Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment of Included Studies
2.5. Statistical Analyses
3. Results
3.1. Selection of Studies
3.2. Study Characteristics and Quality Assessment
3.2.1. Efficacy of TACE Plus TKIs Versus TACE Alone in Terms of Response to Treatment
3.2.2. Efficacy of TACE Plus TKIs Versus TACE Alone in Terms of Survival Outcomes
3.2.3. Efficacy of TACE Plus TKIs Versus TACE in Terms of Survival Outcomes: Subgroup Analysis for OS and PFS
3.2.4. Publication Bias
3.2.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zhong, J.-H.; Wang, Y.-Y.; You, X.-M.; Ma, L.; Xiang, B.-D.; Li, L.-Q. Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin. Transl. Oncol. 2017, 19, 891–897. [Google Scholar] [CrossRef]
- Park, J.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef] [PubMed]
- European association for the study of the liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. American association for the study of liver diseases. Management of hepatocellular carcinoma: An up-date. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Moscatelli, A.; Pellegatta, G.; Vitale, A.; Farinati, F.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am. J. Gastroenterol. 2016, 111, 70–77. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Tajiri, K.; Toyoda, H.; Tada, T.; Ochi, H.; et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig. Dis. 2017, 35, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, G.-S.; Zheng, C.-S.; Zhuo, C.-K. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J. Gastroenterol. 2004, 10, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, Q.; Tan, H.; Pan, S.; Sun, B.; Xu, R.; Sun, X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int. J. Cancer 2007, 121, 416–424. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.X.; Han, Y.W.; Zhang, Y.L.; Hu, J.; Fan, J.; Fu, S.Z.; Xu, S.; Wan, Q. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 2017, 8, 52813–52822. [Google Scholar] [CrossRef]
- Luo, X.-Y.; Wu, K.-M.; He, X.-X. Advances in drug development for hepatocellular carcinoma: Clinical trials and potential therapeutic targets. J. Exp. Clin. Cancer Res. 2021, 40, 1–23. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.-Y.; Takayama, T.; Yoon, J.-H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Kanai, F.; Aramaki, T.; Yamamoto, T.; Tanaka, T.; Yamakado, K.; Kaneko, S.; Kudo, M.; Imanaka, K.; Kora, S.; et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur. J. Cancer 2013, 49, 2832–2840. [Google Scholar] [CrossRef]
- Kudo, M.; Han, G.; Finn, R.S.; Poon, R.T.P.; Blanc, J.-F.; Yan, L.; Yang, J.; Lu, L.; Tak, W.Y.; Yu, X.; et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology 2014, 60, 1697–1707. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.-Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef]
- Kudo, M.; Cheng, A.-L.; Park, J.-W.; Park, J.H.; Liang, P.-C.; Hidaka, H.; Izumi, N.; Heo, J.; Lee, Y.J.; Sheen, I.-S.; et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): A randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol. Hepatol. 2018, 3, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Jin, X.-L.; Yang, C.; Du, P.; Jiang, F.-Q.; Ma, J.-P.; Yang, J.; Xie, P.; Zhang, Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol. Ther. 2017, 18, 433–438. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2019, 69, 1492–1501. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, B.; Mei, L.; Sun, R.; Guo, C.; Zhu, J. Clinical efficacy of TACE combined with Apatinib in the treatment of advanced hepatocellular carcinoma. JBUON 2019, 24, 608–614. [Google Scholar]
- Turpin, A.; de Baere, T.; Heurgué, A.; Le Malicot, K.; Ollivier-Hourmand, I.; Lecomte, T.; Perrier, H.; Vergniol, J.; Sefrioui, D.; Rinaldi, Y.; et al. Liver transarterial chemoembolization and sunitinib for unresectable hepatocellular carcinoma: Results of the PRODIGE 16 study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101464. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, Y. Sorafenib combined with transarterial chemoembolization prolongs survival of patients with advanced hepatocellular carcinoma. JBUON 2020, 25, 945–951. [Google Scholar] [PubMed]
- Duan, X.; Li, H.; Kuang, D.; Chen, P.; Zhang, M.; Li, T.; Jiao, D.; Li, Y.; He, X.; Xing, C.; et al. Comparison of drug-eluting bead transarterial chemoembolization combined with apatinib versus drug-eluting bead transarterial chemoembolization for the treatment of unresectable hepatocellular carcinoma: A randomized, prospective, multicenter phase III trial. Signal Transduct. Target. Ther. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Luo, J.; Zheng, J.; Mao, X.; Tsilimigras, D.I.; Chun, H.J.; Zeng, H. Efficacy and safety of transarterial chemoembolization alone compared to its combination with anlotinib among patients with intermediate or advanced stage hepatocellular carcinoma: A phase II randomized controlled trial. J. Gastrointest. Oncol. 2024, 15, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhu, B.; Chen, S.; Wu, Y.; Zhao, X.; Qiao, L.; Huang, Z.; Tang, R.; Chen, J.; Lau, W.Y.; et al. Survival in Patients With Recurrent Intermediate-Stage Hepatocellular Carcinoma: Sorafenib Plus TACE vs TACE Alone Randomized Clinical Trial. JAMA Oncol. 2024, 10, 1047–1054. [Google Scholar] [CrossRef]
- Ouyang, T.; Kan, X.; Zheng, C. Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: Monotherapies and Combined Therapies. Front. Oncol. 2022, 12, 898964. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Kudo, M.; Erinjeri, J.P.; Qin, S.; Ren, Z.; Chan, S.L.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. Durvalumab with or without bevacizumab with transarterial chemoembolisation in hepatocellular carcinoma (EMERALD-1): A multiregional, randomised, double-blind, placebo-controlled, phase 3 study. Lancet 2025, 405, 216–232. [Google Scholar] [CrossRef]
- Jácome, A.A.; Castro, A.C.G.; Vasconcelos, J.P.S.; Silva, M.H.C.R.; Lessa, M.A.O.; Moraes, E.D.; Andrade, A.C.; Lima, F.M.T.; Farias, J.P.F.; Gil, R.A.; et al. Efficacy and Safety Associated With Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: A Meta-analysis. JAMA Netw. Open 2021, 4, e2136128. [Google Scholar] [CrossRef]
- Kao, T.-W.; Bai, G.-H.; Wang, T.-L.; Shih, I.-M.; Chuang, C.-M.; Lo, C.-L.; Tsai, M.-C.; Chiu, L.-Y.; Lin, C.-C.; Shen, Y.-A. Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance. J. Exp. Clin. Cancer Res. 2023, 42, 1–35. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Liu, J.-H.; Staiculescu, D.; Chen, J. Combination of molecularly targeted therapies and immune checkpoint inhibitors in the new era of unresectable hepatocellular carcinoma treatment. Ther. Adv. Med. Oncol. 2021, 13, 17588359211018026. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Yen, C.-J.; Lin, Y.-J.; Tsai, H.-W.; Tsai, T.-F.; Chang, K.-Y.; Huang, W.-C.; Lin, P.-W.; Chiang, C.-W.; Chang, T.-T.; Bouchard, M. Hepatitis B Virus X Protein Upregulates mTOR Signaling through IKKβ to Increase Cell Proliferation and VEGF Production in Hepatocellular Carcinoma. PLoS ONE 2012, 7, e41931. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Teng, C.; Wu, H.; Tsai, H.; Chuang, H.; Tsai, T.; Hsu, Y.; Huang, W.; Wu, L.; Su, I. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis†. Hepatology 2009, 49, 1962–1971. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Jiang, Y.; Meng, Z.; Lu, M. mTOR Signaling: The Interface Linking Cellular Metabolism and Hepatitis B Virus Replication. Virol. Sin. 2021, 36, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Frenette, C.; Sung, M.; Daniele, B.; Baron, A.; Chan, S.L.; Blanc, J.F.; Tamai, T.; Ren, M.; Lim, H.J.; et al. Baseline Liver Function and Subsequent Outcomes in the Phase 3 REFLECT Study of Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Gong, F.; Wang, Y.; Huang, C.; Wu, J.; Hu, L.; Liu, M.; Qiu, S.; Lu, L.; Lin, Y. Transarterial chemoembolization (TACE) plus tyrosine kinase inhibitors versus TACE in patients with hepatocellular carcinoma: A systematic review and meta-analysis. World J. Surg. Oncol. 2023, 21, 120. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Z.; Hou, Z.; Qiu, G.; Mi, S.; Jin, Z.; Huang, J. Efficacy and safety of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with tyrosine kinase inhibitors (TKIs) in patients with unresectable hepatocellular carcinoma (uHCC): A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102313. [Google Scholar] [CrossRef]
- Facciorusso, A.; Serviddio, G.; Muscatiello, N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J. Hepatol. 2016, 8, 770–778. [Google Scholar] [CrossRef]


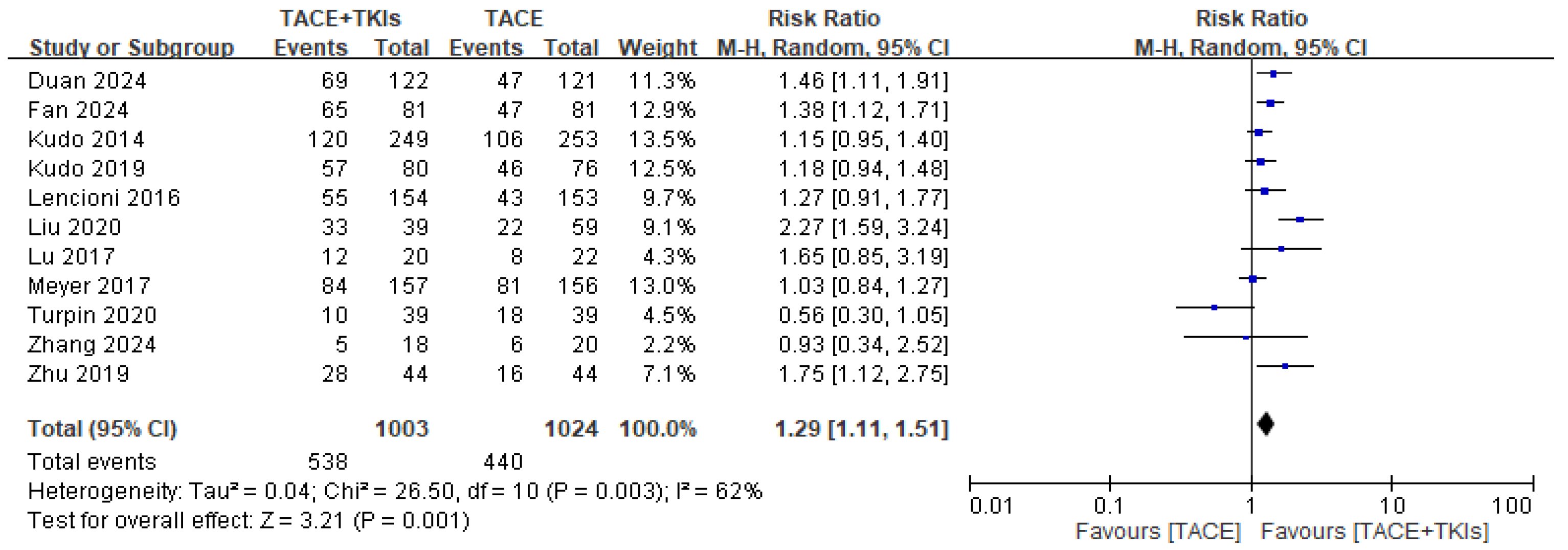

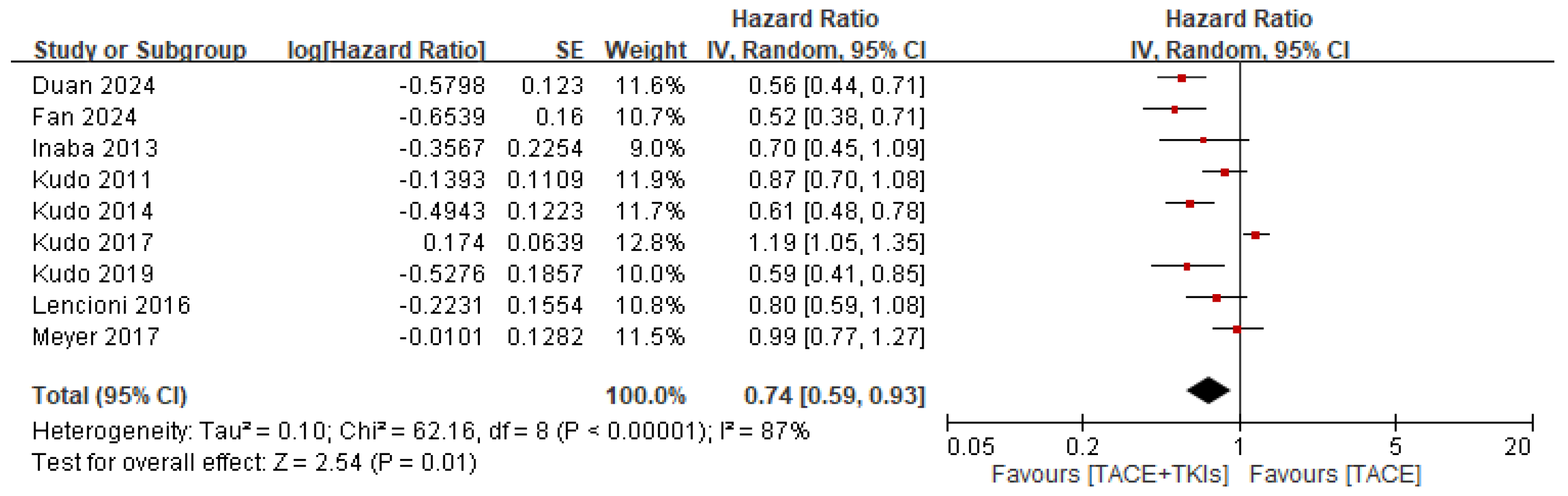
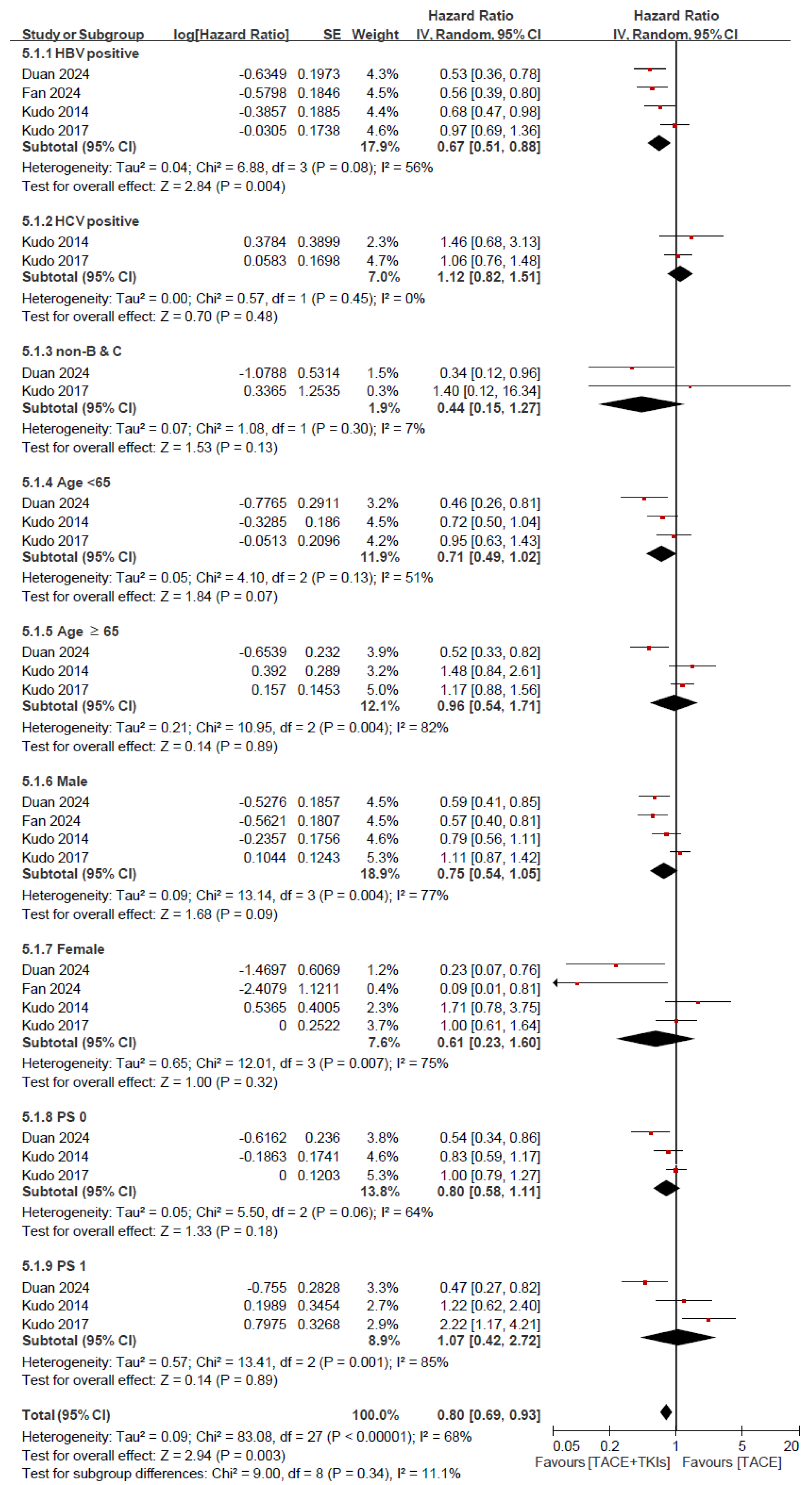

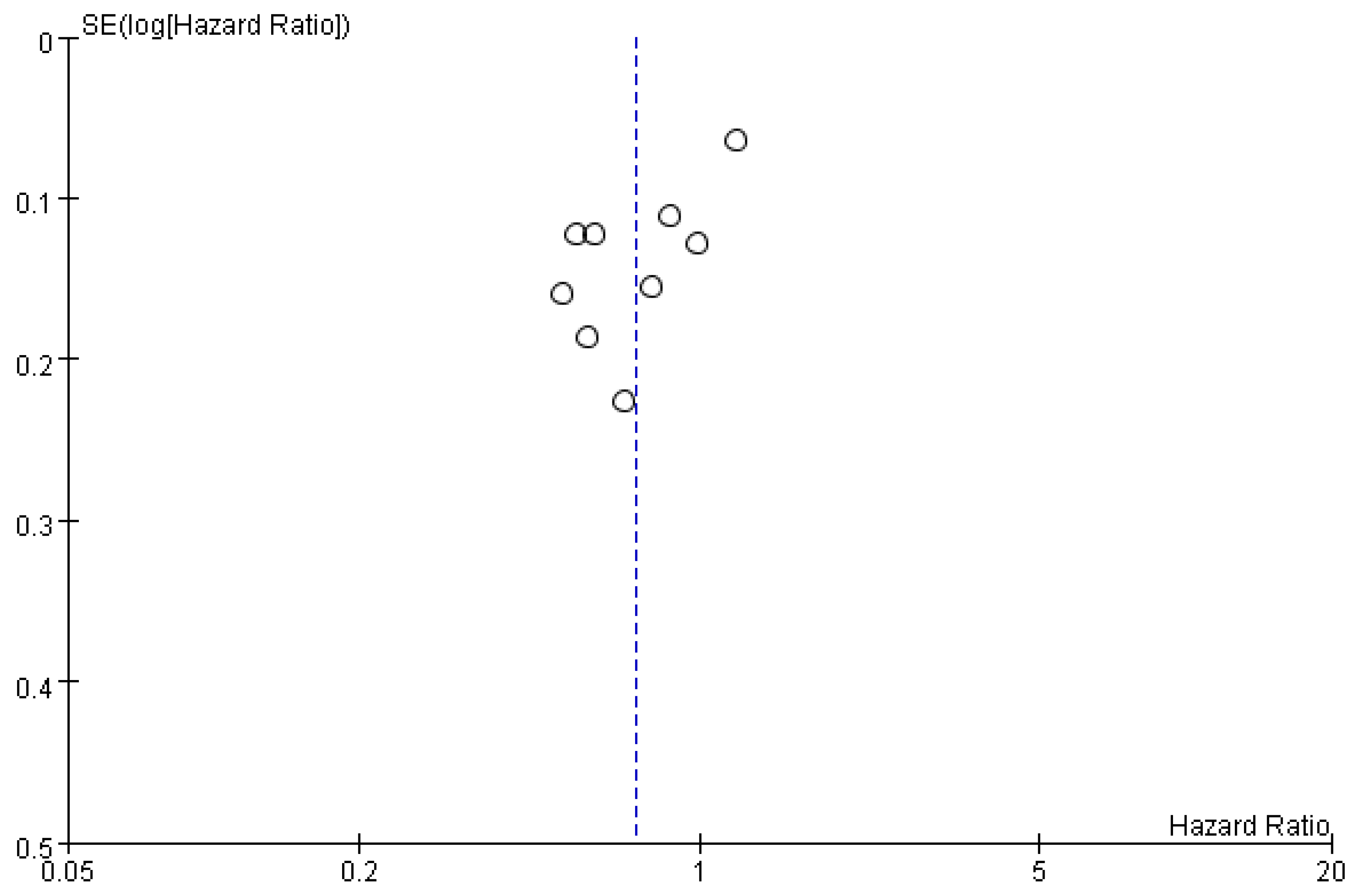
| Study | Country | Intervention | Sample Size | Gender (M/F) | Age (Year) | Child-Pugh Class: A/B/C | ECOG Score: 0–1/2 | BCLC Stage: A/B/C | HBV Infection | HCV Infection | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kudo 2011 [22] | Japan | Sorafenib + TACE | 229 | 174/55 | 69 | 69/0/0 | 229/0 | NA | 21.1% | 61.7% | OS, PFS |
| TACE | 229 | 168/61 | 70 | 70/0/0 | 229/0 | NA | 20.5% | 60.7% | |||
| Inaba 2013 [23] | Japan | Orantinib + TACE | 50 | 39/11 | ≤65:39; >65:11 | 40/9/0 (unknown: 1) | 50/0 | 21/24/5 | 2 | 40 | OS, PFS |
| TACE | 51 | 43/8 | ≤65:42; >65:9 | 45/6/0 | 51/0 | 21/27/2 | 4 | 36 | |||
| Kudo 2014 [24] | Asia, Europe, USA | Brivanib + TACE | 249 | 206/43 | 57 (21–85) | 239/9/1 | 249/0 | 65/129/55 | 158 | 49 | DCR, ORR, OS, PFS |
| TACE | 253 | 216/37 | 59 (22–85) | 231/20/2 | 253/0 | 57/150/46 | 168 | 42 | |||
| Lencioni 2016 [25] | USA | Sorafenib + TACE | 154 | 135/19 | 64.5 | 153/1/0 | NA | NA | 55 | 39 | DCR, ORR, OS, PFS |
| TACE | 153 | 126/27 | 63.0 | 152/0/0 (missing: 1) | NA | NA | 50 | 41 | |||
| Kudo 2017 [26] | Japan, South Korea, Taiwan | Orantinib + TACE | 444 | 363/81 | 66.2 ± 10.2 | 444/0/0 | 444/0 | 158/209/74 | 108 | 193 | OS, PFS |
| TACE | 444 | 364/80 | 65.4 ± 10.0 | 444/0/0 | 444/0 | 135/229/72 | 90 | 165 | |||
| Lu 2017 [27] | China | Apatinib + TACE | 20 | 16/4 | 56.1 ± 10.79 | 18/4/0 | NA | 0/18/2 | 20 | NA | DCR, ORR |
| TACE | 22 | 17/5 | 58.9 ± 9.38 | 17/3/0 | NA | 0/19/3 | 18 | NA | |||
| Meyer 2017 [18] | UK | Sorafenib + TACE | 157 | 139/18 | 65 (57–71) | 145/5/0 (unknown: 7) | 156/NA (unknown: 1) | NA | 7 | 15 | DCR, ORR, OS, PFS |
| TACE | 156 | 138/18 | 68 (63–74) | 148/3/0 (unknown: 5) | 155/NA (unknown: 1) | NA | 7 | 9 | |||
| Kudo 2019 [28] | Japan | Sorafenib + TACE | 80 | 63/17 | 72.0 (36–85) | 79/1/0 | NA | 27/44/9 | 10 | 38 | DCR, ORR, PFS |
| TACE | 76 | 55/21 | 73.0 (55–86) | 71/6/0 | NA | 33/34/9 | 2 | 53 | |||
| Zhu 2019 [29] | China | Apatinib + TACE | 44 | 32/12 | ≤60:29; >60:15 | 38/6/0 | NA | NA | 36 | NA | DCR, ORR |
| TACE | 44 | 34/10 | ≤60:25; >60:19 | 36/8/0 | NA | NA | 34 | NA | |||
| Turpin 2020 [30] | France | Sunitinib + TACE | 39 | 36/3 | 66.0 (46.0–84.7) | 36/2/0 (unknown: 1) | NA | NA/33/NA | 1 | 4 | DCR, ORR |
| TACE | 39 | 35/4 | 67.4 (43.7–84.7) | 37/2/0 | NA | NA/25/NA | 2 | 4 | |||
| Liu 2020 [31] | China | Sorafenib + TACE | 59 | 37/22 | 56.31 ± 9.87 | 43/16/0 | 59/0 | 0/30/29 | NA | NA | DCR, ORR |
| TACE | 59 | 32/27 | 58.11 ± 10.44 | 48/11/0 | 59/0 | 0/36/23 | NA | NA | |||
| Duan 2024 [32] | China | Apatinib + TACE | 122 | 100/22 | 57.5 ± 10.2 | 104/18/0 | 122/0 | 0/48/74 | 99 | 4 | DCR, ORR, OS, PFS |
| TACE | 121 | 107/14 | 58.8 ± 11.1 | 101/20/0 | 121/0 | 0/40/81 | 106 | 2 | |||
| Zhang 2024 [33] | China | Anlotinib + TACE | 18 | 16/2 | 62.2 ± 11.73 | 14/1/3 | 16/NA (unknown: 2) | NA/13/4 (unknown: 1) | 18 | 0 | DCR, ORR |
| TACE | 20 | 18/2 | 63 ± 9.44 | 15/3/2 | 18/NA (unknown: 2) | NA/12/8 | 18 | 0 | |||
| Fan 2024 [34] | China | Sorafenib + TACE | 81 | 72/9 | <50:31; ≥50: 50 | 81/0/0 | 81/0 | NA | 76 | 2 | DCR, ORR, OS, PFS |
| TACE | 81 | 79/2 | <50:28; ≥50: 53 | 81/0/0 | 81/0 | NA | 72 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-R.; Weng, Y.-F.; Wu, T.-W.; Wu, C.-C.; Hsu, C.-L.; Hsu, C.-S. Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 2110. https://doi.org/10.3390/cancers17132110
Peng T-R, Weng Y-F, Wu T-W, Wu C-C, Hsu C-L, Hsu C-S. Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(13):2110. https://doi.org/10.3390/cancers17132110
Chicago/Turabian StylePeng, Tzu-Rong, Yi-Fang Weng, Ta-Wei Wu, Chao-Chuan Wu, Chia-Lu Hsu, and Ching-Sheng Hsu. 2025. "Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis" Cancers 17, no. 13: 2110. https://doi.org/10.3390/cancers17132110
APA StylePeng, T.-R., Weng, Y.-F., Wu, T.-W., Wu, C.-C., Hsu, C.-L., & Hsu, C.-S. (2025). Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 17(13), 2110. https://doi.org/10.3390/cancers17132110








