Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration and Reporting
2.2. Screening and Data Extraction
2.3. Types of Studies and Eligibility Criteria
- 1.
- Population: Patients with histologically confirmed eyelid tumors (benign, premalignant, or malignant).
- 2.
- Intervention: Studies evaluating imiquimod cream as a primary or adjunctive treatment.
- 3.
- Comparison: Studies comparing imiquimod to other standard treatments (surgery, cryotherapy, radiation, PDT, 5-FU, corticosteroids, etc.).
- 4.
- Outcomes: Must report treatment efficacy, recurrence rates, safety, or patient satisfaction.
- 5.
- Study Designs:
- (a)
- Randomized controlled trials (RCTs),
- (b)
- Observational studies (cohort, case–control, or cross-sectional studies).
- 6.
- Language and Publication Status:
- (a)
- Studies published in English,
- (b)
- Full-text available.
- 1.
- Population:
- (a)
- Studies on non-eyelid tumors (e.g., tumors of the face, scalp, or body),
- (b)
- Studies on non-tumor eyelid conditions (e.g., chalazion, blepharitis),
- (c)
- Studies on animal models or in vitro experiments.
- 2.
- Intervention: Studies that do not use imiquimod cream.
- 3.
- Comparison: Studies without a comparator treatment or only descriptive case reports.
- 4.
- Outcomes: Studies that do not report relevant clinical outcomes.
- 5.
- Study Types: Narrative reviews, expert opinions, or letters to the editor without primary data, case reports, and case series (with <5 patients).
- 6.
- Language and Publication Status:
- (a)
- Non-English publications without an available translation,
- (b)
- Unpublished or non-peer-reviewed studies.
2.4. Assessment of the Level of Evidence
2.5. Statistical (Data-Synthesis) Methods
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias and Evidence Certainty
3.4. Primary Outcomes (Efficacy)
3.5. Secondary Outcomes
4. Discussion
4.1. Efficacy and Indications
4.2. Determinants of Response
4.3. Safety and Tolerability
4.4. Cosmesis
4.5. Patient-Centered Outcomes
4.6. Combination Therapy and Emerging Approaches
4.7. Follow-Up and Recurrence Risk
4.8. Limitations of the Evidence Base
4.9. Position of Imiquimod in the Periocular Superficial or Nodular BCC Treatment Algorithm
- 1.
- Low risk tumors (<10 mm, primary, superficial/nodular, away from punctum): Topical imiquimod 5% three to five times weekly for 6–10 weeks.
- 2.
- Intermediate risk lesions (10–20 mm, nodular, proximity to lacrimal drainage or prior surgery): Consider immunocryosurgery or imiquimod neoadjuvant therapy to debulk prior to limited excision [33].
- 3.
- High risk tumors (>20 mm, infiltrative/morpheaform, orbital invasion): Primary Mohs surgery or, where contraindicated, systemic hedgehog inhibitors [7].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, G.M.; Desai, P.; Collin, J.R.O.; Ives, A.; Jones, T.; Hussain, B. Incidence of eyelid basal cell carcinoma in England: 2000–2010. Br. J. Ophthalmol. 2017, 101, 209–212. [Google Scholar] [CrossRef]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Šitum, M.; Buljan, M.; Bulat, V.; Lugović-Mihić, L.; Bolanča, Ž.; Šimić, D. The role of UV radiation in the development of basal cell carcinoma. Coll. Antropol. 2008, 32, 167–170. [Google Scholar] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; McNab, A.; Sullivan, T.; Davis, G.; Selva, D. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005, 112, 717–723. [Google Scholar] [CrossRef]
- Mosterd, K.; Krekels, G.A.; Nieman, F.H.; Ostertag, J.U.; Essers, B.A.; Dirksen, C.D.; Steijlen, P.M.; Vermeulen, A.; Neumann, H.; Kelleners-Smeets, N.W. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008, 9, 1149–1156. [Google Scholar] [CrossRef]
- Bath-Hextall, F.; Ozolins, M.; Armstrong, S.J.; Colver, G.B.; Perkins, W.; Miller, P.S.; Williams, H.C. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 96–105. [Google Scholar] [CrossRef]
- Verkouteren, J.; Ramdas, K.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef]
- Bilu, D.; Sauder, D. Imiquimod: Modes of action. Br. J. Dermatol. 2003, 149, 5–8. [Google Scholar]
- Schön, M.P.; Schön, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157, 8–13. [Google Scholar] [CrossRef]
- Aldara® (Imiquimod) 5% Cream [Prescribing Information]; 3M Pharmaceuticals: Bridgewater, NJ, USA, 1997.
- Bath-Hextall, F.J.; Perkins, W.; Bong, J.; Williams, H.C. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007, CD003412. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003412.pub2/full (accessed on 12 June 2025). [CrossRef] [PubMed]
- Quirk, C.; Gebauer, K.; De’Ambrosis, B.; Slade, H.B.; Meng, T.-C. Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: Results of a prospective 5-year study. Cutis 2010, 85, 318–324. [Google Scholar] [PubMed]
- Prokosch, V.; Thanos, S.; Spaniol, K.; Stupp, T. Long-term outcome after treatment with 5% topical imiquimod cream in patients with basal cell carcinoma of the eyelids. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 121–125. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Library of Medicine. PubMed Help. Search Details. Available online: https://pubmed.ncbi.nlm.nih.gov/help/ (accessed on 12 June 2025).
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef]
- Higgins Jpt, T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- SJ, M. Biology of basal cell carcinoma (Part I). J. Am. Acad. Dermatol. 1991, 24, 1–13. [Google Scholar]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the Criteria for Including Studies and How They Will Be Grouped for the Synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Reeves, B.C.; Deeks, J.J.; Higgins, J.P.; Wells, G.A. Including non-randomized studies. In Cochrane handbook for Systematic Reviews of Interventions: Cochrane Book Series; Cochrane: London, UK, 2008; pp. 389–432. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. (Eds.) PRISMA 2020 Flow Diagram. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Greenhalgh, T. How to Read a Paper: The Basics of Evidence-Based Medicine; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Garcia-Martin, E.; Idoipe, M.; Gil, L.M.; Pueyo, V.; Alfaro, J.; Pablo, L.E.; Zubiri, M.L.; Fernandez, J. Efficacy and tolerability of imiquimod 5% cream to treat periocular basal cell carcinomas. J. Ocul. Pharmacol. Ther. 2010, 26, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Gil-Arribas, L.; Idoipe, M.; Alfaro, J.; Pueyo, V.; Pablo, L.; Fernandez, F. Comparison of imiquimod 5% cream versus radiotherapy as treatment for eyelid basal cell carcinoma. Br. J. Ophthalmol. 2011, 95, 1393–1396. [Google Scholar] [CrossRef]
- Carneiro, R.C.; de Macedo, E.M.S.; Matayoshi, S. Imiquimod 5% cream for the treatment of periocular basal cell carcinoma. Ophthalmic Plast. Reconstr. Surg. 2010, 26, 100–102. [Google Scholar] [CrossRef]
- Macedo, E.M.S.d.; Carneiro, R.C.; de Lima, P.P.; Silva, B.G.; Matayoshi, S. Imiquimod cream efficacy in the treatment of periocular nodular basal cell carcinoma: A non-randomized trial. BMC Ophthalmol. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Gaitanis, G.; Kalogeropoulos, C.D.; Bassukas, I.D. Cryosurgery during imiquimod (Immunocryosurgery) for periocular basal cell carcinomas: An efficacious minimally Invasive treatment alternative. Dermatology 2016, 232, 17–21. [Google Scholar] [CrossRef]
- Cannon, P.S.; O’Donnell, B.; Huilgol, S.C.; Selva, D. The ophthalmic side-effects of imiquimod therapy in the management of periocular skin lesions. Br. J. Ophthalmol. 2011, 95, 1682–1685. [Google Scholar] [CrossRef]
- Singh, M.; Mehta Grewal, A.; Singh, H.; Sharma, M.; Kaur, M.; Gupta, P.; Zadeng, Z. Long-term efficacy and safety of imiquimod 5% and fluorouracil 1% creams in medical monotherapy of complex eyelid basal cell carcinomas. Eur. J. Ophthalmol. 2022, 32, 2093–2100. [Google Scholar] [CrossRef]
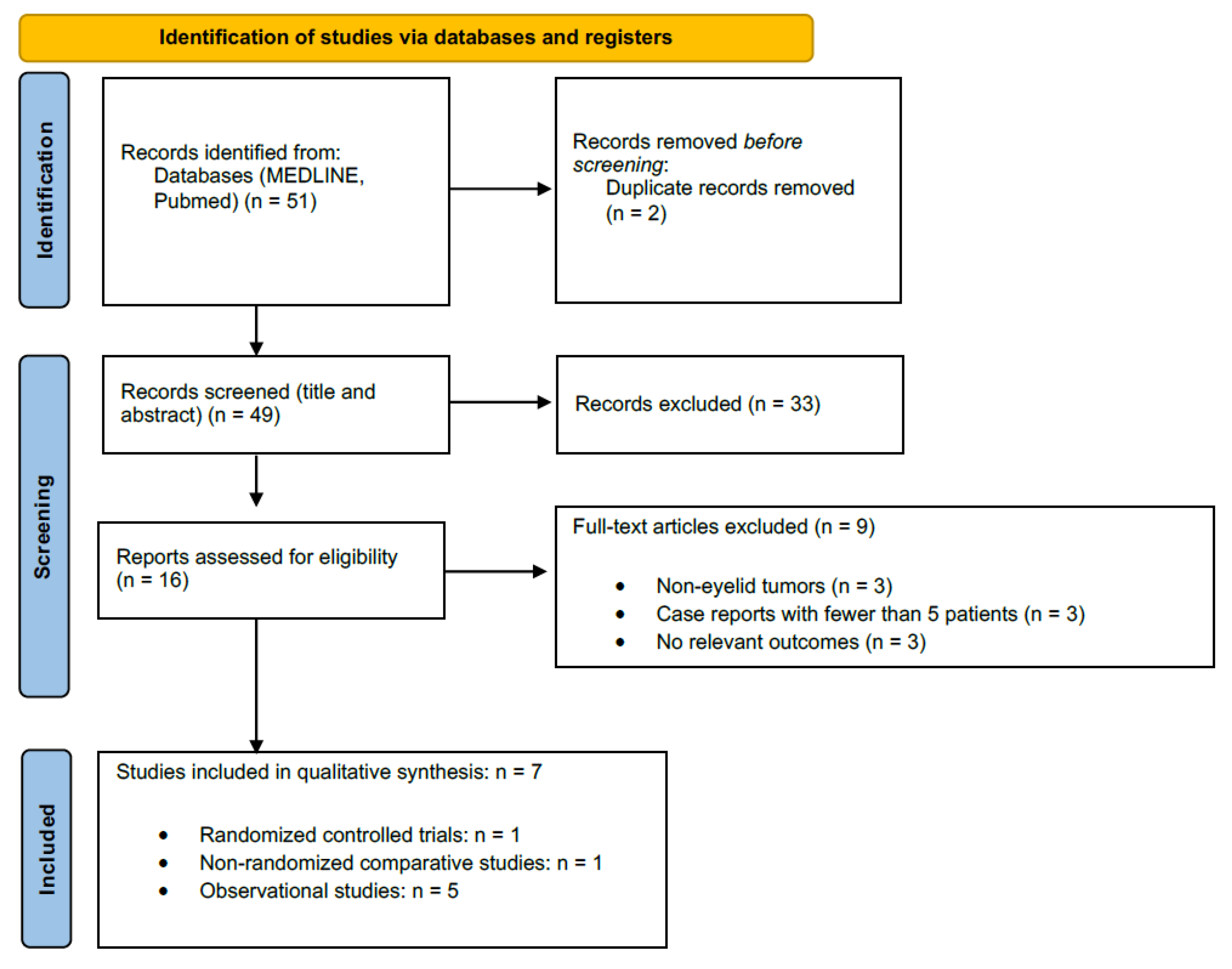
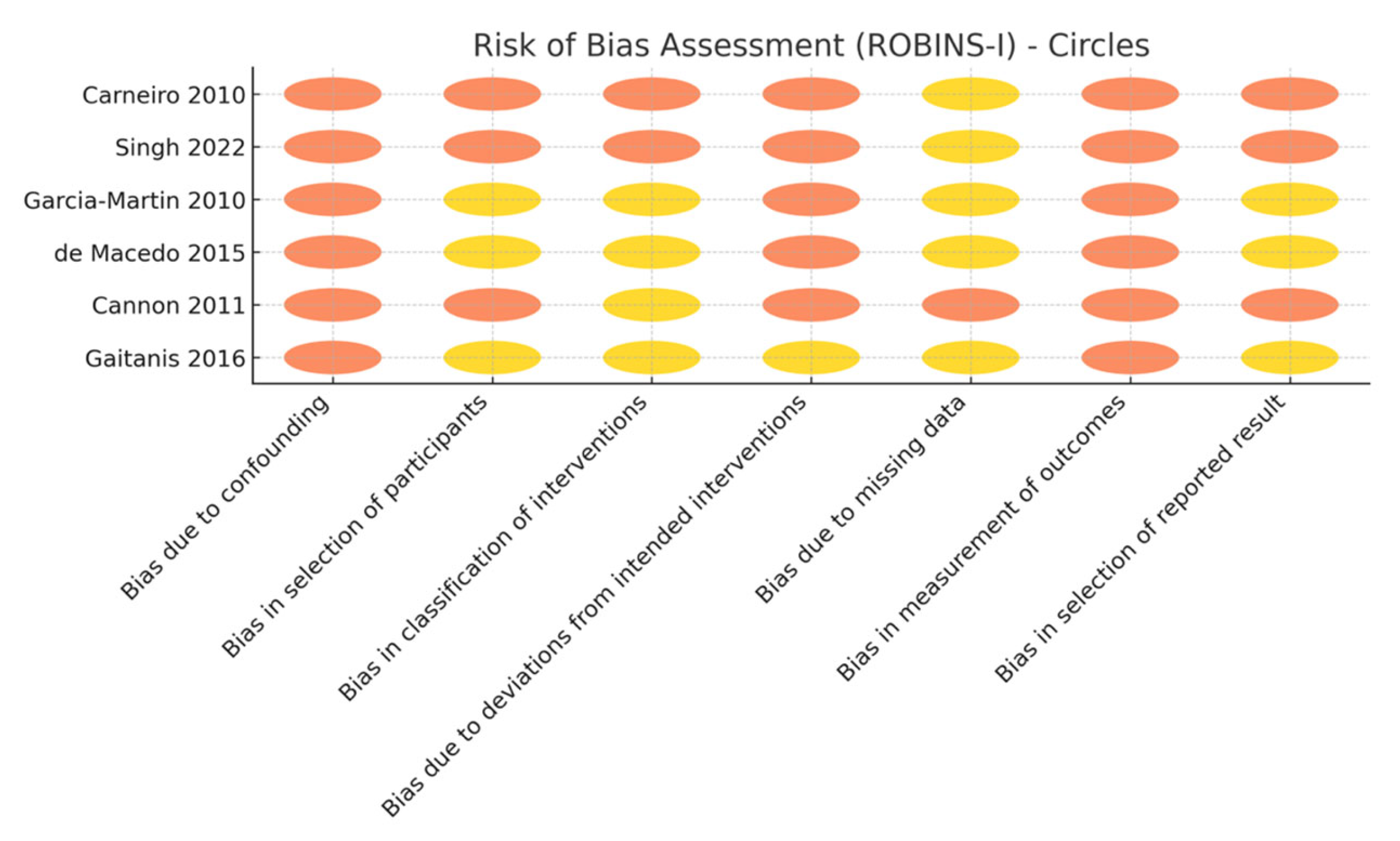
| Outcome | No. Studies/Lesions (Patients) | Study Design(s) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty | Downgrade Rationale |
|---|---|---|---|---|---|---|---|---|---|
| Four studies/64 lesions (56 pts) | A total of 1 RCT + 3 prospective cohorts | Not serious (RoB 2: low concern; cohorts had prospective protocols) | Not serious | Not serious (direct periocular BCC) | Serious (total n < 100; 95% CI 86–97%) | Suspected (small positive trials) | MODERATE | –1 imprecision | |
| Clinical-only clearance | Three studies/79 lesions (58 pts) | Retrospective or uncontrolled series | Serious (no controls and subjective outcome) | Serious (protocols differ) | Serious (indirect surrogate for cure) | Serious (wide CI 57–81%) | Likely | VERY LOW | –1 RoB, –1 indirectness, and –1 imprecision |
| Local periocular skin AEs (erythema, crusting, ulceration) | Six studies/122 pts | Retrospective or uncontrolled series | Serious (selective AE reporting in two studies) | Not serious (all report ≥ 60% events) | Not serious | Serious (denominators unclear in two studies) | Possible | MODERATE | –1 imprecision |
| Ophthalmic AEs(conjunctivitis, keratitis, cellulitis) | Four studies/94 pts | Retrospective or uncontrolled series | Serious (non-masked AE ascertainment) | Serious (rates 0–100%) | Not serious | Serious (event numbers small) | Possible | LOW | –1 RoB and –1 inconsistency/imprecision |
| Cosmetic and PROM outcomes | Two studies/42 pts | RCT + retrospective | Serious (non-validated scales and reporting bias) | Serious (different metrics) | Serious (indirect surrogate of QoL) | Serious (n < 50) | Likely | VERY LOW | –1 RoB, –1 indirectness, and –1 imprecision |
| Study (Design) | N Lesions | Authors’ Term for Success | How Success Was Confirmed | Timing of Assessment |
|---|---|---|---|---|
| Carneiro 2010 [31]—prospective case series | 10 | “clinical and histological resolution” | Slit-lamp/photographic inspection plus repeat 2 mm punch biopsy of every treated lesion | A total of 12 weeks after the end of therapy |
| García-Martín 2010 [29]—prospective case series | 15 | “histopathological remission” (sustained clinical remission reported separately) | Mandatory post-treatment biopsy of the original tumor bed | ≤3 months after starting imiquimod; followed clinically to 24–28 months |
| García-Martín 2011 [30]—randomized comparison IMQ vs. radiotherapy | 15 IMQ, 12 RT | “histopathological remission” | Biopsy of the treated site 6 weeks after completing the 6 week course | Histology at 6 weeks; clinical FU 24 months |
| de Macedo 2015 [32]—nonrandomized cohort | 24 | “75% histological clearance” | Post-treatment biopsy | Not stated |
| Singh 2022 [35]—retrospective series (complex BCC) | 16 IMQ, 14 5-FU | “complete clinical tumor resolution” | External inspection (no routine biopsy); recurrence judged clinically during ≥12 month follow-up | Median 12–16.5 weeks after start; clinical FU ≥ 12 months |
| Gaitanis 2016 [33]—retrospective immunocryosurgery series | 16 | “cleared”/“sustained clinical remission” | Clinical examination only (no biopsy reported) | End of each 5- or 10-week cycle; clinical FU 3–60 months |
| Cannon 2010 [34] —safety series | 47 | Outcome not primary endpoint (34/47 “clinical resolution”) | Clinical assessment; no histology | Mean 16 weeks after therapy |
| Study (Year) | Treated pts/Lesions | Local Periocular Skin Events | Ophthalmic Events (Conjunctiva/Cornea/Orbit) | Systemic Events | Notes on Data Quality |
|---|---|---|---|---|---|
| Carneiro (2010) [31] | 8/10 | Hyperaemia, crusting, ulceration, and bleeding reported in all patients (8/8) | Keratitis punctata ± allergic conjunctivitis (number not stated) | 0 | AEs described qualitatively; counts not provided. |
| García-Martín (2010) [29] | 15/15 | Local inflammatory reactions in 15/15; 7 judged “bad tolerability” | None specifically ocular | 0 | Ocular side-effects not itemized. |
| García-Martín (2011) [30] | 15/15 | Eyelid discomfort when blinking 9/15 | Conjunctival irritation 2/15 | 0 | All reactions resolved after therapy. |
| de Macedo (2015) [32] | 24/24 | Mild local reactions (number not given) | NR | 0 | Only statement that AEs were “mild”; no counts. |
| Singh (2022) [35] | 16/16 | Periocular erythema; skin depigmentation 16/16 | Chemical conjunctivitis 16/16 | 0 | All ocular events reversible. |
| Gaitanis (2016) [33] | 16/16 | Limited morbidity– no cutaneous complications quantified | 0 | 0 | Authors state “minimal” AEs; none detailed. |
| Cannon (2010) [34] | 47/47 | Application-site erythema 47/47; vesicles/ulceration 3 | Conjunctivitis 15; ocular stinging 6; keratitis 1; delayed conjunctivitis 3; pre-septal cellulitis 2 → ≥24/47 unique pts | 0 | AEs listed individually; overlap between events possible. |
| Treatment | Efficacy Rate | Key Advantages | Key Limitations | Citation |
|---|---|---|---|---|
| Imiquimod 5% Cream | 76–88% (varies by study and follow-up) | Non-invasive; suitable for delicate eyelid areas; and cosmetic preservation | Local skin reactions common; variable response; and less effective in nodular/infiltrative types | Singh et al., 2022 [35]; Garcia-Martin et al., 2010 [29] |
| Radiotherapy | ~90–95% | Useful in elderly or inoperable patients; and organ-sparing | Multiple sessions; and risk of long-term skin atrophy or pigmentary changes | Garcia-Martin et al., 2011 [30] |
| Imiquimod + Cryotherapy | ~90% | Potential synergistic effect; and shortened treatment duration | Limited studies; and risk of cryotherapy complications | Gaitanis et al., 2016 [33] |
| Mohs Micrographic Surgery | 97–99% | Tissue-sparing; maximal margin control; and best for recurrent/infiltrative tumors | Limited availability; expensive; and longer procedural time | Standard clinical reference (not from included SR studies) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Węglewicz, L.; Sobolewski, P.; Walecka, I. Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies. Cancers 2025, 17, 2111. https://doi.org/10.3390/cancers17132111
Krajewska-Węglewicz L, Sobolewski P, Walecka I. Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies. Cancers. 2025; 17(13):2111. https://doi.org/10.3390/cancers17132111
Chicago/Turabian StyleKrajewska-Węglewicz, Larysa, Piotr Sobolewski, and Irena Walecka. 2025. "Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies" Cancers 17, no. 13: 2111. https://doi.org/10.3390/cancers17132111
APA StyleKrajewska-Węglewicz, L., Sobolewski, P., & Walecka, I. (2025). Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies. Cancers, 17(13), 2111. https://doi.org/10.3390/cancers17132111








