Navigating Neoplasm Risk in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Clinical Phenotype of PSC-IBD
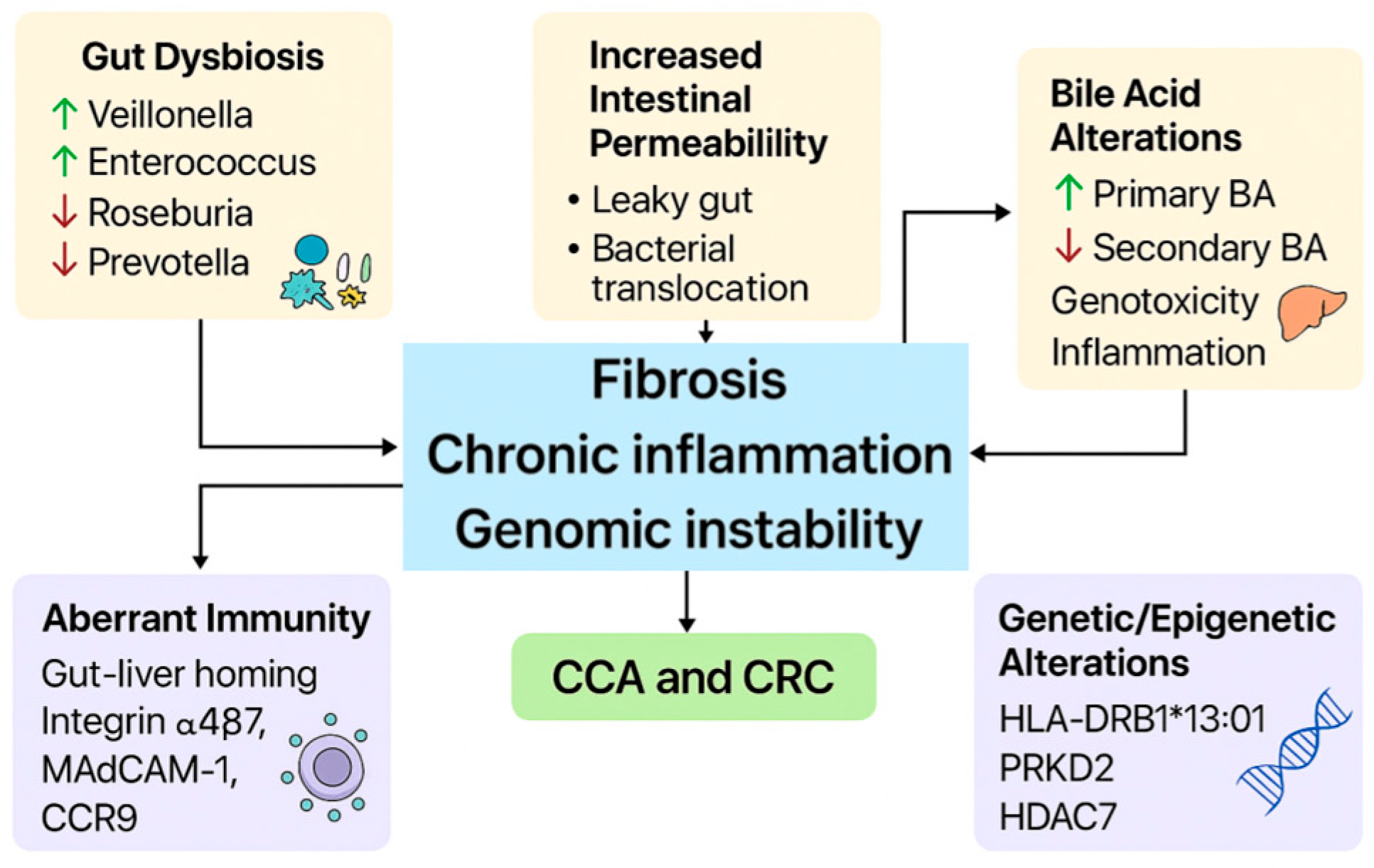
4. Pathophysiological Mechanisms of PSC and Impact on Cancer Development
4.1. Intestinal Permeability and PSC
4.2. Gut Microbioma and PSC
4.3. Bile Acid Metabolism and Lymphocyte Trafficking
4.4. Genetic and HLA Contributions to PSC
4.5. Oncogenic Implications
5. Oncological Risk in PSC-IBD
5.1. Cholangiocarcinoma
5.2. Colorectal Cancer
5.2.1. Epidemiology and Risk Factors of CRC in PSC-IBD
5.2.2. Molecular Characteristics of CRC Associated with PSC-IBD
5.2.3. Histopathological Characteristics of Dysplasia Associated with PSC-IBD
5.2.4. Chemoprevention of CRC in PSC-IBD: The Role of UDCA and 5-ASA
5.3. Other Malignancies
5.3.1. Hepatocellular Carcinoma
5.3.2. Pancreatic Carcinoma
5.3.3. Gallbladder Carcinoma
6. Surveillance Strategies
6.1. Surveillance for Colorectal Cancer
6.2. Surveillance for Cholangiocarcinoma
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Munster, K.N.; Bergquist, A.; Ponsioen, C.Y. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: One Disease or Two? J. Hepatol. 2024, 80, 155–168. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary Sclerosing Cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; Van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; Van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis: Boonstra et Al. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef]
- Durazzo, M.; Ferro, A.; Navarro-Tableros, V.M.; Gaido, A.; Fornengo, P.; Altruda, F.; Romagnoli, R.; Moestrup, S.K.; Calvo, P.L.; Fagoonee, S. Current Treatment Regimens and Promising Molecular Therapies for Chronic Hepatobiliary Diseases. Biomolecules 2025, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef]
- Jrgensen, K.K.; Grzyb, K.; Lundin, K.E.A.; Clausen, O.P.F.; Aamodt, G.; Schrumpf, E.; Vatn, M.H.; Boberg, K.M. Inflammatory Bowel Disease in Patients with Primary Sclerosing Cholangitis: Clinical Characterization in Liver Transplanted and Nontransplanted Patients. Inflamm. Bowel Dis. 2012, 18, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Van Erpecum, K.J.; Van Nieuwkerk, K.M.J.; Drenth, J.P.H.; Poen, A.C.; Witteman, B.J.M.; Tuynman, H.A.R.E.; Beuers, U.; Ponsioen, C.Y. Primary Sclerosing Cholangitis Is Associated with a Distinct Phenotype of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2270–2276. [Google Scholar] [CrossRef]
- Joo, M.; Abreu-e-Lima, P.; Farraye, F.; Smith, T.; Swaroop, P.; Gardner, L.; Lauwers, G.Y.; Odze, R.D. Pathologic Features of Ulcerative Colitis in Patients with Primary Sclerosing Cholangitis: A Case-Control Study. Am. J. Surg. Pathol. 2009, 33, 854–862. [Google Scholar] [CrossRef]
- Sano, H.; Nakazawa, T.; Ando, T.; Hayashi, K.; Naitoh, I.; Okumura, F.; Miyabe, K.; Yoshida, M.; Takahashi, S.; Ohara, H.; et al. Clinical Characteristics of Inflammatory Bowel Disease Associated with Primary Sclerosing Cholangitis. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 154–161. [Google Scholar] [CrossRef]
- Stumme, F.; Steffens, N.; Steglich, B.; Mathies, F.; Nawrocki, M.; Sabihi, M.; Soukou-Wargalla, S.; Göke, E.; Kempski, J.; Fründt, T.; et al. A Protective Effect of Inflammatory Bowel Disease on the Severity of Sclerosing Cholangitis. Front. Immunol. 2024, 15, 1307297. [Google Scholar] [CrossRef]
- Gui, W.; Hole, M.J.; Molinaro, A.; Edlund, K.; Jørgensen, K.K.; Su, H.; Begher-Tibbe, B.; Gaßler, N.; Schneider, C.V.; Muthukumarasamy, U.; et al. Colitis Ameliorates Cholestatic Liver Disease via Suppression of Bile Acid Synthesis. Nat. Commun. 2023, 14, 3304. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Halliday, J.S.; Djordjevic, J.; Lust, M.; Culver, E.L.; Braden, B.; Travis, S.P.L.; Chapman, R.W.G. A Unique Clinical Phenotype of Primary Sclerosing Cholangitis Associated with Crohn’s Disease. J. Crohn’s Colitis 2012, 6, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Dozois, R.; Tremaine, W.; Sandborn, W.; LaRusso, N.; Schleck, C.; Ilstrup, D. Pouchitis after Ileal Pouch-Anal Anastomosis for Ulcerative Colitis Occurs with Increased Frequency in Patients with Associated Primary Sclerosing Cholangitis. Gut 1996, 38, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.P.; Urquhart, S.A.; Janssens, L.P.; Lennon, R.J.; Chedid, V.G.; Raffals, L.E. Primary Sclerosing Cholangitis–Associated Pouchitis: A Distinct Clinical Phenotype. Clin. Gastroenterol. Hepatol. 2022, 20, e964–e973. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Jones, M.P.; Callaghan, G.; Fairlie, T.; Ma, X.; Culver, E.L.; Stuart, K.; De Cruz, P.; O’Beirne, J.; Tabibian, J.H.; et al. Efficacy and Safety of Biologics in Primary Sclerosing Cholangitis with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Hepatol. Commun. 2024, 8, e0347. [Google Scholar] [CrossRef]
- Berg, R.D. Bacterial Translocation from the Gastrointestinal Tract. In Mechanisms in the Pathogenesis of Enteric Diseases 2; Paul, P.S., Francis, D.H., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 473, pp. 11–30. ISBN 978-1-4613-6858-8. [Google Scholar]
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef]
- Blesl, A.; Stadlbauer, V. The Gut-Liver Axis in Cholestatic Liver Diseases. Nutrients 2021, 13, 1018. [Google Scholar] [CrossRef]
- Lichtman, S.N.; Keku, J.; Schwab, J.H.; Sartor, R.B. Hepatic Injury Associated with Small Bowel Bacterial Overgrowth in Rats Is Prevented by Metronidazole and Tetracycline. Gastroenterology 1991, 100, 513–519. [Google Scholar] [CrossRef]
- Dhillon, A.K.; Kummen, M.; Trøseid, M.; Åkra, S.; Liaskou, E.; Moum, B.; Vesterhus, M.; Karlsen, T.H.; Seljeflot, I.; Hov, J.R. Circulating Markers of Gut Barrier Function Associated with Disease Severity in Primary Sclerosing Cholangitis. Liver Int. 2019, 39, 371–381. [Google Scholar] [CrossRef]
- Björnsson, E.; Cederborg, A.; Åkvist, A.; Simren, M.; Stotzer, P.; Bjarnason, I. Intestinal Permeability and Bacterial Growth of the Small Bowel in Patients with Primary Sclerosing Cholangitis. Scand. J. Gastroenterol. 2005, 40, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Crawford, D.; Burger, D.; Martin, N.; Walker, M.; Talley, N.J.; Tallis, C.; Jones, M.; Stuart, K.; Keely, S.; et al. Effects of Antibiotic Therapy in Primary Sclerosing Cholangitis with and without Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Semin. Liver Dis. 2019, 39, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Iqbal, T.; Chan, J.; Constantinidou, C.; Trivedi, P.; Ferguson, J.; Adams, D.H.; Pallen, M.; et al. The Gut-Adherent Microbiota of PSC–IBD Is Distinct to That of IBD. Gut 2017, 66, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut Microbiome in Primary Sclerosing Cholangitis: A Review. WJG 2020, 26, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van Der Merwe, S.; Vermeire, S.; et al. Primary Sclerosing Cholangitis Is Characterised by Intestinal Dysbiosis Independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B.; et al. The Gut Microbial Profile in Patients with Primary Sclerosing Cholangitis Is Distinct from Patients with Ulcerative Colitis without Biliary Disease and Healthy Controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef]
- Liwinski, T.; Zenouzi, R.; John, C.; Ehlken, H.; Rühlemann, M.C.; Bang, C.; Groth, S.; Lieb, W.; Kantowski, M.; Andersen, N.; et al. Alterations of the Bile Microbiome in Primary Sclerosing Cholangitis. Gut 2020, 69, 665–672. [Google Scholar] [CrossRef]
- Lemoinne, S.; Kemgang, A.; Ben Belkacem, K.; Straube, M.; Jegou, S.; Corpechot, C.; Saint-Antoine IBD Network; Chazouillères, O.; Housset, C.; Sokol, H. Fungi Participate in the Dysbiosis of Gut Microbiota in Patients with Primary Sclerosing Cholangitis. Gut 2020, 69, 92–102. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Acharjee, A.; Beggs, A.D.; Horniblow, R.; Tselepis, C.; Gkoutos, G.; Ghosh, S.; Rossiter, A.E.; Loman, N.; Van Schaik, W.; et al. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease with Bile Acid Pathways. J. Crohn’s Colitis 2020, 14, 935–947. [Google Scholar] [CrossRef]
- Torres, J.; Bao, X.; Goel, A.; Colombel, J.-F.; Pekow, J.; Jabri, B.; Williams, K.M.; Castillo, A.; Odin, J.A.; Meckel, K.; et al. The Features of Mucosa-associated Microbiota in Primary Sclerosing Cholangitis. Aliment. Pharmacol. Ther. 2016, 43, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg Induction by a Rationally Selected Mixture of Clostridia Strains from the Human Microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; Boonstra, K.; D’Haens, G.R.; Heilig, H.G.; Zoetendal, E.G.; De Vos, W.M.; Ponsioen, C.Y. The Mucosa-Associated Microbiota of PSC Patients Is Characterized by Low Diversity and Low Abundance of Uncultured Clostridiales II. J. Crohn’s Colitis 2015, 9, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Aho, V.; Arola, J.; Boyd, S.; Jokelainen, K.; Paulin, L.; Auvinen, P.; Färkkilä, M. Bile Microbiota in Primary Sclerosing Cholangitis: Impact on Disease Progression and Development of Biliary Dysplasia. PLoS ONE 2017, 12, e0182924. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, N.; Sasaki, N.; Aoki, R.; Miyamoto, K.; Suda, W.; Teratani, T.; Suzuki, T.; Koda, Y.; Chu, P.-S.; Taniki, N.; et al. Gut Pathobionts Underlie Intestinal Barrier Dysfunction and Liver T Helper 17 Cell Immune Response in Primary Sclerosing Cholangitis. Nat. Microbiol. 2019, 4, 492–503. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z. Fecal Microbiota Transplantation in Patients with Primary Sclerosing Cholangitis: The Next Steps in This Promising Story. Am. J. Gastroenterol. 2019, 114, 1354–1355. [Google Scholar] [CrossRef]
- Lv, L.; Fang, D.; Shi, D.; Chen, D.; Yan, R.; Zhu, Y.; Chen, Y.; Shao, L.; Guo, F.; Wu, W.; et al. Alterations and Correlations of the Gut Microbiome, Metabolism and Immunity in Patients with Primary Biliary Cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286. [Google Scholar] [CrossRef]
- Zhang, R.; Lauwers, G.Y.; Choi, W.-T. Increased Risk of Non-Conventional and Invisible Dysplasias in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 1825–1834. [Google Scholar] [CrossRef]
- Trottier, J.; Białek, A.; Caron, P.; Straka, R.J.; Heathcote, J.; Milkiewicz, P.; Barbier, O. Metabolomic Profiling of 17 Bile Acids in Serum from Patients with Primary Biliary Cirrhosis and Primary Sclerosing Cholangitis: A Pilot Study. Dig. Liver Dis. 2012, 44, 303–310. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Vuppalanchi, R.; Levy, C.; Floreani, A.; Andreone, P.; LaRusso, N.F.; Shrestha, R.; Trotter, J.; Goldberg, D.; Rushbrook, S.; et al. A Randomized, Placebo-Controlled, Phase II Study of Obeticholic Acid for Primary Sclerosing Cholangitis. J. Hepatol. 2020, 73, 94–101. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorakova, K.; Garewal, H. Bile Acids as Carcinogens in Human Gastrointestinal Cancers. Mutat. Res. Rev. Mutat. Res. 2005, 589, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.Y.; Emond, M.J.; Haggitt, R.C.; Bronner, M.P.; Kimmey, M.B.; Kowdley, K.V.; Brentnall, T.A. Ursodiol Use Is Associated with Lower Prevalence of Colonic Neoplasia in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis. Ann. Intern. Med. 2001, 134, 89–95. [Google Scholar] [CrossRef]
- Brentnall, T.; Haggitt, R.; Rabinovitch, P.; Kimmey, M.; Bronner, M.; Levine, D.; Kowdley, K.; Stevens, A.; Crispin, D.; Emond, M.; et al. Risk and Natural History of Colonic Neoplasia in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. Gastroenterology 1996, 110, 331–338. [Google Scholar] [CrossRef]
- De Krijger, M.; Wildenberg, M.E.; De Jonge, W.J.; Ponsioen, C.Y. Return to Sender: Lymphocyte Trafficking Mechanisms as Contributors to Primary Sclerosing Cholangitis. J. Hepatol. 2019, 71, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.; Curion, F.; Yeung, H.-Y.; Fryer, E.; Slater, A.; Ferry, H.; Attar, M.; Slawinski, H.; Chapman, R.W.G.; Bowden, R.; et al. PS-127-Analysis of Liver Infiltrating Lymphocytes in Primary Sclerosing Cholangitis by Surface Antigen and Single Cell RNAseq. J. Hepatol. 2019, 70, e78–e79. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Tickle, J.; Vesterhus, M.N.; Eddowes, P.J.; Bruns, T.; Vainio, J.; Parker, R.; Smith, D.; Liaskou, E.; Thorbjørnsen, L.W.; et al. Vascular Adhesion Protein-1 Is Elevated in Primary Sclerosing Cholangitis, Is Predictive of Clinical Outcome and Facilitates Recruitment of Gut-Tropic Lymphocytes to Liver in a Substrate-Dependent Manner. Gut 2018, 67, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.D.; Chapman, R.W.; Keshav, S.; Montano-Loza, A.J.; Mason, A.L.; Kremer, A.E.; Vetter, M.; De Krijger, M.; Ponsioen, C.Y.; Trivedi, P.; et al. Effects of Vedolizumab in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 179–187.e6. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Boberg, K.M. Update on Primary Sclerosing Cholangitis. J. Hepatol. 2013, 59, 571–582. [Google Scholar] [CrossRef]
- Næss, S.; Shiryaev, A.; Hov, J.R.; Franke, A.; Karlsen, T.H. Genetics in Primary Sclerosing Cholangitis. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 325–333. [Google Scholar] [CrossRef]
- Cortes, A.; Brown, M.A. Promise and Pitfalls of the Immunochip. Arthritis Res. Ther. 2010, 13, 101. [Google Scholar] [CrossRef]
- Henriksen, E.K.K.; Viken, M.K.; Wittig, M.; Holm, K.; Folseraas, T.; Mucha, S.; Melum, E.; Hov, J.R.; Lazaridis, K.N.; Juran, B.D.; et al. HLA Haplotypes in Primary Sclerosing Cholangitis Patients of Admixed and non-European Ancestry. HLA 2017, 90, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Næss, S.; Lie, B.A.; Melum, E.; Olsson, M.; Hov, J.R.; Croucher, P.J.P.; Hampe, J.; Thorsby, E.; Bergquist, A.; Traherne, J.A.; et al. Refinement of the MHC Risk Map in a Scandinavian Primary Sclerosing Cholangitis Population. PLoS ONE 2014, 9, e114486. [Google Scholar] [CrossRef] [PubMed]
- Wiencke, K.; Karlsen, T.H.; Boberg, K.M.; Thorsby, E.; Schrumpf, E.; Lie, B.A.; Spurkland, A. Primary Sclerosing Cholangitis Is Associated with Extended HLA-DR3 and HLA-DR6 Haplotypes. Tissue Antigens 2007, 69, 161–169. [Google Scholar] [CrossRef]
- Beggs, A.D.; James, J.; Caldwell, G.; Prout, T.; Dilworth, M.P.; Taniere, P.; Iqbal, T.; Morton, D.G.; Matthews, G. Discovery and Validation of Methylation Biomarkers for Ulcerative Colitis Associated Neoplasia. Inflamm. Bowel Dis. 2018, 24, 1503–1509. [Google Scholar] [CrossRef]
- Hu, W.; Yang, Y.; Li, X.; Huang, M.; Xu, F.; Ge, W.; Zhang, S.; Zheng, S. Multi-Omics Approach Reveals Distinct Differences in Left- and Right-Sided Colon Cancer. Mol. Cancer Res. 2018, 16, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Terjung, B.; Sohne, J.; Lechtenberg, B.; Gottwein, J.; Muennich, M.; Herzog, V.; Mahler, M.; Sauerbruch, T.; Spengler, U. P-ANCAs in Autoimmune Liver Disorders Recognise Human-Tubulin Isotype 5 and Cross-React with Microbial Protein FtsZ. Gut 2010, 59, 808–816. [Google Scholar] [CrossRef]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef]
- Saab, M.; Mestivier, D.; Sohrabi, M.; Rodriguez, C.; Khonsari, M.R.; Faraji, A.; Sobhani, I. Characterization of Biliary Microbiota Dysbiosis in Extrahepatic Cholangiocarcinoma. PLoS ONE 2021, 16, e0247798. [Google Scholar] [CrossRef]
- Moeini, A.; Haber, P.K.; Sia, D. Cell of Origin in Biliary Tract Cancers and Clinical Implications. JHEP Rep. 2021, 3, 100226. [Google Scholar] [CrossRef]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the Pathogenesis of Primary Sclerosing Cholangitis and Development of Cholangiocarcinoma. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Tenca, A.; Jokelainen, K.; Mustonen, H.; Krogerus, L.; Arola, J.; Färkkilä, M. Screening Primary Sclerosing Cholangitis and Biliary Dysplasia with Endoscopic Retrograde Cholangiography and Brush Cytology: Risk Factors for Biliary Neoplasia. Endoscopy 2016, 48, 432–439. [Google Scholar] [CrossRef]
- Sokol, H.; Cosnes, J.; Chazouilleres, O.; Beaugerie, L.; Tiret, E.; Poupon, R.; Seksik, P. Disease Activity and Cancer Risk in Inflammatory Bowel Disease Associated with Primary Sclerosing Cholangitis. WJG 2008, 14, 3497. [Google Scholar] [CrossRef]
- Claessen, M.M.H.; Lutgens, M.W.M.D.; Van Buuren, H.R.; Oldenburg, B.; Stokkers, P.C.F.; Van Der Woude, C.J.; Hommes, D.W.; De Jong, D.J.; Dijkstra, G.; Van Bodegraven, A.A.; et al. More Right-Sided IBD-Associated Colorectal Cancer in Patients with Primary Sclerosing Cholangitis. Inflamm. Bowel Dis. 2009, 15, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Gringeri, E.; Burra, P.; Gambato, M. Primary Sclerosing Cholangitis-Associated Cholangiocarcinoma: From Pathogenesis to Diagnostic and Surveillance Strategies. Cancers 2023, 15, 4947. [Google Scholar] [CrossRef]
- Fung, B.M.; Lindor, K.D.; Tabibian, J.H. Cancer Risk in Primary Sclerosing Cholangitis: Epidemiology, Prevention, and Surveillance Strategies. WJG 2019, 25, 659–671. [Google Scholar] [CrossRef]
- Gulamhusein, A.F.; Eaton, J.E.; Tabibian, J.H.; Atkinson, E.J.; Juran, B.D.; Lazaridis, K.N. Duration of Inflammatory Bowel Disease Is Associated with Increased Risk of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis and IBD. Am. J. Gastroenterol. 2016, 111, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.R.; Tabibian, J.H.; Bowlus, C.L. The Evolution of Natural History of Primary Sclerosing Cholangitis. Curr. Opin. Gastroenterol. 2017, 33, 71–77. [Google Scholar] [CrossRef]
- Fevery, J.; Verslype, C.; Lai, G.; Aerts, R.; Van Steenbergen, W. Incidence, Diagnosis, and Therapy of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis. Dig. Dis. Sci. 2007, 52, 3123–3135. [Google Scholar] [CrossRef]
- Burak, K.; Angulo, P.; Pasha, T.M.; Egan, K.; Petz, J.; Lindor, K.D. Incidence and Risk Factors for Cholangiocarcinoma in Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2004, 99, 523–526. [Google Scholar] [CrossRef]
- Souza, M.; Lima, L.C.V.; Al-Sharif, L.; Huang, D.Q. Incidence of Hepatobiliary Malignancies in Primary Sclerosing Cholangitis: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2024, S1542356524010437. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Refsum, E.; Helsingen, L.M.; Folseraas, T.; Ploner, A.; Wieszczy, P.; Barua, I.; Jodal, H.C.; Melum, E.; Løberg, M.; et al. Risk of Hepato-Pancreato-Biliary Cancer Is Increased by Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Population-Based Cohort Study. United Eur. Gastroenterol. J. 2022, 10, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V. Intra-Hepatic and Extra-Hepatic Cholangiocarcinoma: New Insight into Epidemiology and Risk Factors. WJGO 2010, 2, 407. [Google Scholar] [CrossRef] [PubMed]
- Claessen, M.M.H.; Vleggaar, F.P.; Tytgat, K.M.A.J.; Siersema, P.D.; Van Buuren, H.R. High Lifetime Risk of Cancer in Primary Sclerosing Cholangitis. J. Hepatol. 2009, 50, 158–164. [Google Scholar] [CrossRef]
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S.; Blackstone, M.O. Increased Risk of Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis: A Meta-Analysis. Gastrointest. Endosc. 2002, 56, 48–54. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Jiang, X.-L. Increased Risk of Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease: A Meta-Analysis of 16 Observational Studies. Eur. J. Gastroenterol. Hepatol. 2016, 28, 383–390. [Google Scholar] [CrossRef]
- Jess, T.; Loftus, E.V.; Velayos, F.S.; Winther, K.V.; Tremaine, W.J.; Zinsmeister, A.R.; Scott Harmsen, W.; Langholz, E.; Binder, V.; Munkholm, P.; et al. Risk Factors for Colorectal Neoplasia in Inflammatory Bowel Disease: A Nested Case? Control Study from Copenhagen County, Denmark and Olmsted County, Minnesota. Am. J. Gastroenterol. 2007, 102, 829–836. [Google Scholar] [CrossRef]
- Rao, B.B.; Lashner, B.; Kowdley, K.V. Reviewing the Risk of Colorectal Cancer in Inflfromtory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis. Inflamm. Bowel Dis. 2018, 24, 269–276. [Google Scholar] [CrossRef]
- Navaneethan, U.; Rai, T.; Venkatesh, P.G.; Kiran, R.P. Primary Sclerosing Cholangitis and the Risk of Colon Neoplasia in Patients with Crohn’s Colitis. Gastroenterol. Rep. 2016, 4, 226–231. [Google Scholar] [CrossRef][Green Version]
- Das, T.S.; Ho, K.; Udaikumar, J.; Chen, B.; Delau, O.; Shaukat, A.; Jacobson, I.; Sarwar, R. Risk of Colorectal Cancer in Patients with Primary Sclerosing Cholangitis and Concomitant Inflammatory Bowel Disease Compared with Primary Sclerosing Cholangitis Only. Hepatol. Res. 2024, 54, 807–816. [Google Scholar] [CrossRef]
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hurley, E.H.; Park, Y.; Ko, S. Primary Sclerosing Cholangitis (PSC) and Inflammatory Bowel Disease (IBD): A Condition Exemplifying the Crosstalk of the Gut–Liver Axis. Exp. Mol. Med. 2023, 55, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Sanchez, W.; Gores, G.J. Long-Term Probability of and Mortality From De Novo Malignancy After Liver Transplantation. Gastroenterology 2009, 137, 2010–2017. [Google Scholar] [CrossRef]
- Vera, A.; Gunson, B.K.; Ussatoff, V.; Nightingale, P.; Candinas, D.; Radley, S.; David Mayer, A.; Buckels, J.A.C.; McMaster, P.; Neuberger, J.; et al. Colorectal Cancer in Patients with Inflammatory Bowel Disease after Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation 2003, 75, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Varayil, J.E.; Loftus, E.V.; Talwalkar, J.A. Incidence of Colorectal Cancer after Liver Transplantation for Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Liver Transplant. 2013, 19, 1361–1369. [Google Scholar] [CrossRef]
- Jørgensen, K.K.; Lindström, L.; Cvancarova, M.; Castedal, M.; Friman, S.; Schrumpf, E.; Foss, A.; Isoniemi, H.; Nordin, A.; Holte, K.; et al. Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis Undergoing Liver Transplantation: A Nordic Multicenter Study. Scand. J. Gastroenterol. 2012, 47, 1021–1029. [Google Scholar] [CrossRef]
- Ståhlberg, D.; Veress, B.; Tribukait, B.; Broomé, U. Atrophy and Neoplastic Transformation of the Ileal Pouch Mucosa in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis: A Case Control Study. Dis. Colon Rectum 2003, 46, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, M.; Cleland, J.; Rahman, M.; Christian, A.; Doyle, J.; Gaunt, R.; Travis, S.; Mortensen, N.; Chapman, R. Outcomes after Ileal Pouch Anal Anastomosis in Patients with Primary Sclerosing Cholangitis. J. Crohn’s Colitis 2014, 8, 662–670. [Google Scholar] [CrossRef]
- Barnes, E.L.; Holubar, S.D.; Herfarth, H.H. Systematic Review and Meta-Analysis of Outcomes After Ileal Pouch-Anal Anastomosis in Primary Sclerosing Cholangitis and Ulcerative Colitis. J. Crohn’s Colitis 2021, 15, 1272–1278. [Google Scholar] [CrossRef]
- Marchesa, P.; Lashner, B.A.; Lavery, I.C.; Milsom, J.; Hull, T.L.; Strong, S.A.; Church, J.M.; Navarro, G.; Fazio, V.W. The Risk of Cancer and Dysplasia among Ulcerative Colitis Patients with Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 1997, 92, 1285–1288. [Google Scholar]
- Imam, M.H.; Eaton, J.E.; Puckett, J.S.; Loftus, E.V.; Mathis, K.L.; Gossard, A.A.; Talwalkar, J.A.; Lindor, K.D. Neoplasia in the Ileoanal Pouch Following Colectomy in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis. J. Crohn’s Colitis 2014, 8, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- De Krijger, M.; Carvalho, B.; Rausch, C.; Bolijn, A.S.; Delis-van Diemen, P.M.; Tijssen, M.; Van Engeland, M.; Mostafavi, N.; Bogie, R.M.M.; Dekker, E.; et al. Genetic Profiling of Colorectal Carcinomas of Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1309–1320. [Google Scholar] [CrossRef]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease−Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef]
- Yaeger, R.; Shah, M.A.; Miller, V.A.; Kelsen, J.R.; Wang, K.; Heins, Z.J.; Ross, J.S.; He, Y.; Sanford, E.; Yantiss, R.K.; et al. Genomic Alterations Observed in Colitis-Associated Cancers Are Distinct from Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology 2016, 151, 278–287.e6. [Google Scholar] [CrossRef]
- Dhir, M.; Montgomery, E.A.; Glöckner, S.C.; Schuebel, K.E.; Hooker, C.M.; Herman, J.G.; Baylin, S.B.; Gearhart, S.L.; Ahuja, N. Epigenetic Regulation of WNT Signaling Pathway Genes in Inflammatory Bowel Disease (IBD) Associated Neoplasia. J. Gastrointest. Surg. 2008, 12, 1745–1753. [Google Scholar] [CrossRef]
- Choi, W.-T.; Salomao, M.; Zhao, L.; Alpert, L.; Setia, N.; Liao, X.; Drage, M.G.; Westerhoff, M.; Cheng, J.; Lauwers, G.Y.; et al. Hypermucinous, Goblet Cell-Deficient and Crypt Cell Dysplasias in Inflammatory Bowel Disease Are Often Associated with Flat/Invisible Endoscopic Appearance and Advanced Neoplasia on Follow-Up. J. Crohn’s Colitis 2022, 16, 98–108. [Google Scholar] [CrossRef]
- Andersen, S.N.; Lovig, T.; Clausen, O.P.F.; Bakka, A.; Fausa, O.; Rognum, T.O. Villous, Hypermucinous Mucosa in Long Standing Ulcerative Colitis Shows High Frequency of K-Ras Mutations. Gut 1999, 45, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Köbel, M.; Ferraz, J.G.; Iacucci, M.; Ghosh, S.; Liu, S.; Ou, Y.; Perizzolo, M.; Winkfein, R.J.; Rambau, P.; et al. Histological and Molecular Diversity and Heterogeneity of Precancerous Lesions Associated with Inflammatory Bowel Diseases. J. Clin. Pathol. 2020, 73, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rabinovitch, P.S.; Mattis, A.N.; Lauwers, G.Y.; Choi, W. Non-conventional Dysplasia in Inflammatory Bowel Disease Is More Frequently Associated with Advanced Neoplasia and Aneuploidy than Conventional Dysplasia. Histopathology 2021, 78, 814–830. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Rabinovitch, P.S.; Huang, D.; Small, T.; Mattis, A.N.; Kakar, S.; Choi, W.-T. Association of Aneuploidy and Flat Dysplasia with Development of High-Grade Dysplasia or Colorectal Cancer in Patients with Inflammatory Bowel Disease. Gastroenterology 2017, 153, 1492–1495.e4. [Google Scholar] [CrossRef]
- Ashraf, I.; Choudhary, A.; Arif, M.; Matteson, M.L.; Hammad, H.T.; Puli, S.R.; Bechtold, M.L. Ursodeoxycholic Acid in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis for Prevention of Colon Cancer: A Meta-Analysis. Indian J. Gastroenterol. 2012, 31, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, S.; Pardi, D.S.; Loftus, E.V.; Talwalkar, J.A. Effect of Ursodeoxycholic Acid Use on the Risk of Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2013, 19, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Sinakos, E.; Marschall, H.-U.; Kowdley, K.V.; Befeler, A.; Keach, J.; Lindor, K. Bile Acid Changes After High-Dose Ursodeoxycholic Acid Treatment in Primary Sclerosing Cholangitis: Relation to Disease Progression. Hepatology 2010, 52, 197–203. [Google Scholar] [CrossRef]
- Eaton, J.E.; Silveira, M.G.; Pardi, D.S.; Sinakos, E.; Kowdley, K.V.; Luketic, V.A.C.; Harrison, E.M.; McCashland, T.; Befeler, A.S.; Harnois, D.; et al. High-Dose Ursodeoxycholic Acid Is Associated with the Development of Colorectal Neoplasia in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2011, 106, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Smyrk, T.C.; Imam, M.; Pardi, D.S.; Loftus, E.V.; Owens, V.L.; Talwalkar, J.A. The Fate of Indefinite and Low-Grade Dysplasia in Ulcerative Colitis and Primary Sclerosing Cholangitis Colitis before and after Liver Transplantation. Aliment. Pharmacol. Ther. 2013, 38, 977–987. [Google Scholar] [CrossRef][Green Version]
- Harnois, D.M.; Gores, G.J.; Ludwig, J.; Steers, J.L.; LaRusso, N.F.; Wiesner, R.H. Are Patients with Cirrhotic Stage Primary Sclerosing Cholangitis at Risk for the Development of Hepatocellular Cancer? J. Hepatol. 1997, 27, 512–516. [Google Scholar] [CrossRef]
- Ali, A.H.; Tabibian, J.H.; Nasser-Ghodsi, N.; Lennon, R.J.; DeLeon, T.; Borad, M.J.; Hilscher, M.; Silveira, M.G.; Carey, E.J.; Lindor, K.D. Surveillance for Hepatobiliary Cancers in Patients with Primary Sclerosing Cholangitis. Hepatology 2018, 67, 2338–2351. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD Practice Guidance on Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Murad, M.H.; Ananthakrishnan, A.N.; Malekzadeh, R.; Talley, N.J. Meta-analysis: Risk of Pancreatic Cancer in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2024, 59, 918–927. [Google Scholar] [CrossRef]
- Said, K.; Glaumann, H.; Bergquist, A. Gallbladder Disease in Patients with Primary Sclerosing Cholangitis. J. Hepatol. 2008, 48, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.T.; Talwalkar, J.A.; Rosen, C.B.; Smyrk, T.C.; Abraham, S.C. Prevalence and Risk Factors for Gallbladder Neoplasia in Patients with Primary Sclerosing Cholangitis: Evidence for a Metaplasia-Dysplasia-Carcinoma Sequence. Am. J. Surg. Pathol. 2007, 31, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Thackeray, E.W.; Lindor, K.D. Likelihood of Malignancy in Gallbladder Polyps and Outcomes Following Cholecystectomy in Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2012, 107, 431–439. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; De Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- Kiesslich, R.; Goetz, M.; Lammersdorf, K.; Schneider, C.; Burg, J.; Stolte, M.; Vieth, M.; Nafe, B.; Galle, P.R.; Neurath, M.F. Chromoscopy-Guided Endomicroscopy Increases the Diagnostic Yield of Intraepithelial Neoplasia in Ulcerative Colitis. Gastroenterology 2007, 132, 874–882. [Google Scholar] [CrossRef]
- Al Sulais, E.; AlAmeel, T.; Alenzi, M.; Shehab, M.; AlMutairdi, A.; Al-Bawardy, B. Colorectal Neoplasia in Inflammatory Bowel Disease. Cancers 2025, 17, 665. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Navaneethan, U.; Njei, B.; Vargo, J.J.; Parsi, M.A. Diagnostic Yield of Bile Duct Brushings for Cholangiocarcinoma in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2014, 79, 783–789. [Google Scholar] [CrossRef]
- Bangarulingam, S.Y.; Bjornsson, E.; Enders, F.; Barr Fritcher, E.G.; Gores, G.; Halling, K.C.; Lindor, K.D. Long-Term Outcomes of Positive Fluorescence in Situ Hybridization Tests in Primary Sclerosing Cholangitis. Hepatology 2010, 51, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Iyer, R.K.; Juran, B.D.; McCauley, B.M.; Atkinson, E.J.; Eaton, J.E.; Ali, A.H.; Lazaridis, K.N. Predicting Cholangiocarcinoma in Primary Sclerosing Cholangitis: Using Artificial Intelligence, Clinical and Laboratory Data. BMC Gastroenterol. 2023, 23, 129. [Google Scholar] [CrossRef]
- El-Matary, W.; Guthery, S.L.; Amir, A.Z.; DiGuglielmo, M.; Draijer, L.G.; Furuya, K.N.; Gupta, N.; Hochberg, J.T.; Horslen, S.; Kerkar, N.; et al. Colorectal Dysplasia and Cancer in Pediatric-Onset Ulcerative Colitis Associated with Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1067–1070.e2. [Google Scholar] [CrossRef]
- Samaan, M.A.; Forsyth, K.; Segal, J.P.; De Jong, D.; Vleugels, J.L.A.; Elkady, S.; Kabir, M.; Campbell, S.; Kok, K.; Armstrong, D.G.; et al. Current Practices in Ileal Pouch Surveillance for Patients with Ulcerative Colitis: A Multinational, Retrospective Cohort Study. J. Crohn’s Colitis 2019, 13, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.M.; Shahid, M.W.; Heckman, M.G.; McNeil, R.B.; Cleveland, P.; Gill, K.R.; Schore, A.; Ghabril, M.; Raimondo, M.; Gross, S.A.; et al. High-Definition Colonoscopy Detects Colorectal Polyps at a Higher Rate Than Standard White-Light Colonoscopy. Clin. Gastroenterol. Hepatol. 2010, 8, 364–370. [Google Scholar] [CrossRef]
- Mohamed, M.F.H.; Marino, D.; Elfert, K.; Beran, A.; Nayfeh, T.; Abdallah, M.A.; Sultan, S.; Shah, S.A. Dye Chromoendoscopy Outperforms High-Definition White Light Endoscopy in Dysplasia Detection for Patients with Inflammatory Bowel Disease: An Updated Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2024, 119, 719–726. [Google Scholar] [CrossRef]
- Subramanian, V.; Mannath, J.; Ragunath, K.; Hawkey, C.J. Meta-Analysis: The Diagnostic Yield of Chromoendoscopy for Detecting Dysplasia in Patients with Colonic Inflammatory Bowel Disease: Meta-Analysis: Chromoendoscopy for IBD Surveillance. Aliment. Pharmacol. Ther. 2011, 33, 304–312. [Google Scholar] [CrossRef] [PubMed]
- El-Dallal, M.; Chen, Y.; Lin, Q.; Rakowsky, S.; Sattler, L.; Foromera, J.; Grossberg, L.; Cheifetz, A.S.; Feuerstein, J.D. Meta-Analysis of Virtual-Based Chromoendoscopy Compared with Dye-Spraying Chromoendoscopy Standard and High-Definition White Light Endoscopy in Patients with Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Inflamm. Bowel Dis. 2020, 26, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, F.J.C.; Fockens, P.; Van Eeden, S.; Reitsma, J.B.; Hardwick, J.C.H.; Stokkers, P.C.F.; Dekker, E. Endoscopic Tri-Modal Imaging for Surveillance in Ulcerative Colitis: Randomised Comparison of High-Resolution Endoscopy and Autofluorescence Imaging for Neoplasia Detection; and Evaluation of Narrow-Band Imaging for Classification of Lesions. Gut 2008, 57, 1083–1089. [Google Scholar] [CrossRef]
- Hu, A.B.; Burke, K.E.; Kochar, B.; Ananthakrishnan, A.N. Yield of Random Biopsies During Colonoscopies in Inflammatory Bowel Disease Patients Undergoing Dysplasia Surveillance. Inflamm. Bowel Dis. 2021, 27, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Kochhar, G.; Venkatesh, P.G.K.; Bennett, A.E.; Rizk, M.; Shen, B.; Kiran, R.P. Random Biopsies during Surveillance Colonoscopy Increase Dysplasia Detection in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. J. Crohn’s Colitis 2013, 7, 974–981. [Google Scholar] [CrossRef]
- Shah, S.C.; Ten Hove, J.R.; Castaneda, D.; Palmela, C.; Mooiweer, E.; Colombel, J.-F.; Harpaz, N.; Ullman, T.A.; Van Bodegraven, A.A.; Jansen, J.M.; et al. High Risk of Advanced Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis Associated with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1106–1113.e3. [Google Scholar] [CrossRef]
- Guerrero Vinsard, D.; Fetzer, J.R.; Agrawal, U.; Singh, J.; Damani, D.N.; Sivasubramaniam, P.; Poigai Arunachalam, S.; Leggett, C.L.; Raffals, L.E.; Coelho-Prabhu, N. Development of an Artificial Intelligence Tool for Detecting Colorectal Lesions in Inflammatory Bowel Disease. iGIE 2023, 2, 91–101.e6. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of Serum Tumor Markers, Imaging, and Biliary Cytology for Detecting Cholangiocarcinoma in Primary Sclerosing Cholangitis†. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Kowdley, K.V.; Harrison, E.M. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2015, 110, 646–659. [Google Scholar] [CrossRef]
- Wannhoff, A.; Gotthardt, D.N. Recent Developments in the Research on Biomarkers of Cholangiocarcinoma in Primary Sclerosing Cholangitis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bangarulingam, S.Y.; Gossard, A.A.; Petersen, B.T.; Ott, B.J.; Lindor, K.D. Complications of Endoscopic Retrograde Cholangiopancreatography in Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2009, 104, 855–860. [Google Scholar] [CrossRef]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.; Lourdusamy, V.; Sanaka, M.R. Endoscopic Ultrasound in the Diagnosis of Cholangiocarcinoma as the Etiology of Biliary Strictures: A Systematic Review and Meta-Analysis. Gastroenterol. Rep. 2015, 3, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Aabakken, L.; Karlsen, T.H.; Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.-M.; Färkkilä, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; et al. Role of Endoscopy in Primary Sclerosing Cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J. Hepatol. 2017, 66, 1265–1281. [Google Scholar] [CrossRef]
- Arnelo, U.; Von Seth, E.; Bergquist, A. Prospective Evaluation of the Clinical Utility of Single-Operator Peroral Cholangioscopy in Patients with Primary Sclerosing Cholangitis. Endoscopy 2015, 47, 696–702. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary Sclerosing Cholangitis—A Comprehensive Review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef]
| Feature | PSC-IBD Associated CRC | Sporadic CRC |
|---|---|---|
| Onset age | Younger | Older |
| Tumor location | Right colon predominance | Left-sided predominance |
| Dysplasia type | Invisible, flat, multifocal | Visible, polypoid |
| Molecular alterations | p53 early mutation, low APC/KRAS | APC/KRAS mutations common |
| Microsatellite instability (MSI) | Rare | Present in Lynch/MSI-H sporadic |
| CpG island methylation (CIMP) | Less common | More common in serrated pathway |
| Tumor Type | Relative Risk Increase | Risk Factors | Guideline Surveillance |
|---|---|---|---|
| Colorectal Cancer (CRC) | 4–10× in PSC-IBD vs. IBD alone | Colonic involvement, duration of disease, male sex, backwash ileitis | Annual colonoscopy from IBD diagnosis (ECCO, BSG, EASL) |
| Cholangiocarcinoma (CCA) | ~160× vs. general population | Older age, dominant strictures, elevated bilirubin, concurrent IBD | MRI/MRCP every 6–12 months ± CA 19-9 (BSG, EASL) |
| Gallbladder Cancer (GBC) | Increased; particularly with polyps > 8 mm | Gallbladder polyps, chronic inflammation | Ultrasound every 6–12 months (EASL) |
| Hepatocellular Carcinoma (HCC) | Increased only in cirrhotic PSC | Advanced fibrosis, cirrhosis | Ultrasound every 6 months in cirrhotic patients (EASL) |
| Pancreatic Cancer | >5× in PSC-IBD vs. IBD alone | Older age, chronic inflammation | Not routinely recommended |
| Cancer Type | Risk Group | Surveillance Modality | Frequency | Notes |
|---|---|---|---|---|
| Colorectal Cancer (CRC) | PSC with inflammatory bowel disease (IBD) | Colonoscopy with biopsies | Annually starting at IBD diagnosis | Increased CRC risk with PSC-IBD; earlier and more frequent screening recommended |
| Cholangiocarcinoma (CCA) | All PSC patients | MRCP with CA 19-9 | Annually | May help detect CCA early, although evidence regarding survival benefit remains inconclusive |
| Category | Surveillance Strategy | Frequency | Notes |
|---|---|---|---|
| Patients with PSC-IBD (UC or CD with colonic involvement) | High-definition colonoscopy (HD-WLC) with random biopsies or chromoendoscopy (DCE/VCE) | Annually, starting at IBD diagnosis | PSC-IBD has higher CRC risk than IBD alone; invisible dysplasia is frequent; chromoendoscopy improves detection (~7% more than HD-WLC) |
| Advanced endoscopy techniques | DCE with indigo carmine/methylene blue; NBI/VCE; CLE; AFI | Based on patient risk and lesion suspicion | DCE preferred for high-risk patients; NBI/VCE have similar sensitivity with faster execution; CLE increases detection (×4.75); AFI sensitive for early dysplasia |
| Artificial Intelligence (AI) | AI-assisted image analysis (e.g., IBD-CADe) | In development for future protocols | May improve dysplasia detection (95.1% sensitivity, 98.8% specificity); promising adjunct for endoscopic surveillance |
| Surveillance in IPAA (post-colectomy) | Pouchoscopy with systematic biopsies (pre-pouch ileum, pouch body, anastomosis) | Annually, even if no prior dysplasia | PSC-IBD patients have increased risk of pouch neoplasia and pouchitis; systematic biopsies essential even without prior dysplasia |
| Patients with confirmed dysplasia | Intensified surveillance or colectomy | Based on dysplasia grade (LGD vs. HGD) | PSC-IBD dysplasia (esp. invisible) has high CRC progression risk; aneuploidy in >80% of HGD cases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitoni, D.; Dal Buono, A.; Gabbiadini, R.; Ronca, V.; Colapietro, F.; Pugliese, N.; Ribaldone, D.G.; Bezzio, C.; Lleo, A.; Armuzzi, A. Navigating Neoplasm Risk in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Cancers 2025, 17, 2165. https://doi.org/10.3390/cancers17132165
Pitoni D, Dal Buono A, Gabbiadini R, Ronca V, Colapietro F, Pugliese N, Ribaldone DG, Bezzio C, Lleo A, Armuzzi A. Navigating Neoplasm Risk in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Cancers. 2025; 17(13):2165. https://doi.org/10.3390/cancers17132165
Chicago/Turabian StylePitoni, Demis, Arianna Dal Buono, Roberto Gabbiadini, Vincenzo Ronca, Francesca Colapietro, Nicola Pugliese, Davide Giuseppe Ribaldone, Cristina Bezzio, Ana Lleo, and Alessandro Armuzzi. 2025. "Navigating Neoplasm Risk in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis" Cancers 17, no. 13: 2165. https://doi.org/10.3390/cancers17132165
APA StylePitoni, D., Dal Buono, A., Gabbiadini, R., Ronca, V., Colapietro, F., Pugliese, N., Ribaldone, D. G., Bezzio, C., Lleo, A., & Armuzzi, A. (2025). Navigating Neoplasm Risk in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Cancers, 17(13), 2165. https://doi.org/10.3390/cancers17132165









