the treatment of breast cancer. Eur. J. Surg. Oncol. 1995, 21, 604–606. [Google Scholar] [CrossRef]
- Jogerst, K.; Thomas, O.; Kosiorek, H.E.; Gray, R.; Cronin, P.; Casey W3rd Rebecca, A.; Craner, R.; Young-Fadok, T.; Pockaj, B. Same-Day Discharge After Mastectomy: Breast Cancer Surgery in the Era of ERAS®. Ann. Surg. Oncol. 2020, 27, 3436–3445. [Google Scholar] [CrossRef]
- Margolese, R.G.; Lasry, J.C.M. Ambulatory surgery for breast cancer patients. Ann. Surg. Oncol. 2000, 7, 181–187. [Google Scholar] [CrossRef]
- Horgan, K.; Benson, E.A.; Miller, A.; Robertson, A. Early discharge with drain in situ following axillary lymphadenectomy for breast cancer. Breast 2000, 9, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.M.; Berchery, D.; Campion, L.; Pioud, R.; Dravet, F.; Robard, S. Randomized clinical trial comparing axillary padding with closed suction drainage for the axillary wound after lymphadenectomy for breast cancer. Br. J. Surg. 2006, 93, 820–824. [Google Scholar] [CrossRef]
- Srivastava, V.; Basu, S.; Shukla, V.K. Seroma formation after breast cancer surgery: What we have learned in the last two decades. J. Breast Cancer 2012, 15, 373–380. [Google Scholar] [CrossRef]
- Droeser, R.A.; Frey, D.M.; Oertli, D.; Kopelman, D.; Baas-Vrancken Peeters, M.J.; Giuliano, A.E.; Dalberg, K.; Kallam, R.; Nordmann, A. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: A meta-analysis. Breast 2009, 18, 109–114. [Google Scholar] [CrossRef]
- Cameron, A.E.; Ebbs, S.R.; Wylie, F.; Baum, M. Suction drainage of the axilla: A prospective randomized trial. Br. J. Surg. 1998, 75, 1211. [Google Scholar] [CrossRef]
- Somers, R.G.; Jablon, L.K.; Kaplan, M.J.; Sandler, G.L.; Rosenblatt, N.K. The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer: A prospective randomized trial. Ann. Surg. 1992, 215, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Zavotsky, J.; Jones, R.C.; Brennan, M.B.; Giuliano, A.E. Evaluation of axillary lymphadenectomy without axillary drainage for patients undergoing breast-conserving therapy. Ann. Surg. Oncol. 1998, 5, 227–231. [Google Scholar] [CrossRef]
- Soon, P.S.H.; Clark, J.; Magarey, C.J. Seroma formation after axillary lymphadenectomy with and without the use of drains. Breast 2005, 14, 103–107. [Google Scholar] [CrossRef]
- He, X.D.; Guo, Z.H.; Tian, J.H.; Yang, K.H.; Xie, X.D. Whether drainage should be used after surgery for breast cancer? A systematic review of randomized controlled trials. Med. Oncol. 2011, 28, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Troost, M.S.; Kempees, C.J.; De Roos, M.A.J. Breast cancer surgery without drains: No influence on seroma formation. Int. J. Surg. 2015, 13, 170–174. [Google Scholar] [CrossRef]
- López Gordo, S.; Ruiz-Edo, N.; Fernández-Planas, M.T.; Viscaya-Martín, S.; Serra-Serra, C. Seroma control in axillary lymphadenectomy with Glubran 2® without drain. Multicenter, prospective, randomized, clinical trial. GALA-ND study (Glubran, Axillary Lymphadenectomy, Ambulatory, No Drain). Trials 2024, 25, 142. [Google Scholar] [CrossRef]
- Jeffrey, S.S.; Goodson, W.H.; Ikeda, D.M.; Birdwell, R.L.; Bogetz, M.S. Axillary Lymphadenectomy for Breast Cancer Without Axillary Drainage. Arch. Surg. 1995, 130, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Garbay, J.R.; Thoury, A.; Moinon, E.; Cavalcanti, A.; Palma, M.D.; Karsenti, G.; Leymarie, N.; Sarfati, B.; Rimareix, F.; Mazouni, C. Axillary padding without drainage after axillary lymphadenectomy—A prospective study of 299 patients with early breast cancer. Breast Care 2012, 7, 231–235. [Google Scholar] [CrossRef]
- Conversano, A.; Mazouni, C.; Thomin, A.; Gaudin, A.; Fournier, M.; Rimareix, F.; Bonastre, J. Use of Low-Thrombin Fibrin Sealant Glue After Axillary Lymphadenectomy for Breast Cancer to Reduce Hospital Length and Seroma. Clin. Breast Cancer 2017, 17, 293–297. [Google Scholar] [CrossRef]
- Purushotham, A.D.; McLatchie, E.; Young, D.; George, W.D.; Stallard, S.; Doughty, J.; Brown, D.C.; Farish, C.; Walker, A.; Millar, K.; et al. Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer. Br. J. Surg. 2002, 89, 286–292. [Google Scholar] [CrossRef]
- Cortadellas, T.; Córdoba, O.; Espinosa-Bravo, M.; Mendoza-Santin, C.; Rodríguez-Fernández, J.; Esgueva, A.; Alvarez-Vinuesa, M.; Rubio, I.T.; Xercavins, J. Electrothermal bipolar vessel sealing system in axillary dissection: A prospective randomized clinical study. Int. J. Surg. 2011, 9, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Basso, S.M.M.; Santeufemia, D.A.; Bonamini, M.; Chiara, G.B. Ultrasonic dissection system technology in breast cancer: A case-control study in a large cohort of patients requiring axillary dissection. Breast Cancer Res. Treat. 2013, 142, 399–404. [Google Scholar] [CrossRef]
- Thomson, D.R.; Sadideen, H.; Furniss, D. Wound drainage after axillary dissection for carcinoma of the breast. Cochrane Database Syst. Rev. 2013, 2013, CD006823. [Google Scholar] [CrossRef] [PubMed]
- Buch-Villa, E.; Castañer-Puga, C.; Delgado-Garcia, S.; Fuster-Diana, C.; Vidal-Herrador, B.; Ripoll-Orts, F.; Galeote-Quecedo, T.; Prat, A.; Andrés-Matias, M.; Jimeno-Fraile, J.; et al. Clinical and cost outcomes of a polyethylene glycol (PEG)-coated patch versus drainage after axillary lymph node dissection in breast cancer: Results from a multicentre randomized clinical trial. Br. J. Surg. 2023, 110, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Al-Masri, M.; Alawneh, F.; Daoud, F.; Ebous, A.; Hamdan, B.; Al-Najjar, H.; Al-Masri, R.; Abufara, M. Effectiveness of Cyanoacrylate in Reducing Seroma Formation in Breast Cancer Patients Post-Axillary Dissection: A Randomized Controlled Trial. Front. Oncol. 2021, 10, 580861. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef]
- Moore, M.; Burak, W.E., Jr.; Nelson, E.; Kearney, T.; Simmons, R.; Mayers, L.; Spotnitz, W.D. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: A randomized prospective clinical trial. J. Am. Coll. Surg. 2001, 192, 591–599. [Google Scholar] [CrossRef]
- Gilly, F.N.; François, Y.; Sayag-Beaujard, A.C.; Glehen, O.; Brachet, A.; Vignal, J. Prevention of lymphorrhea by means of fibrin glue after axillary lymphadenectomy in breast cancer: Prospective randomized trial. Eur. Surg. Res. 1998, 30, 439–443. [Google Scholar] [CrossRef]
- Ruggiero, R.; Docimo, G.; Gubitosi, A.; Conzo, G.; Tolone, S.; Gili, S.; Bosco, A.; Docimo, L. Axillary lymphadenectomy for breast cancer and fibrin glue. Ann. Ital. Chir. 2014, 85, 88–92. [Google Scholar]
- Mustonen, P.K.; Härmä, M.A.; Eskelinen, M.J. The effect of fibrin sealant combined with fibrinolysis inhibitor on reducing the amount of lymphatic leakage after axillary evacuation in breast cancer. A prospective randomized clinical trial Background and Aims: One third of women undergoing mastectomy. Scand. J. Surg. 2004, 93, 209–212. [Google Scholar] [CrossRef]
- Vaxman, F.; Kolbe, A.; Stricher, F.; Zund, D.; Volkmar, P.; Gros, D.; Grenier, J.F. Does fibrin glue improve drainage after axillary lymph node dissection? Prospective and randomized study in humans. Eur. Surg. Res. 1995, 27, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Benevento, R.; Santoriello, A.; Pellino, G.; Sciaudone, G.; Candilio, G.; De Fatico, G.S.; Selvaggi, F.; EBSQ Colo; Canonico, S. The effects of low-thrombin fibrin sealant on wound serous drainage, seroma formation and length of postoperative stay in patients undergoing axillary node dissection for breast cancer: A randomized controlled trial. Int. J. Surg. 2014, 12, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Shih, S.L.; Loh, E.W.; Tam, K.W. Effects of Fibrin Sealant on Seroma Reduction for Patients with Breast Cancer Undergoing Axillary Dissection: Meta-Analysis of Randomized Controlled Trials. Ann. Surg. Oncol. 2020, 27, 5286–5295. [Google Scholar] [CrossRef]
- Vasileiadou, K.; Kosmidis, C.; Anthimidis, G.; Miliaras, S.; Kostopoulos, I.; Fahantidis, E. Cyanoacrylate Adhesive Reduces Seroma Production After Modified Radical Mastectomy or Quadrantectomy with Lymph Node Dissection—A Prospective Randomized Clinical Trial. Clin. Breast Cancer 2017, 17, 595–600. [Google Scholar] [CrossRef]
- Esposito, E.; Siani, C.; Donzelli, I.; Crispo, A.; Coluccia, S.; Di Gennaro, P.; Luongo, A.; Avino, F.; Fucito, A.; Marone, U.; et al. Cyanoacrylate glue in breast surgery: The GLUBREAST Trial. Front. Oncol. 2004, 14, 1473157. [Google Scholar] [CrossRef]
- Clement, Z.; Shin, P.; Hoffmann, C.; Eaton, M.; McLeay, W. A Double-Blinded Prospective Randomised Controlled Trial to Assess the Efficacy of Glubran-2 in Reducing Seroma Formation after a Mastectomy with or without Axillary Dissection. Adv. Breast Cancer Res. 2017, 06, 117–128. [Google Scholar] [CrossRef]
- Vinchant, M.; Bonneau, C.; Lesavre, M.; Akerman, G.; Raiffort, C.; Barranger, E.; Bricou, A. Intérêt d’un combipatch de thrombine et de fibrinogène dans la prévention des lymphocèles après curage axillaire. Gynecol. Obstet. Fertil. 2013, 41, 583–587. [Google Scholar] [CrossRef]
- Lacoste, C.; Ouldamer, L.; Body, G.; Marret, H. Does the use of TachoSil allow to reduce the morbidity of axillary dissection? Gynecol. Obstet. Fertil. 2013, 41, 141–143. [Google Scholar] [CrossRef]
- Tagaya, N.; Kubota, K. Experience with endoscopic axillary lymphadenectomy using needlescopic instruments in patients with breast cancer: A preliminary report. Surg. Endosc. Other Interv. Tech. 2002, 16, 307–309. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, Z.; Xu, L.; Chen, C.; Fu, Q.; Teng, R.; Chen, J.; Xie, S.; Wang, L.; Yu, X.F.; et al. Contrast of Mastoscopic and Conventional Axillary Lymph Node Dissection of Patients with Breast Cancer: Meta-Analysis. Cancer Control 2020, 27, 1073274820932987. [Google Scholar] [CrossRef]
- Suzanne, F.; Emering, C.; Wattiez, A.; Bournazeau, J.A.; Bruhat, M.A.; Jacquetin, B. Le curage axillaire par lipo-aspiration et prélèvement endoscopique. A propos de 72 cas [Axillary lymphadenectomy by lipo-aspiration and endoscopic picking. Apropos of 72 cases]. Chirurgie 1997, 122, 138–142. [Google Scholar] [PubMed]
- Cangiotti, L.; Poiatti, R.; Taglietti, L.; Re, P.; Carrara, B. A mini-invasive technique for axillary lymphadenectomy in early breast cancer: A study of 15 patients. J. Exp. Clin. Cancer Res. 1999, 18, 295–298. [Google Scholar] [PubMed]
- Kamprath, S.; Bechler, J.; Kühne-Heid, R.; Krause, N.; Schneider, A. Endoscopic axillary lymphadenectomy without prior liposuction: Development of a technique and initial experience. Surg. Endosc. 1999, 13, 1226–1229. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gang, T.R.; Wu, S.S.; Lu, C.; Gao, G.X.; Xu, W.; Ding, G.Q.; Qu, X.; Zhang, Z.T. Single-port endoscopic-sentinel lymph node biopsy combined with indocyanine green and carbon nanoparticles in breast cancer. Surg. Endosc. 2023, 37, 7591–7599. [Google Scholar] [CrossRef]
- Yao, C.C.; Liu, C.; Xian, J. Comparison of single-pore non-liposuction near-infrared laparoscopy with conventional open surgery for axillary sentinel lymph node biopsy in patients with early breast cancer: A single-center, small-sample retrospective study. World J. Surg. Oncol. 2023, 21, 66. [Google Scholar] [CrossRef]
- Aponte-Rueda, M.E.; Cárdenas, R.A.S.; Saade Aure, M.J. Endoscopic axillary dissection: A systematic review of the literature. Breast 2009, 18, 150–158. [Google Scholar] [CrossRef]
- De Wilde, R.L.; Schmidt, E.H.; Hesseling, M.; Mildner, R.; Frank, V.; Tenger, M. Comparison of classic and endoscopic lymphadenectomy for staging breast cancer. J. Am. Assoc. Gynecol. Laparosc. 2003, 10, 75–79. [Google Scholar] [CrossRef]
- Luo, C.; Guo, W.; Yang, J.; Sun, Q.; Wei, W.; Wu, S.; Fang, S.; Zeng, Q.; Zhao, Z.; Meng, F.; et al. Comparison of mastoscopic and conventional axillary lymph node dissection in breast cancer: Long-term results from a randomized, multicenter trial. Mayo Clin. Proc. 2012, 87, 1153–1161. [Google Scholar] [CrossRef]
- López Gordo, S.; Borisova, I.; Ruiz-Edo, N.; López-Cano, D.; De la Iglésia, M.; Giner Pichel, M.; Salcedo-Pujantell, M.; Serra-Serra, C. Indocyanine green for axillary sentinel lymph node biopsy in patients with breast cancer (INSEAN study). Cir. Esp. 2025, 103, 173–178. [Google Scholar] [CrossRef]
- Chengyu, L.; Yongqiao, Z.; Hua, L.; Xiaoxin, J.; Chen, G.; Jing, L.; Jian, Z. A standardized surgical technique for mastoscopic axillary lymph node dissection. Surg. Laparosc. Endosc. Percutan Tech. 2005, 15, 153–159. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, J.M.; Choi, S.B.; Go, J.; Lee, J.; Kim, J.Y.; Park, H.S. Early experience of robotic axillary lymph node dissection in patients with node-positive breast cancer. Breast Cancer Res. Treat. 2023, 198, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; MBeeraka, N.; Zhang, J.; Reshetov, I.V.; Nikolenko, V.N.; Sinelnikov, M.Y.; Mikhaleva, L.M. Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer—A comparative study. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2307. [Google Scholar] [CrossRef]
- Basch, E.; Torda, P.; Adams, K. Standards for patient-reported outcome-based performance measures. JAMA 2013, 310, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA-J. Am. Med. Assoc. 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA-J. Am. Med. Assoc. 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (alliance) clinical trial. JAMA-J. Am. Med. Assoc. 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–263. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Alvarez-Pardo, S.; Romero-Pérez, E.M.; Camberos-Castañeda, N.; de Paz, J.A.; Horta-Gim, M.A.; González-Bernal, J.J.; Mielgo-Ayuso, J.; Simón-Vicente, L.; Fernández-Solana, J.; González-Santos, J. Quality of Life in Breast Cancer Survivors in Relation to Age, Type of Surgery and Length of Time since First Treatment. Int. J. Environ. Res. Public Health 2022, 19, 16229. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Djordjevic, N.; Djordjevic, L.; Ignjatovic, N.; Djordjevic, M.; Karanikolic, V. Assessment of the quality of life in breast cancer depending on the surgical treatment. Support. Care Cancer 2021, 29, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.E.; Lei, X.; Roubaud, M.S.; DeSnyder, S.M.; Caudle, A.S.; Shaitelman, S.F.; Hoffman, K.E.; Smith, G.L.; Jagsi, R.; Peterson, S.K.; et al. Long-Term Quality of Life in Patients with Breast Cancer after Breast Conservation vs Mastectomy and Reconstruction. JAMA Surg. 2022, 157, e220631. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, U.; Glickman, M.; Winter, M.; Clark, M.A. Long-term breast cancer survivors’ symptoms and morbidity: Differences by sexual orientation? J. Cancer Surviv. 2013, 7, 203–210. [Google Scholar] [CrossRef]
- Laws, A.; Lagendijk, M.; Grossmith, S.; Hughes, M.; Lin, N.U.; Mittendorf, E.A.; Eliassen, A.H.; King, T.A.; Dominici, L.S. Long-Term Patient-Reported Arm Symptoms in Breast Cancer Survivors. Ann. Surg. Oncol. 2024, 31, 1623–1633. [Google Scholar] [CrossRef]
- Enien, M.; Ibrahim, N.; Makar, W.; Darwish, D.; Gaber, M. Health-related quality of life: Impact of surgery and treatment modality in breast cancer. J. Cancer Res. Ther. 2018, 14, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Fontein, D.B.; Meershoek-Klein Kranenbarg, E.; Nortier, J.W.; Rutgers, E.J.; Marang-van de Mheen, P.J.; van de Velde, C.J. Long term effects of extended adjuvant endocrine therapy on quality of life in breast cancer patients. Breast 2015, 24, 224–229. [Google Scholar] [CrossRef]
- Hopwood, P.; Haviland, J.; Mills, J.; Sumo, G.; Bliss, J.M. The impact of age and clinical factors on quality of life in early breast cancer: An analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial). Breast 2007, 16, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Del Bianco, P.; Zavagno, G.; Burelli, P.; Scalco, G.; Barutta, L.; Carraro, P.; Pietrarota, P.; Meneghini, G.; Morbin, T.; Tacchetti, G.; et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: Results of the sentinella-GIVOM Italian randomised clinical trial. Eur. J. Surg. Oncol. 2008, 34, 508–513. [Google Scholar] [CrossRef]
- Belmonte, R.; Garin, O.; Segura, M.; Pont, A.; Escalada, F.; Ferrer, M. Quality-of-life impact of sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer patients. Value Health 2012, 15, 907–915. [Google Scholar] [CrossRef]
- Dabakuyo, T.S.; Fraisse, J.; Causeret, S.; Gouy, S.; Padeano, M.M.; Loustalot, C.; Cuisenier, J.; Sauzedde, J.M.; Smail, M.; Combier, J.P.; et al. A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection. Ann. Oncol. 2009, 20, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Al Nakib, M.; Buttarelli, M.; Huiart, L.; Martino, M.; Tarpin, C.; Extra, J.M.; Tallet, A.; Houvenaeghel, G. Quality of life at 2 years follow-up after sentinel lymph node biopsy, immediate or delayed axillary dissection for breast cancer. Breast J. 2010, 16, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Barranger, E.; Dubernard, G.; Fleurence, J.; Antoine, M.; Darai, E.; Uzan, S. Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer. J. Surg. Oncol. 2005, 92, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.A.; Donker, M.; Poncet, C.; Sauvé, N.; Straver, M.E.; van de Velde, C.J.; Mansel, R.E.; Blanken, C.; Orzalesi, L.; Klinkenbijl, J.H.; et al. Radiotherapy or Surgery of the Axilla after a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J. Clin. Oncol. 2023, 41, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Kootstra, J.J.; Hoekstra-Weebers, J.E.; Rietman, J.S.; de Vries, J.; Baas, P.C.; Geertzen, J.H.; Hoekstra, H.J.A. A longitudinal comparison of arm morbidity in stage I-II breast cancer patients treated with sentinel lymph node biopsy, sentinel lymph node biopsy followed by completion lymph node dissection, or axillary lymph node dissection. Ann. Surg. Oncol. 2010, 17, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Rietman, J.S.; Geertzen, J.H.; Hoekstra, H.J.; Baas, P.; Dolsma, W.V.; de Vries, J.; Groothoff, J.W.; Eisma, W.H.; Dijkstra, P.U. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur. J. Surg. Oncol. 2006, 32, 148–152. [Google Scholar] [CrossRef]
| Outcome | Drainage | Sealants |
|---|---|---|
| Seroma formation | Common | Variable (higher in some studies, lower in others) |
| Hospital stay | Prolonged | Reduced in some sealant groups |
| Need for postoperative aspirations | Frequent | Reduced in some sealant groups |
| Pain and discomfort | Higher due to drains | Lower in most sealant groups |
| Cost-effectiveness | Increased outpatient visits | Potential cost savings |
| Study/Year | Patients, N | Pain | Arm Swelling | Dysesthesias | QoL |
|---|---|---|---|---|---|
| Barranger E et al., 2005 [79] | SNB, n = 54 | 21.2% | 0% | 5.7% | 7.6 |
| ALND, n = 61 | 52.9% | 21.6% | 51% | 7.6 | |
| SNB + ALND, n = 10 | 60% | 10% | 50% | 7.7 | |
| Belmonte et al., 2012 [80] | SNB, n = 64 | ND | 11.8% | 9.8% | 119.05 |
| ALND, n = 29 | 35.5% | 69.2% | 111.89 | ||
| Dabakuyo et al., 2009 [81] | SNB, n = 222 | ND | ND | ND | 75.9 |
| ALND, n = 235 | 74.5 | ||||
| SNB + ALND, n = 61 | 68.0 | ||||
| Al Nakib et al., 2010 [82] | SNB, n = 212 | 39.4% | 9.9% | 40.1% | 73.6 |
| ALND, n = 131 | 67.7% | 33.6% | 83.1% | 68.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Gordo, S.; Jimeno-Fraile, J.; García-Monferrer, A.; Nicolau, P.; Ruiz-Edo, N.; Ramirez-Maldonado, E.; Rojas, S.; Serra-Serra, C. Breaking Dogmas in Axillary Lymphadenectomy and Quality of Life. Cancers 2025, 17, 2201. https://doi.org/10.3390/cancers17132201
López Gordo S, Jimeno-Fraile J, García-Monferrer A, Nicolau P, Ruiz-Edo N, Ramirez-Maldonado E, Rojas S, Serra-Serra C. Breaking Dogmas in Axillary Lymphadenectomy and Quality of Life. Cancers. 2025; 17(13):2201. https://doi.org/10.3390/cancers17132201
Chicago/Turabian StyleLópez Gordo, Sandra, Jaime Jimeno-Fraile, Anna García-Monferrer, Pau Nicolau, Neus Ruiz-Edo, Elena Ramirez-Maldonado, Santiago Rojas, and Cristina Serra-Serra. 2025. "Breaking Dogmas in Axillary Lymphadenectomy and Quality of Life" Cancers 17, no. 13: 2201. https://doi.org/10.3390/cancers17132201
APA StyleLópez Gordo, S., Jimeno-Fraile, J., García-Monferrer, A., Nicolau, P., Ruiz-Edo, N., Ramirez-Maldonado, E., Rojas, S., & Serra-Serra, C. (2025). Breaking Dogmas in Axillary Lymphadenectomy and Quality of Life. Cancers, 17(13), 2201. https://doi.org/10.3390/cancers17132201








