Prognostic Features of Recurrent Midline and H3 K27M-Mutant Glioma
Simple Summary
Abstract
1. Introduction
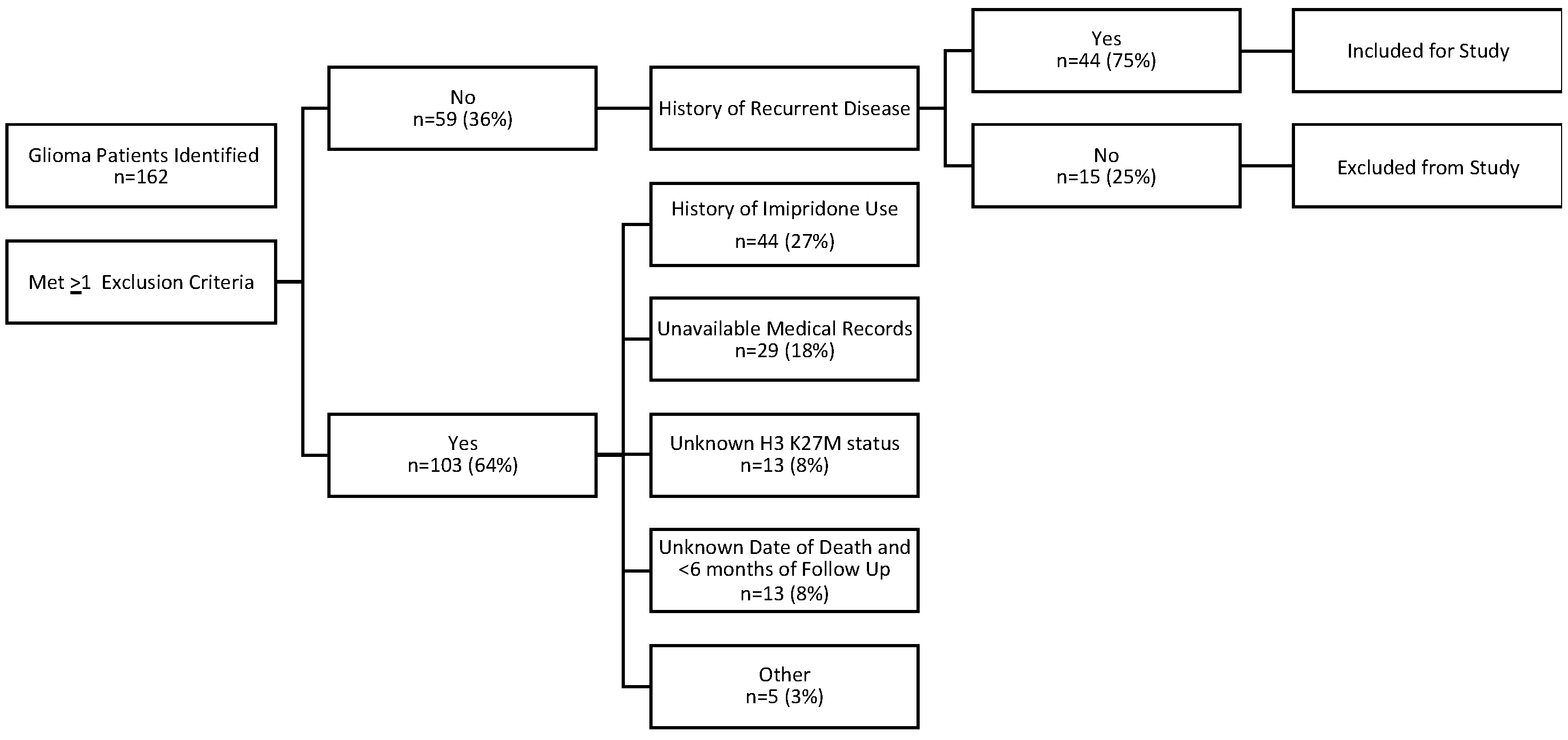
2. Materials and Methods
- Diagnosis of H3 K27M-mutant and/or midline glioma, initially diagnosed in 2012 or later.
- Known tissue-proven H3 K27 status (H3 K27M-mutant or wild-type).
- Medical records (including clinic notes and/or electronic databases) relating to glioma diagnosis and treatments received must be available for review. (Minimum information included demographics, disease characteristics, histology, disease history [diagnosis, tumor location, recurrences], radiation and other treatment history, survival status, and death date if applicable.)
- Presence of recurrent disease after standard-of-care therapy.
- No Prior Treatment with ONC201 or ONC 206.
2.1. Patients
2.2. Evaluation and Statistical Methods
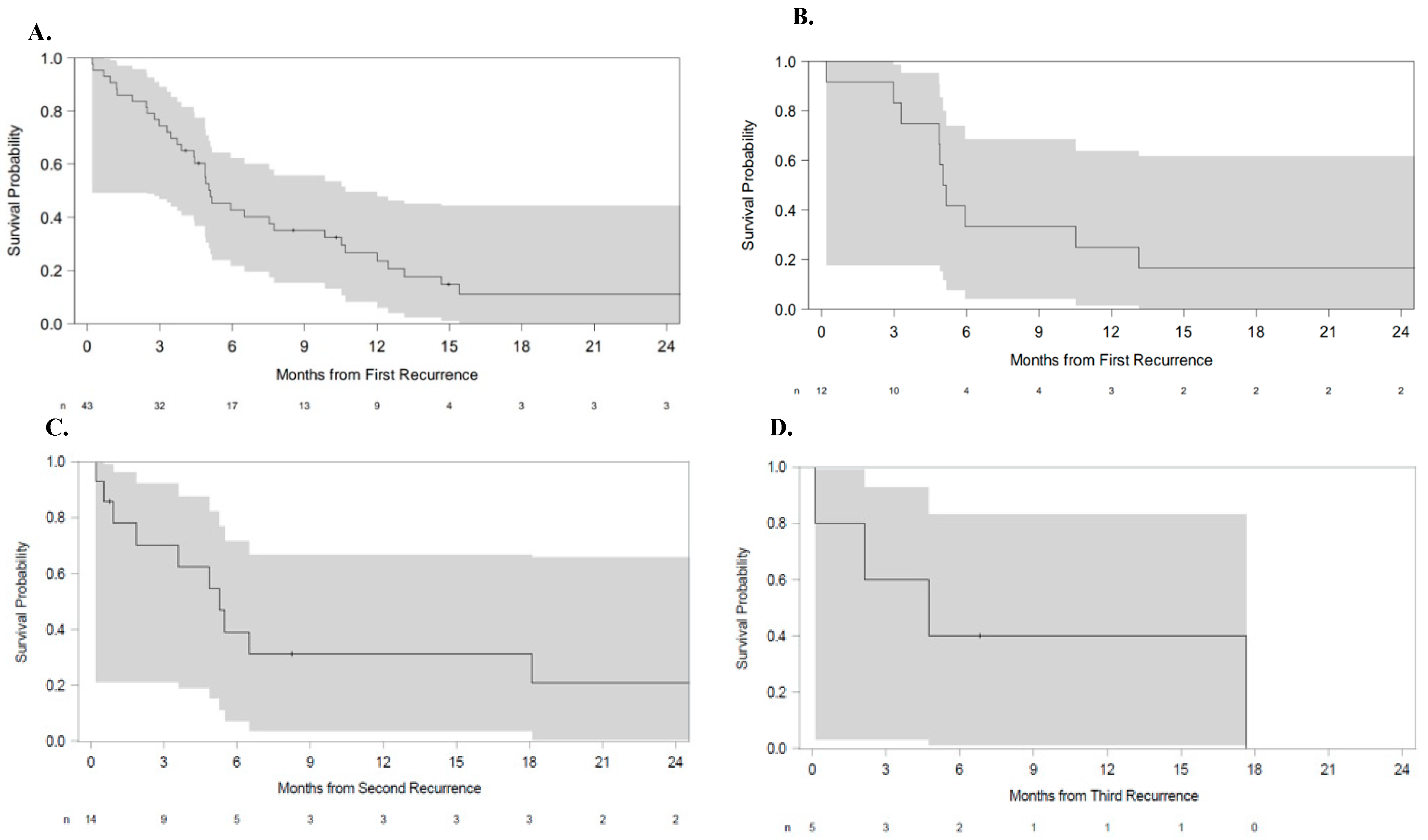
3. Results
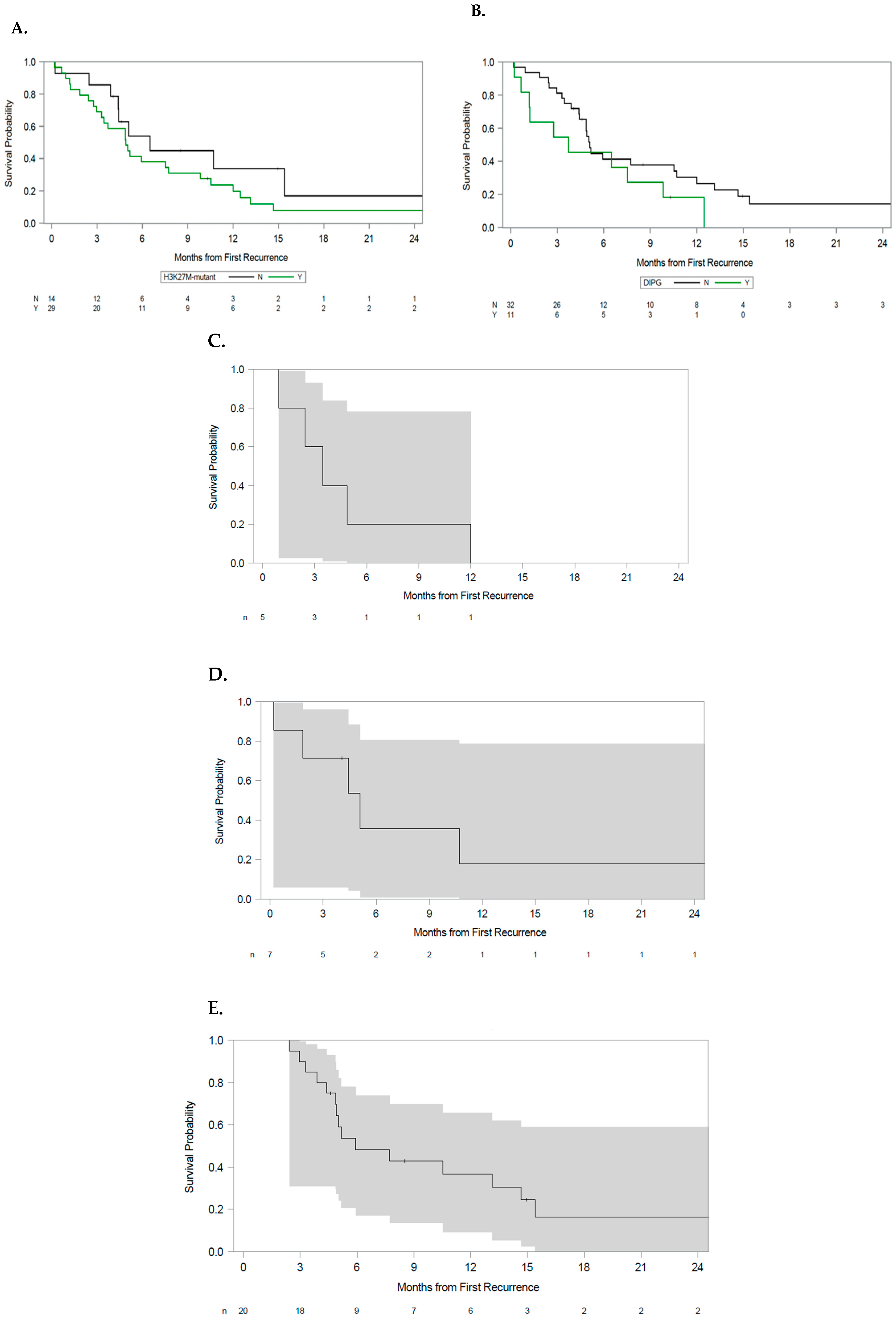
3.1. Analysis by H3 K27M Status
3.2. Analysis by Tumor Anatomic Origin
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PI Name | Institution | IRB | IRB Study Code |
| Yazmin Odia | Miami Cancer Institute | Advarra IRB | SSU00180912 |
| Kevin Bielamowicz | Arkansas Children’s Hospital | University of Arkansas for Medical Sciences IRB | 274102 |
| Lauren Weinbtraub | Albany Medical Center | Advarra IRB | SSU00180912 |
| Xiao-Tang Kong | University of California, Irvine | University of California, Irvine Office of Research IRB | UCI IRB #769 |
| Joe Mendez | The University of Utah, Huntsman Cancer Institute | The University of Utah IRB | IRB_00124158 |
| Akanksha Sharma | Providence Saint John’s Health Center | Advarra IRB | SSU00180912 |
| Yoshie Umemura | University of Michigan Health System | University of Michigan Medical School IRB | HUM00188956 |
| Nicholas Butowski | University of California San Francisco | University of California San Francisco Human Research Protection Program IRB | 257857 |
| Isabel Arrillaga | Massachusetts General Hospital | Dana Farber Cancer Institute Office for Human Research Studies IRB | 20–639 |
| Stephen Bagley | University of Pennsylvania Pearlman Center for Advance Medicine | Office of the Institutional Review Board University of Pennsylvania | 850834 |
References
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Khuong-Quang, D.-A.; Buczkowicz, P.; Rakopoulos, P.; Liu, X.-Y.; Fontebasso, A.M.; Bouffet, E.; Bartels, U.; Albrecht, S.; Schwartzentruber, J.; Letourneau, L.; et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Khuong-Quang, D.-A.; Tönjes, M.; et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231, Erratum in Nature 2012, 484, 130. [Google Scholar] [CrossRef]
- Saratsis, A.M.; Kambhampati, M.; Snyder, K.; Yadavilli, S.; Devaney, J.M.; Harmon, B.; Hall, J.; Raabe, E.H.; An, P.; Weingart, M.; et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuropathol. 2013, 127, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Karremann, M.; Gielen, G.H.; Hoffmann, M.; Wiese, M.; Colditz, N.; Warmuth-Metz, M.; Bison, B.; Claviez, A.; van Vuurden, D.G.; O von Bueren, A.; et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro-Oncology 2017, 20, 123–131. [Google Scholar] [CrossRef]
- Erker, C.; Lane, A.; Chaney, B.; Leary, S.; E Minturn, J.; Bartels, U.; Packer, R.J.; Dorris, K.; Gottardo, N.G.; E Warren, K.; et al. Characteristics of patients ≥10 years of age with diffuse intrinsic pontine glioma: A report from the International DIPG/DMG Registry. Neuro-Oncology 2021, 24, 141–152. [Google Scholar] [CrossRef]
- Aihara, K.; Mukasa, A.; Gotoh, K.; Saito, K.; Nagae, G.; Tsuji, S.; Tatsuno, K.; Yamamoto, S.; Takayanagi, S.; Narita, Y.; et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro-Oncology 2013, 16, 140–146. [Google Scholar] [CrossRef]
- Meyronet, D.; Esteban-Mader, M.; Bonnet, C.; Joly, M.-O.; Uro-Coste, E.; Amiel-Benouaich, A.; Forest, F.; Rousselot-Denis, C.; Burel-Vandenbos, F.; Bourg, V.; et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro-Oncology 2017, 19, 1127–1134. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Skardelly, M.; Schuhmann, M.U.; Ebinger, M.; Reuss, D.; Neumann, M.; Tabatabai, G.; Kohlhof-Meinecke, P.; Schittenhelm, J. High frequency of H3 K27M mutations in adult midline gliomas. J. Cancer Res. Clin. Oncol. 2019, 145, 839–850. [Google Scholar] [CrossRef]
- Lu, V.M.; Alvi, M.A.; McDonald, K.L.; Daniels, D.J. Impact of the H3K27M mutation on survival in pediatric high-grade glioma: A systematic review and meta-analysis. J. Neurosurg. Pediatr. 2019, 23, 308–316. [Google Scholar] [CrossRef]
- Pagès, M.; Beccaria, K.; Boddaert, N.; Saffroy, R.; Besnard, A.; Castel, D.; Fina, F.; Barets, D.; Barret, E.; Lacroix, L.; et al. Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol. 2016, 28, 103–111. [Google Scholar] [CrossRef]
- Joyon, N.; Tauziède-Espariat, A.; Alentorn, A.; Giry, M.; Castel, D.; Capelle, L.; Zanello, M.; Varlet, P.; Bielle, F. K27M mutation in H3F3A in ganglioglioma grade I with spontaneous malignant transformation extends the histopathological spectrum of the histone H3 oncogenic pathway. Neuropathol. Appl. Neurobiol. 2017, 43, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mosaab, A.; El-Ayadi, M.; Khorshed, E.N.; Amer, N.; Refaat, A.; El-Beltagy, M.; Hassan, Z.; Soror, S.H.; Zaghloul, M.S.; El-Naggar, S. Histone H3K27M Mutation Overrides Histological Grading in Pediatric Gliomas. Sci. Rep. 2020, 10, 8368. [Google Scholar] [CrossRef] [PubMed]
- Findlay, I.J.; De Iuliis, G.N.; Duchatel, R.J.; Jackson, E.R.; Vitanza, N.A.; Cain, J.E.; Waszak, S.M.; Dun, M.D. Pharmaco-proteogenomic profiling of pediatric diffuse midline glioma to inform future treatment strategies. Oncogene 2021, 41, 461–475. [Google Scholar] [CrossRef]
- Solomon, D.A.; Wood, M.D.; Tihan, T.; Bollen, A.W.; Gupta, N.; Phillips, J.J.J.; Perry, A. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol. 2015, 26, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Langmoen, I.A.; Lundar, T.; Storm-Mathisen, I.; Lie, S.O.; Hovind, K.H. Management of pediatric pontine gliomas. Child’s Nerv. Syst. 1991, 7, 13–15. [Google Scholar] [CrossRef]
- Hoffman, L.M.; van Zanten, S.E.V.; Colditz, N.; Baugh, J.; Chaney, B.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; Bartels, U.; et al. HG-75 Clinical, Radiological, and Histo-Genetic Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma: A Collaborative Report from the International and SIOP-E Dipg Registries. Neuro-Oncology 2016, 18, iii65–iii66. [Google Scholar] [CrossRef]
- Baugh, J.; Colditz, N.; Janssens, G.; Dietzsch, S.; Hargrave, D.; von Bueren, A.; Kortmann, R.-D.; Bison, B.; van Vuurden, D.; van Zanten, S.V.; et al. DIPG-77 Treatment Extent and the Effect on Survival in Diffuse Intrinsic Pontine Glioma. Neuro-Oncology 2020, 22, iii302. [Google Scholar] [CrossRef]
- Schüller, U.; Iglauer, P.; Dorostkar, M.M.; Mawrin, C.; Herms, J.; Giese, A.; Glatzel, M.; Neumann, J.E. Mutations within FGFR1 are associated with superior outcome in a series of 83 diffuse midline gliomas with H3F3A K27M mutations. Acta Neuropathol. 2021, 141, 323–325. [Google Scholar] [CrossRef]
- Kulinich, D.P.; Sheppard, J.P.; Nguyen, T.; Kondajji, A.M.; Unterberger, A.; Duong, C.; Enomoto, A.; Patel, K.; Yang, I. Radiotherapy versus combination radiotherapy-bevacizumab for the treatment of recurrent high-grade glioma: A systematic review. Acta Neurochir. 2021, 163, 1921–1934. [Google Scholar] [CrossRef]
- Shariff, N.; Moreno, A.S.; Bennett, J.; Ramaswamy, V.; Das, A.; Liu, A.P.; Huang, A.; Tabori, U.; Hawkins, C.; Dirks, P.; et al. Re-irradiation for children with diffuse intrinsic pontine glioma and diffuse midline glioma. Radiother. Oncol. 2025, 207, 110865. [Google Scholar] [CrossRef] [PubMed]
- Furth, N.; Algranati, D.; Dassa, B.; Beresh, O.; Fedyuk, V.; Morris, N.; Kasper, L.H.; Jones, D.; Monje, M.; Baker, S.J.; et al. H3-K27M-mutant nucleosomes interact with MLL1 to shape the glioma epigenetic landscape. Cell Rep. 2022, 39, 110836. [Google Scholar] [CrossRef]
- Wang, J.; Huang, T.Y.-T.; Hou, Y.; Bartom, E.; Lu, X.; Shilatifard, A.; Yue, F.; Saratsis, A. Epigenomic landscape and 3D genome structure in pediatric high-grade glioma. Sci. Adv. 2021, 7, eabg4126. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Hakkim, F.L.; Day, C.A.; Grigore, F.; Langfald, A.; Entin, I.; Hinchcliffe, E.H.; Robinson, J.P. H3F3A K27M mutations drive a repressive transcriptome by modulating chromatin accessibility independent of H3K27me3 in Diffuse Midline Glioma. Epigenet. Chromatin 2025, 18, 23. [Google Scholar] [CrossRef]
- Saaid, A.; Monticelli, M.; Ricci, A.A.; Orlando, G.; Botta, C.; Zeppa, P.; Bianconi, A.; Osella-Abate, S.; Bruno, F.; Pellerino, A.; et al. Prognostic Analysis of the IDH1 G105G (rs11554137) SNP in IDH-Wildtype Glioblastoma. Genes 2022, 13, 1439. [Google Scholar] [CrossRef]
- Vuong, H.G.; Le, H.T.; Jea, A.; McNall-Knapp, R.; Dunn, I.F. Risk stratification of H3 K27M–mutant diffuse midline gliomas based on anatomical locations: An integrated systematic review of individual participant data. J. Neurosurg. Pediatr. 2022, 30, 99–106. [Google Scholar] [CrossRef]
- Vuong, H.G.; Ngo, T.N.M.; Le, H.T.; Dunn, I.F. The prognostic significance of HIST1H3B/C and H3F3A K27M mutations in diffuse midline gliomas is influenced by patient age. J. Neuro-Oncol. 2022, 158, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gong, J.; Yu, T.; Zou, Y.; Zhang, M.; Nie, L.; Chen, X.; Yue, Q.; Liu, Y.; Mao, Q.; et al. Diffuse Midline Gliomas With Histone H3 K27M Mutation in Adults and Children. Am. J. Surg. Pathol. 2022, 46, 863–871. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.-L.; Ji, P.-G.; Liu, J.-H.; Guo, S.-C.; Zhai, Y.-L.; Sankey, E.W.; Wang, Y.; Xue, Y.-R.; Wang, N.; et al. Clinical Features and Molecular Markers on Diffuse Midline Gliomas With H3K27M Mutations: A 43 Cases Retrospective Cohort Study. Front. Oncol. 2021, 10, 602553. [Google Scholar] [CrossRef]
- Manjunath, N.; Jha, P.; Singh, J.; Raheja, A.; Kaur, K.; Suri, A.; Garg, A.; Sharma, M.C.; Sarkar, C.; Mohan, M.; et al. Clinico-pathological and molecular characterization of diffuse midline gliomas: Is there a prognostic significance? Neurol. Sci. 2020, 42, 925–934. [Google Scholar] [CrossRef]
| Demographic Feature | Number of Patients (%) |
|---|---|
| Age (years), median (range) | 28 (4–68) |
| Pediatric, (age < 18 y) | 16 (36.4%) |
| Female | 22 (50%) |
| Race Asian Black White Multiple Not reported | 5 (11.4%) 5 (11.4%) 30 (68.2%) 1 (2.3%) 3 (6.8%) |
| Ethnicity Hispanic or Latino Not Hispanic or Latino Unknown Not reported | 7 (15.9%) 35 (79.5%) 1 (2.3%) 1 (2.3%) |
| Karnosfsky Performance Status (KPS) 100 90 80 70 60 50 40 Unknown | 1 (2.3%) 3 (6.8%) 3 (6.8%) 10 (22.7%) 2 (4.5%) 3 (6.8%) 2 (4.5% 20 (45.5%) |
| H3 K27M mutation | 30 (68.2%) |
| CSF dissemination * | 5 (11.4%) |
| Leptomeningeal spread # | 10 (22.7%) |
| Resection Gross total resection Near gross resection Sub-total resection None | 2 (4.5%) 3 (6.8%) 16 (36.4%) 23 (52.3%) |
| Primary spinal tumor | 5 (11.4%) |
| DIPG | 12 (27.3%) |
| Multifocal disease † | 12 (27.2%) |
| Number of recurrences 1 2 3 5 | 30 (68.2%) 9 (20.5%) 4 (9.1%) 1 (2.3%) |
| Contrast enhancement | 36 (81.8%) |
| Steroid use (≥1.5 mg/day, dex) | 18 (40.9%) |
| Parameter | Pr > ChiSq | Hazard Ratio | 95% CI for Hazard Ratio |
|---|---|---|---|
| Primary tumor location: DIPG | 0.0078 | 3.64 | 1.41–9.45 |
| Primary tumor location: Non-DIPG brainstem | 0.44 | 0.53 | 0.11–2.66 |
| Primary tumor location: Non-DIPG spinal tumor | 0.0092 | 4.69 | 1.47–14.99 |
| Extent of resection: Sub-total resection | 0.052 | 0.43 | 0.19–1.01 |
| Extent of resection: Near gross resection | 0.21 | 2.30 | 0.63–8.40 |
| Extent of resection: Gross total resection | 0.39 | 1.96 | 0.42–9.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagley, S.J.; Umemura, Y.; Mendez, J.S.; Arrillaga-Romany, I.; Bielamowicz, K.J.; Butowski, N.; Hutchins, K.; Kong, X.-T.; Odia, Y.; Sharma, A.; et al. Prognostic Features of Recurrent Midline and H3 K27M-Mutant Glioma. Cancers 2025, 17, 2107. https://doi.org/10.3390/cancers17132107
Bagley SJ, Umemura Y, Mendez JS, Arrillaga-Romany I, Bielamowicz KJ, Butowski N, Hutchins K, Kong X-T, Odia Y, Sharma A, et al. Prognostic Features of Recurrent Midline and H3 K27M-Mutant Glioma. Cancers. 2025; 17(13):2107. https://doi.org/10.3390/cancers17132107
Chicago/Turabian StyleBagley, Stephen J., Yoshie Umemura, Joe S. Mendez, Isabel Arrillaga-Romany, Kevin J. Bielamowicz, Nick Butowski, Kelley Hutchins, Xiao-Tang Kong, Yazmin Odia, Akanksha Sharma, and et al. 2025. "Prognostic Features of Recurrent Midline and H3 K27M-Mutant Glioma" Cancers 17, no. 13: 2107. https://doi.org/10.3390/cancers17132107
APA StyleBagley, S. J., Umemura, Y., Mendez, J. S., Arrillaga-Romany, I., Bielamowicz, K. J., Butowski, N., Hutchins, K., Kong, X.-T., Odia, Y., Sharma, A., Weintraub, L., Koschmann, C., Wen, P. Y., Saratsis, A. M., Brundage, T., Ramage, S. C., Tarapore, R. S., Knowles, T., Yang, D., ... Cloughesy, T. (2025). Prognostic Features of Recurrent Midline and H3 K27M-Mutant Glioma. Cancers, 17(13), 2107. https://doi.org/10.3390/cancers17132107






