Artery-First Approach During Minimally Invasive Pancreatoduodenectomy for Pancreatic Cancer: Outcomes from a Single Center and Comparison Between Laparoscopic and Robotic Approaches
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Surgical Technique and Perioperative Management
2.2.1. LPD
2.2.2. Robotic PD (RPD)
2.3. Definitions and Variables
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Selection
3.2. Operative and Postoperative Outcomes
3.3. Pathological and Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Pancreatoduodenectomy |
| SMA | Superior Mesenteric Artery |
| SMV | Superior Mesenteric Vein |
| AFA | Artery-first Approach |
| IPDA | Inferior Pancreatico-duodenal Artery |
| MIPD | Minimally Invasive Pancreaticoduodenectomy |
| PC | Pancreatic Cancer |
| LPD | Laparoscopic Pancreaticoduodenectomy |
| FRS | Fistula Risk Score |
| BMI | Body Mass Index |
| LN | Lymph Node |
| IAFA | Ineffective Artery-first Approach |
References
- Jiang, X.; Yu, Z.; Ma, Z.; Deng, H.; Ren, W.; Shi, W.; Jiao, Z. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int. J. Surg. 2020, 73, 14–24. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Mackay, T.M.; Tol, J.A.M.G.; van Delden, O.M.; van Lienden, K.P.; Nio, C.Y.; Phoa, S.S.K.S.; Fockens, P.; van Hooft, J.E.; Verheij, J.; et al. Impact of expanding indications on surgical and oncological outcome in 1434 consecutive pancreatoduodenectomies. HPB 2019, 21, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Fernandes Ede, S.M.; Strobel, O.; Girão, C.; Moraes-Junior, J.M.A.; Torres, O.J.M. What do surgeons need to know about the mesopancreas. Langenbecks Arch. Surg. 2021, 406, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.R.; Ahmad, S.A.; Katz, M.H.; Cioffi, J.L.; Zyromski, N.J. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB 2016, 18, 305–311. [Google Scholar] [CrossRef]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; Reid-Lombardo, K.M.; Truty, M.J.; Nagorney, D.M.; Kendrick, M.L. Pancreaticoduodenectomy with Major Vascular Resection: A Comparison of Laparoscopic Versus Open Approaches. J. Gastrointest. Surg. 2015, 19, 189–194. [Google Scholar] [CrossRef]
- Kendrick, M.L.; Sclabas, G.M. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB 2011, 13, 454–458. [Google Scholar] [CrossRef]
- Khatkov, I.E.; Izrailov, R.E.; Khisamov, A.A.; Tyutyunnik, P.S.; Fingerhut, A. Superior mesenteric-portal vein resection during laparoscopic pancreatoduodenectomy. Surg. Endosc. 2017, 31, 1488–1495. [Google Scholar] [CrossRef]
- Weitz, J.; Rahbari, N.; Koch, M.; Büchler, M.W. The “artery first” approach for resection of pancreatic head cancer. J. Am. Coll. Surg. 2010, 210, e1–e4. [Google Scholar] [CrossRef]
- Varty, P.P.; Yamamoto, H.; Farges, O.; Belghiti, J.; Sauvanet, A. Early retropancreatic dissection during pancreaticoduodenectomy. Am. J. Surg. 2005, 189, 488–491. [Google Scholar] [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. “Artery-first” approaches to pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef]
- van Hilst, J.; de Graaf, N.; Abu Hilal, M.; Besselink, M.G. The Landmark Series: Minimally Invasive Pancreatic Resection. Ann. Surg. Oncol. 2021, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, Y.; Watanabe, Y.; Kozono, S.; Boggi, U.; Palanivelu, C.; Liu, R.; Wang, S.E.; He, J.; Nishino, H.; Ohtsuka, T.; et al. Study group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP surgery). Surgical approaches to the superior mesenteric artery during minimally invasive pancreaticoduodenectomy: A systematic review. J. Hepatobiliary Pancreat. Sci. 2022, 29, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Uijterwijk, B.A.; Lemmers, D.H.; Fusai, G.K.; Groot Koerkamp, B.; Koek, S.; Zerbi, A.; Sparrelid, E.; Boggi, U.; Luyer, M.; Ielpo, B.; et al. Different Periampullary Types and Subtypes Leading to Different Perioperative Outcomes of Pancreatoduodenectomy: Reality and Not a Myth; An International Multicenter Cohort Study. Cancers 2024, 16, 899. [Google Scholar] [CrossRef]
- He, C.; Mao, Y.; Wang, J.; Duan, F.; Lin, X.; Li, S. Nomograms predict long-term survival for patients with periampullary adenocarcinoma after pancreatoduodenectomy. BMC Cancer 2018, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573. [Google Scholar] [CrossRef]
- Mazzola, M.; Giani, A.; Crippa, J.; Morini, L.; Zironda, A.; Bertoglio, C.L.; De Martini, P.; Magistro, C.; Ferrari, G. Totally laparoscopic versus open pancreaticoduodenectomy: A propensity score matching analysis of short-term outcomes. Eur. J. Surg. Oncol. 2021, 47, 674–680. [Google Scholar] [CrossRef]
- Mazzola, M.; Giani, A.; Bertoglio, C.L.; Carnevali, P.; De Martini, P.; Benedetti, A.; Giusti, I.; Magistro, C.; Ferrari, G. Standardized right artery first approach during laparoscopic pancreaticoduodenectomy for periampullary neoplasms: Technical aspects and perioperative outcomes. Surg. Endosc. 2023, 37, 759–765. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Hosokawa, Y.; Sahara, Y.; Takishita, C.; Hijikata, Y.; Osakabe, H.; Nakajima, T.; Shirota, T.; Katsumata, K.; Nakamura, M.; et al. Approaching the superior mesenteric artery from the right side using the proximal-dorsal jejunal vein preisolation method during laparoscopic pancreaticoduodenectomy. Surg. Endosc. 2018, 32, 4044–4051. [Google Scholar] [CrossRef]
- Callery, M.P.; Pratt, W.B.; Kent, T.S.; Chaikof, E.L.; Vollmer, C.M., Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J. Am. Coll. Surg. 2013, 216, 1–14. [Google Scholar] [CrossRef]
- Mazzola, M.; Giani, A.; Veronesi, V.; Bernasconi, D.P.; Benedetti, A.; Magistro, C.; Bertoglio, C.L.; De Martini, P.; Ferrari, G. Multidimensional evaluation of the learning curve for totally laparoscopic pancreaticoduodenectomy: A risk-adjusted cumulative summation analysis. HPB 2023, 25, 507–517. [Google Scholar] [CrossRef]
- Salvia, R.; Marchegiani, G.; Andrianello, S.; Balduzzi, A.; Masini, G.; Casetti, L.; Esposito, A.; Landoni, L.; Malleo, G.; Paiella, S.; et al. Redefining the Role of Drain Amylase Value for a Risk-Based Drain Management after Pancreaticoduodenectomy: Early Drain Removal Still Is Beneficial. J. Gastrointest. Surg. 2021, 25, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K. AJCC Cancer Staging Manual; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Pedrazzoli, S. Extent of lymphadenectomy to associate with pancreaticoduodenectomy in patients with pancreatic head cancer for better tumor staging. Cancer Treat. Rev. 2015, 41, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Müller, P.C.; Müller, B.P.; Hackert, T. Contemporary artery-first approaches in pancreatoduodenectomy. Br. J. Surg. 2023, 110, 1570–1573. [Google Scholar] [CrossRef]
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Deprés, J.P. Sex differences in the relation of visceral adipose tissue acculumation to total body fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef]
- Takagi, K.; Yamada, M.; Fuji, T.; Yasui, K.; Nishiyama, T.; Nagai, Y. Impact of visceral fat area on surgical difficulty during robotic distal pancreatectomy (TAKUMI-2). Surg. Endosc. 2025, 39, 3137–3145. [Google Scholar] [CrossRef]
- Mungroop, T.H.; Klompmaker, S.; Wellner, U.F.; Steyeberg, E.W.; Coratti, A.; D’Hondt, M.; de Pastena, M.; Dokmak, S.; Khatkov, I.; Saint-Marc, O.; et al. Consortium on minimally invasive pancreatic surgery (E-MIPS). Updated alternative fistula risk score (ua-FRS) to include minimally invasive pancreaticoduodenectomy: Pan-European validation. Ann. Surg. 2021, 273, 334–340. [Google Scholar] [CrossRef]
- Sabater, L.; Cugat, E.; Serrablo, A.; Suarez-Artacho, G.; Diez-Valladares, L.; Santoyo-Santoyo, J.; Martín-Pérez, E.; Ausania, F.; Lopez-Ben, S.; Jover-Navalon, J.M.; et al. Does the artery-first approach improve the rate of R0 resection in pancreatoduodenectomy? Ann. Surg. 2019, 270, 738–746. [Google Scholar] [CrossRef]
- Morales, E.; Zimmitti, G.; Codignola, C.; Manzoni, A.; Garatti, M.; Sega, V.; Rosso, E. Follow “the superior mesenteric artery”: Laparoscopic approach for total mesopancreas excision during pancreaticoduodenectomy. Surg. Endosc. 2019, 33, 4186–4191. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Yamamoto, H.; Kainuma, O. Tips of laparoscopic pancreaticoduodenectomy: Superior mesenteric artery first approach (with video). J. Hepatobiliary Pancreat. Sci. 2014, 21, e19–e21. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Liu, Y.-Y.; Wang, S.-Y.; Liu, K.-H.; Yeh, C.-N.; Yeh, T.-S. The feasibility of laparoscopic pancreaticoduodenectomy-a stepwise procedure and learning curve. Langenbecks Arch. Surg. 2017, 402, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Gao, P.; Li, Y.; Wang, X.; Peng, B. Laparoscopic pancreaticoduodenectomy with major venous resection and reconstruction: Anterior superior mesenteric artery first approach. Surg. Endosc. 2018, 32, 4209–4215. [Google Scholar] [CrossRef]
- Wang, X.M.; Sun, W.D.; Hu, M.H.; Wang, G.N.; Jiang, Y.Q.; Fang, X.S.; Han, M. Inferoposterior duodenal approach for laparoscopic pancreaticoduodenectomy. World J. Gastroenterol. 2016, 22, 2142–2148. [Google Scholar] [CrossRef]
- Ielpo, B.; Anselmo, A.; Masuda, Y.; Xuan, M.Y.H.; Burdio, F.; De Blasi, V.; Sanchez-Velazquez, P.; Giuliani, A.; Azagra, J.S.; Viola, G.M.; et al. Superior Mesenteric Artery First Approach for Minimally Invasive Pancreaticoduodenectomy: A Step-By-Step Surgical Technique Video. Ann. Surg. Oncol. 2023, 30, 1500–1503. [Google Scholar] [CrossRef]
- Sun, R.; Yu, J.; Zhang, Y.; Liang, Z.; Han, X. Perioperative and oncological outcomes following minimally invasive versus open pancreaticoduodenectomy for pancreatic duct adenocarcinoma. Surg. Endosc. 2021, 35, 2273–2285. [Google Scholar] [CrossRef]
- Giani, A.; Mazzola, M.; Paterno, M.; Zironda, A.; Calcagno, P.; Zuppi, E.; De Martini, P.; Ferrari, G. Oncological Outcomes of Open Versus Minimally Invasive Surgery for Ductal Adenocarcinomas of Pancreatic Head: A Propensity Score Matching Analysis. Curr. Oncol. 2024, 31, 6096–6109. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Nassour, I.; Adam, M.A.; Winters, S.; Paniccia, A.; Zureikat, A.H. National Trends in Robotic Pancreas Surgery. J. Gastrointest. Surg. 2021, 25, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Nota, C.L.; Zwart, M.J.; Fong, Y.; Hagendoorn, J.; Hogg, M.E.; Koerkamp, B.G.; Besselink, M.G.; Molenaar, I.Q. Dutch Pancreatic Cancer Group. Developing a robotic pancreas program: The Dutch experience. J. Visc. Surg. 2017, 3, 106. [Google Scholar] [CrossRef] [PubMed]
- Emmen, A.M.L.H.; Görgec, B.; Zwart, M.J.W.; Daams, F.; Erdmann, J.; Festen, S.; Gouma, D.J.; van Gulik, T.M.; van Hilst, J.; Kazemier, G.; et al. Impact of shifting from laparoscopic to robotic surgery during 600 minimally invasive pancreatic and liver resections. Surg. Endosc. 2023, 37, 3291–3292. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef]
- Boggi, U.; Donisi, G.; Napoli, N.; Partelli, S.; Esposito, A.; Ferrari, G.; Butturini, G.; Morelli, L.; Abu Hilal, M.; Viola, M.; et al. Prospective minimally invasive pancreatic resections from the IGOMIPS registry: A snapshot of daily practice in Italy on 1191 between 2019 and 2022. Updates Surg. 2023, 75, 1439–1456. [Google Scholar] [CrossRef]
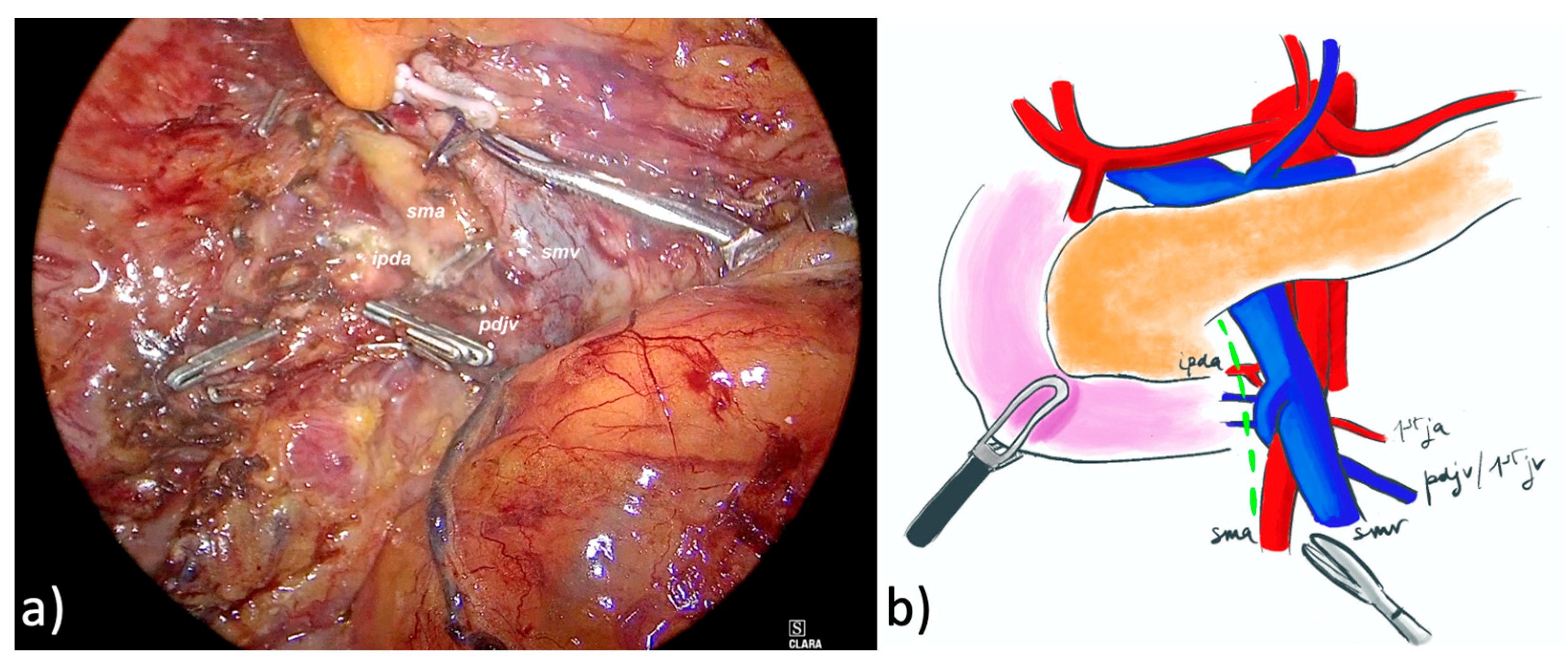
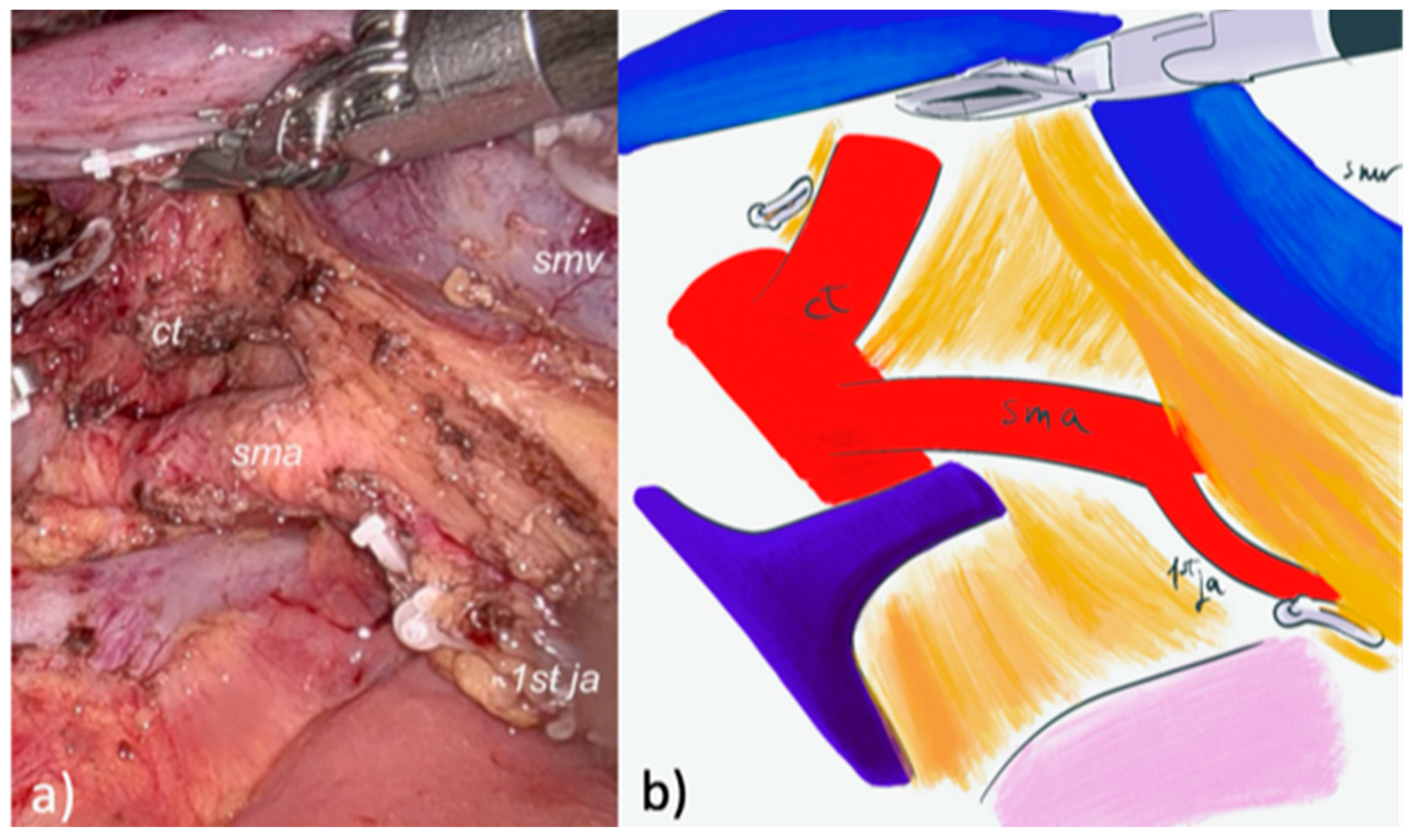
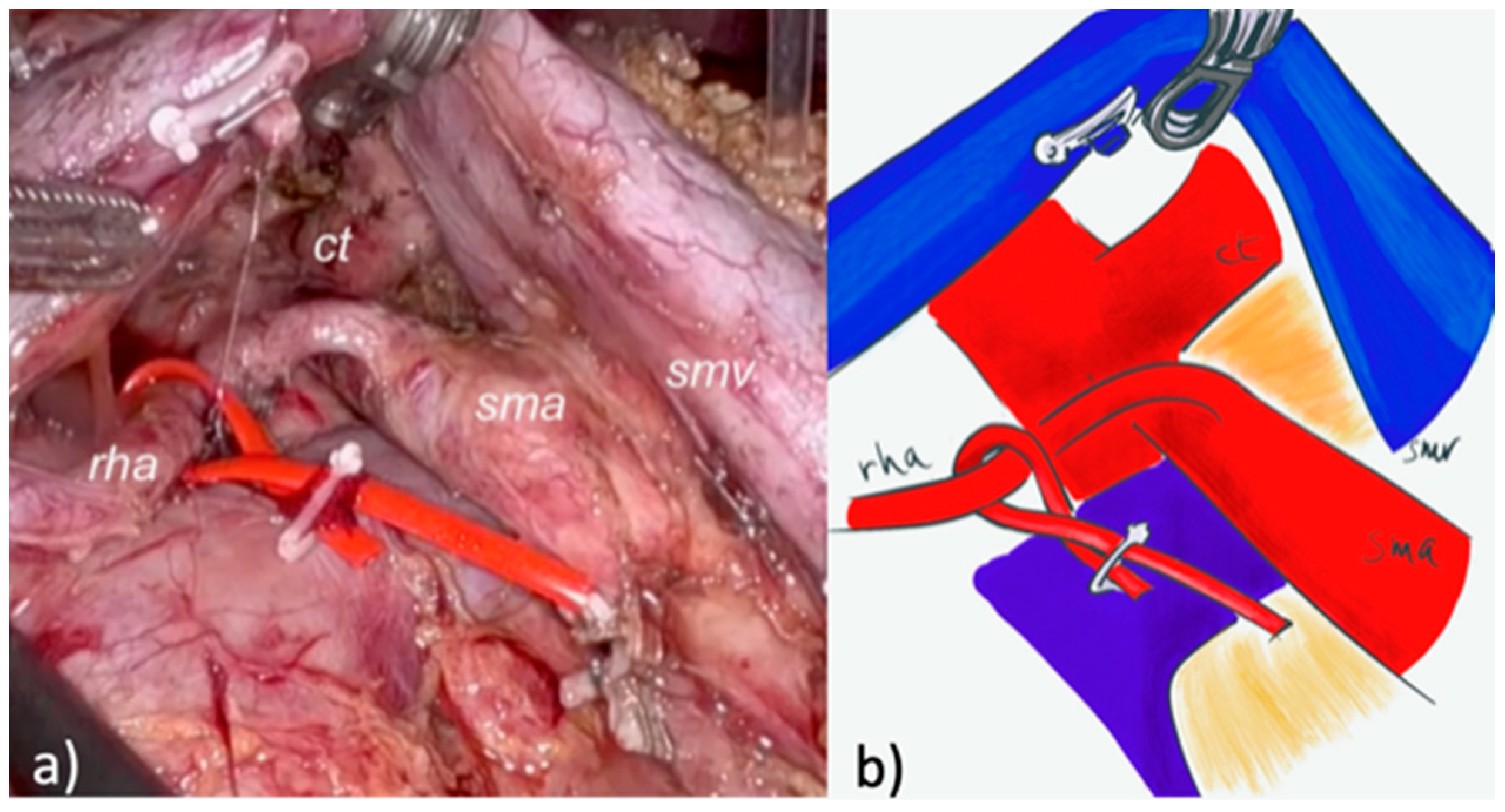
| Characteristic | Overall (71) | LPD (32) | RPD (39) | p |
|---|---|---|---|---|
| Sex (F) | 39 (54.9) | 19 (59.4) | 20 (51.3) | 0.495 |
| Age (years) * | 72 (65–80) | 73 (60.5–80.7) | 71 (65–79) | 0.556 |
| BMI (kg/m2) * | 24.7 (22.5–27.6) | 25.6 (22.6–27.8) | 24.6 (22.0–26.7) | 0.291 |
| ACCI * | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.610 |
| ASA | 0.741 | |||
| ASA 1 | 3 (4.2) | 2 (6.2) | 1 (2.5) | |
| ASA 2 | 52 (73.2) | 23 (71.9) | 29 (74.4) | |
| ASA 3 | 16 (22.6) | 7 (21.9) | 9 (23.1) | |
| Previous abdominal surgery | 32 (45.1) | 11 (34.4) | 21 (53.8) | 0.100 |
| Neoadjuvant therapy | 9 (12.7) | 0 (0) | 9 (23.1) | 0.0036 |
| Venous involvement | 12 (16.9) | 4 (12.5) | 8 (20.5) | 0.370 |
| Vascular anomalies | 18 (25.3) | 9 (28.1) | 9 (23.1) | 0.626 |
| Preoperative biliary drainage | 36 (50.7) | 14 (43.8) | 22 (56.4) | 0.288 |
| Characteristic | Overall (71) | LPD (32) | RPD (39) | p |
|---|---|---|---|---|
| Vascular resection | 0.059 | |||
| No | 66 (92.9) | 32 (100) | 34 (87.2) | |
| PV | 1 (1.5) | 0 (0) | 1 (2.5) | |
| SMV | 4 (5.6) | 0 (0) | 4 (10.3) | |
| Operative time * | 545 (500–600) | 555 (510–597.5) | 540 (480–620) | 0.561 |
| Resection time * | 310 (265–350) | 305 (271.2–328.7) | 318 (260–370) | 0.708 |
| Conversion | 0 (0) | 0 (0) | 0 (0) | / |
| Wirsung diameter (mm) * | 4 (2–5) | 4 (2–5) | 4 (2–5) | 0.276 |
| Blood loss (mL) | 0.231 | |||
| <400 | 49 (69.0) | 25 (78.1) | 24 (61.5) | |
| 401–700 | 13 (18.4) | 4 (12.5) | 9 (23.1) | |
| 701–1000 | 6 (8.4) | 3 (9.4) | 3 (7.7) | |
| >1000 | 3 (4.2) | 0 (0) | 3 (7.7) | |
| Blood loss (mL) * | 250 (150–500) | 300 (200–487.5) | 200 (150–600) | 0.561 |
| Pancreatic texture | 0.283 | |||
| Soft | 24 (33.8) | 9 (28.1) | 15 (38.5) | |
| Intermediate | 7 (9.9) | 5 (15.6) | 2 (5.1) | |
| Firm | 40 (56.3) | 18 (56.3) | 22 (56.4) | |
| FRS * | 3 (1–5) | 2 (0.2–5) | 3 (1–4) | 0.706 |
| FRS moderate-high | 35 (49.3) | 15 (46.9) | 20 (51.3) | 0.711 |
| Class of FRS | 0.146 | |||
| Negligible (0 pt) | 15 (21.1) | 8 (25.0) | 7 (17.9) | 0.640 |
| Low (1–2 pt) | 20 (28.2) | 9 (28.1) | 11 (28.2) | |
| Moderate (3–6 pt) | 31 (43.7) | 14 (43.8) | 17 (43.6) | |
| High (7–10 pt) | 5 (7.0) | 1 (3.1) | 4 (10.3) |
| Characteristic | Overall (71) | LPD (32) | RPD (39) | p |
|---|---|---|---|---|
| Length of stay (day) * | 11 (8–16) | 10.5 (8–19.2) | 11 (8–16) | 0.893 |
| Overall complications | 32 (45.1) | 13 (40.6) | 19 (48.7) | 0.495 |
| Reoperation | 4 (5.6) | 3 (9.4) | 1 (2.5) | 0.215 |
| Complications: | 0.551 | |||
| 1 | 40 (56.3) | 20 (62.6) | 20 (51.3) | |
| 2 | 14 (19.7) | 4 (12.5) | 10 (25.7) | |
| 3a | 10 (14.1) | 4 (12.5) | 6 (15.5) | |
| 3b | 3 (4.2) | 2 (6.2) | 1 (2.5) | |
| 4 | 1 (1.5) | 0 (0) | 1 (2.5) | |
| 90-day mortality | 3 (4.2) | 2 (6.2) | 1 (2.5) | 0.442 |
| Comprehensive CI * | 0 (0–25.7) | 0 (0–24.9) | 0 (0–25.7) | 0.587 |
| Severe complications | 15 (21.1) | 8 (25.0) | 7 (17.9) | 0.468 |
| POPF | 6 (8.4) | 3 (9.4) | 3 (7.7) | 0.799 |
| POPF grade | 0.691 | |||
| Grade B | 3 (4.2) | 1 (3.1) | 2 (5.0) | |
| Grade C | 3 (4.2) | 2 (6.2) | 1 (2.5) | |
| DGE | 11 (15.5) | 5 (15.6) | 6 (15.4) | 0.977 |
| DGE grade | 0.330 | |||
| Grade A | 4 (5.6) | 3 (9.4) | 1 (2.5) | |
| Grade B | 7 (9.9) | 2 (6.2) | 5 (12.8) | |
| Grade C | 0 (0) | 0 (0) | 0 (0) | |
| Biliary leak | 4 (5.6) | 3 (9.4) | 1 (2.5) | 0.215 |
| Biliary leak grade | 0.280 | |||
| Grade A | 2 (2.8) | 1 (3.1) | 1 (2.5) | |
| Grade B | 2 (2.8) | 2 (6.2) | 0 (0) | |
| Grade C | 0 (0) | 0 (0) | 0 (0) | |
| PPH | 7 (9.9) | 3 (9.4) | 4 (10.3) | 0.901 |
| PPH grade | 0.669 | |||
| Grade A | 1 (1.5) | 0 (0) | 1 (2.5) | |
| Grade B | 3 (4.2) | 1 (3.1) | 2 (5.0) | |
| Grade C | 3 (4.2) | 2 (6.2) | 1 (2.5) | |
| 90 day-readmission | 6 (8.4) | 2 (6.2) | 4 (10.3) | 0.545 |
| IAFA | 9 (12.7) | 3 (9.4) | 6 (15.4) | 0.499 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex (male) | 5.181 (0.994–27.027) | 0.035 | 5.181 (1.105–37.037) | 0.036 |
| Age > 70 | 1.029 (0.252–4.203) | 0.968 | ||
| BMI > 30 | 0.982 (0.106–9.067) | 0.987 | ||
| ASA > 2 | 3.333 (0.776–14.325) | 0.092 | 3.310 (0.687–15.605) | 0.131 |
| ACCI > 5 | 1.620 (0.397–6.620) | 0.498 | ||
| Previous abdominal surgery | 2.245 (0.387–13.013) | 0.319 | ||
| Vascular involvement | 0.580 (0.066–5.120) | 0.620 | ||
| RHA anomalies | 0.821 (0.154–4.369) | 0.817 | ||
| Preoperative biliary drainage | 2.133 (0.489–9.303) | 0.305 | ||
| Ca 19.9 > 500 U/mL | 0.680 (0.075–6.124) | 0.730 | ||
| Neoadjuvant chemotherapy | 2.245 (0.387–13.013) | 0.319 | ||
| Robotic approach | 1.758 (0.403–7.667) | 0.449 | ||
| Tumor size > 25 mm | 1.620 (0.397–6.620) | 0.380 | ||
| Positive lymph nodes (yes) | 0.844 (0.206–3.457) | 0.814 | ||
| Characteristic | Overall (71) | LPD (32) | RPD (39) | p |
|---|---|---|---|---|
| Tumor size (mm) * | 25 (20–30) | 25 (20–30) | 25 (21–30) | 0.847 |
| Grading | 0.281 | |||
| not applicable | 3 (4.2) | 0 (0) | 3 (7.7) | |
| Gx | 1 (1.5) | 0 (0) | 1 (2.5) | |
| G1 | 0 (0) | 0 (0) | 0 (0) | |
| G2 | 36 (50.7) | 16 (50.0) | 20 (51.3) | |
| G3 | 31 (43.7) | 16 (50.0) | 15 (38.5) | |
| Perineural invasion | 47 (66.2) | 25 (78.1) | 28 (71.8) | 0.593 |
| Lymphovascular invasion | 37 (52.1) | 16 (50.0) | 21 (53.8) | 0.746 |
| Lymph nodes harvested * | 22 (15–27) | 17 (15–24.7) | 24 (17–28) | 0.023 |
| Positive lymph nodes * | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.630 |
| pN+ | 42 (59.1) | 21 (65.7) | 21 (53.8) | 0.315 |
| Lymph node ratio * | 0.05 (0–0.12) | 0.06 (0–0.16) | 0.04 (0–0.11) | 0.291 |
| R0 | 53 (74.6) | 26 (81.2) | 27 (69.2) | 0.246 |
| R1 | 0.108 | |||
| Posterior margin | 14 (19.7) | 6 (100) | 8 (66.7) | |
| Medial margin | 4 (5.6) | 0 (0) | 4 (33.3) | |
| pT | 0.590 | |||
| pT0 † | 2 (2.8) | 0 (0) | 2 (5.1) | |
| pTis | 1 (1.5) | 0 (0) | 1 (2.5) | |
| pT1a | 3 (4.2) | 1 (3.1) | 2 (5.1) | |
| pT1b | 3 (4.2) | 1 (3.1) | 2 (5.1) | |
| pT1c | 15 (21.1) | 6 (18.7) | 9 (23.1) | |
| pT2 | 43 (60.6) | 21 (65.7) | 22 (56.4) | |
| pT3 | 4 (5.6) | 3 (9.4) | 1 (2.5) | |
| pN | 0.570 | |||
| pN0 | 29 (40.9) | 11 (34.4) | 18 (46.2) | |
| pN1 | 29 (40.9) | 15 (46.9) | 14 (35.9) | |
| pN2 | 13 (18.4) | 6 (18.7) | 7 (17.9) | |
| TNM Stage > IIa | 41 (57.8) | 21 (65.7) | 20 (51.3) | 0.223 |
| TNM Stage | 0.577 | |||
| Ia | 16 (22.6) | 5 (15.6) | 11 (28.2) | |
| Ib | 13 (18.4) | 6 (18.7) | 7 (17.9) | |
| IIa | 1 (1.5) | 0 (0) | 1 (2.5) | |
| IIb | 28 (39.1) | 15 (46.9) | 13 (33.5) | |
| III | 13 (18.4) | 6 (18.7) | 7 (17.9) | |
| Performed adjuvant therapy | 40/67 †† (59.7) | 20 (62.6) | 20/35 † (57.1) | 0.655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzola, M.; Paterno, M.; Giani, A.; Calcagno, P.; Zironda, A.; Mucci, G.; Franzetti, C.; De Martini, P.; Ferrari, G. Artery-First Approach During Minimally Invasive Pancreatoduodenectomy for Pancreatic Cancer: Outcomes from a Single Center and Comparison Between Laparoscopic and Robotic Approaches. Cancers 2025, 17, 2103. https://doi.org/10.3390/cancers17132103
Mazzola M, Paterno M, Giani A, Calcagno P, Zironda A, Mucci G, Franzetti C, De Martini P, Ferrari G. Artery-First Approach During Minimally Invasive Pancreatoduodenectomy for Pancreatic Cancer: Outcomes from a Single Center and Comparison Between Laparoscopic and Robotic Approaches. Cancers. 2025; 17(13):2103. https://doi.org/10.3390/cancers17132103
Chicago/Turabian StyleMazzola, Michele, Michele Paterno, Alessandro Giani, Pietro Calcagno, Andrea Zironda, Gaia Mucci, Camillo Franzetti, Paolo De Martini, and Giovanni Ferrari. 2025. "Artery-First Approach During Minimally Invasive Pancreatoduodenectomy for Pancreatic Cancer: Outcomes from a Single Center and Comparison Between Laparoscopic and Robotic Approaches" Cancers 17, no. 13: 2103. https://doi.org/10.3390/cancers17132103
APA StyleMazzola, M., Paterno, M., Giani, A., Calcagno, P., Zironda, A., Mucci, G., Franzetti, C., De Martini, P., & Ferrari, G. (2025). Artery-First Approach During Minimally Invasive Pancreatoduodenectomy for Pancreatic Cancer: Outcomes from a Single Center and Comparison Between Laparoscopic and Robotic Approaches. Cancers, 17(13), 2103. https://doi.org/10.3390/cancers17132103








