Lymphoproliferations in People Living with HIV: Oncogenic Pathways, Diagnostic Challenges, and New Therapeutic Opportunities
Simple Summary
Abstract
1. Introduction
2. Pathogenesis of HIV-Associated Lymphoproliferative Disorders
2.1. Indirect Mechanisms
2.2. Direct Mechanisms
3. Pathologic Features of HIV-Associated Lymphoproliferations
3.1. Diffuse Large B Cell Lymphoma (DLBCL)
3.2. Classic Hodgkin Lymphoma (HL) in PLWH
3.3. Epstein–Barr Virus-Positive Marginal Zone Lymphoma (EBV-MZL)
3.4. HIV-Associated Burkitt Lymphoma (BL)
3.5. Other Rare HIV-Associated Lymphomas
4. Treatment Strategies
4.1. Current Treatment Strategies for HIV-Related NHL
| Treatment | Study Design | Patients N° | Histology % | CR Rate % | PFS % | OS % | I.Death % | Ref. |
|---|---|---|---|---|---|---|---|---|
| HIV-NHL | ||||||||
| R-CHOP-R vs. CHOP | Phase 3 | 150 | DLBCL 81 | 58 vs. 47 | 11.3 vs. 9.5 mos | 28 vs. 35 | 14 vs. 2 | [170] |
| R-CHOP | Phase 2 | 61 | DLBCL 72 | 77 | 69 (2 yr) | 75 (2 yr) | 2 | [166] |
| R-CHOP | Phase 2 | 95 | DLBCL 81 | 69 | NA | 56 (3 yr) | 7 | [169] |
| R-CDE | Phase 2 | 74 | DLBCL 72 | 70 | EFS (2 yr) 52 | 64 (2 yr) | 7 | [165] |
| R-EPOCH vs. EPOCH-R | Rand. Phase 2 | 106 | DLBCL 80 | 73 vs. 55 | 66 vs. 63 (2 yr) | 70 vs. 67 (2 yr) | 10 vs. 7 | [167] |
| SC-EPOCH-RR | Phase 2 | 33 | DLBCL 100 | 91 | 84 (5 yr) | 68 (5 yr) | [168] | |
| Vorinostat-R-EPOCH | Rand. Phase 2 | 90 | DLBCL 100 | 58 vs. 74 | EFS (3 yr) 63 vs. 69 | 70 vs. 77 (3 yr) | NA | [173] |
| R-CODOX-M/IVAC | Phase 2 | 34 | BL 100 | NA | 69 (1 yr) | 69 (2 yr) | 3 | [177] |
| DA-EPOCH-R | Phase 2 | 28 HIV | BL 100 | NA | 85 (4 yr) | NA | NA | [179] |
| HIV-cHL | ||||||||
| ABVD | Retrospective | 93 | cHL | 74 | EFS (5-yr) 59 | 81 (5 yr) | <1 | [182] |
| (a) EF: ABVD (b) EU: BEACOPP (c) Adv: BEACOPP or ABVD | Phase 2 | 108 | cHL | (a) EF: 96 (b) EU: 100 (c) Adv: 86 | 90 (2 yr) | 91 (2 yr) | EF: 4 Adv: 7 | [183] |
| BV-AVD | Phase 2 | 41 | cHL | 76 | 86 (2 yr) | 92 (2 yr) | <1 | [184] |
4.2. Current Treatment Strategies for HIV-Related Classic Hodgkin Lymphoma
4.3. Current Treatment Strategies for HIV-Related Multicentric Castleman Disease
5. Immunotherapeutic Perspectives for Lymphomas in PLWH
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABC | Activated B-cell Like |
| AICDA | Activation-Induced Cytidine Deaminase |
| ASCT | Autologous stem cell transplantation |
| Allo-HCT | Allogeneic Hematopoietic Cell Transplantation |
| ASCT | Autologous Stem Cell Transplantation |
| ATLL | Adult T-cell leukemia/lymphoma |
| BART | BamHI-A region rightward transcript |
| BCR | B Cell Receptor |
| BHRF1 | BamHI-H rightward fragment 1 |
| BST2 | Bone marrow stromal antigen 2 |
| BL | Burkitt Lymphoma |
| cART | Combined Anti-Retroviral Therapy |
| CAR | Chimeric Antigen Receptor |
| CR | Complete Response |
| DA | Dose-adjusted |
| DDI | Drug–Drug Interactions |
| DLBCL | Diffuse Large B Cell Lymphoma |
| EFS | Event-Free Survival |
| EBNA6 | EBV nuclear antigen 6 |
| EBV | Epstein–Barr Virus |
| EC | Extra cavitary |
| EGFR | Epithelial growth factor receptor |
| EFS | Event-free survival |
| GCB | Germinal Center B-cell Like |
| HDC | High-Dose Chemotherapy |
| HHV-8 | Human Herpesvirus-8 |
| HIV | Human Immunodeficiency Virus |
| HL | Hodgkin Lymphoma |
| HR | Hazard ratio |
| HSCT | Hematopoietic Stem Cell Transplantation |
| HTLV-1 | Human T-cell Lymphotropic Virus type 1 |
| KICS | Kaposi’s Sarcoma Inflammatory Cytokine Syndrome |
| KSHV | Kaposi’s Sarcoma-associated Herpes Virus |
| ICIs | Immune Checkpoint Inhibitors |
| IDO | Indoleamine 2,3-dioxygenase |
| IPI | International Prognostic Index |
| irAEs | Immune-Related Adverse Events |
| IRIS | Immune Reconstitution Inflammatory Syndrome |
| LANA | Latency-Associated Nuclear Antigen |
| LMP1 | Latent Membrane Protein 1 |
| MCD | Multicentric Castleman Disease |
| MZL | Marginal Zone Lymphoma |
| NK | Natural killer |
| OS | Overall survival |
| P17 | HIV Matrix Protein p17 |
| PFS | Progression-Free Survival |
| PBL | Plasmablastic Lymphoma |
| PD-1 | Programmed Cell Death 1 |
| PD-L1 | Programmed Cell Death Ligand 1 |
| PEL | Primary Effusion Lymphoma |
| PI3K | Phosphoinositide 3-kinase |
| PLWH | People Living With HIV |
| PCNSL | Primary central Nervous System Lymphoma |
| TAM | Tumor-Associated Macrophage |
| TCR | T cell receptor |
| TME | Tumor Microenvironment |
| TRM | Treatment-related mortality |
| VEGF | Vascular endothelial growth factor |
| vFLIP | Viral FLICE inhibitory protein |
| vIL-6 | Viral interleukin 6 |
| vIRF-3 | Viral interferon regulatory factor 3 |
| Vpu | HIV viral protein U |
| WHO | World Health Organization |
References
- Carbone, A.; Vaccher, E.; Gloghini, A. Hematologic cancers in individuals infected by HIV. Blood 2022, 139, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, R.; Chia, G.; Dalla Pria, A.; Pinato, D.J.; Parker, K.; Nelson, M.; Bower, M. Evolution of HIV-Associated Lymphoma Over 3 Decades. J. Acquir. Immune Defic. Syndr. 2016, 72, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ramírez, R.U.; Shiels, M.S.; Dubrow, R.; Engels, E.A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV 2017, 4, e495–e504. [Google Scholar] [CrossRef]
- Kimani, S.M.; Painschab, M.S.; Horner, M.J.; Muchengeti, M.; Fedoriw, Y.; Shiels, M.S.; Gopal, S. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV 2020, 7, e641–e651. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Grulich, A.E.; Vajdic, C.M. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin. Oncol. 2015, 42, 247–257. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Carbone, A.; Dolcetti, R. Microenvironment and HIV-related lymphomagenesis. Semin. Cancer Biol. 2015, 34, 52–57. [Google Scholar] [CrossRef]
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016, 127, 1403–1409. [Google Scholar] [CrossRef]
- Armani-Tourret, M.; Bone, B.; Tan, T.S.; Sun, W.; Bellefroid, M.; Struyve, T.; Louella, M.; Yu, X.G.; Lichterfeld, M. Immune targeting of HIV-1 reservoir cells: A path to elimination strategies and cure. Nat. Rev. Microbiol. 2024, 22, 328–344. [Google Scholar] [CrossRef]
- Benito, J.M.; Restrepo, C.; García-Foncillas, J.; Rallón, N. Immune checkpoint inhibitors as potential therapy for reverting T-cell exhaustion and reverting HIV latency in people living with HIV. Front. Immunol. 2023, 14, 1270881. [Google Scholar] [CrossRef]
- Makgoeng, S.B.; Bolanos, R.S.; Jeon, C.Y.; Weiss, R.E.; Arah, O.A.; Breen, E.C.; Martínez-Maza, O.; Hussain, S.K. Markers of Immune Activation and Inflammation, and Non-Hodgkin Lymphoma: A Meta-Analysis of Prospective Studies. JNCI Cancer Spectr. 2018, 2, pky082. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Abreu, C.M.; Shirk, E.N.; Gama, L.; Clements, J.E. HIV replication and latency in monocytes and macrophages. Semin. Immunol. 2021, 51, 101472. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.S.; Katsura, C.; Skasko, M.A.; Fitzpatrick, K.; Lau, D.; Ruiz, A.; Stephens, E.B.; Margottin-Goguet, F.; Benarous, R.; Guatelli, J.C. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009, 5, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Jadhav, S.; Arif Khan, A.; Aggarwal, S.K.; Choudhari, R.; Verma, S.; Aggarwal, S.; Gupta, V.; Singh, A.; Nain, S.; et al. APOBEC3, TRIM5α, and BST2 polymorphisms in healthy individuals of various populations with special references to its impact on HIV transmission. Microb. Pathog. 2022, 162, 105326. [Google Scholar] [CrossRef]
- Lê-Bury, G.; Niedergang, F. Defective Phagocytic Properties of HIV-Infected Macrophages: How Might They Be Implicated in the Development of Invasive Salmonella Typhimurium? Front. Immunol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Yao, Z.Q.; Moorman, J.P.; Ning, S. MicroRNA regulation of viral immunity, latency, and carcinogenesis of selected tumor viruses and HIV. Rev. Med. Virol. 2015, 25, 320–341. [Google Scholar] [CrossRef]
- Planès, R.; Bahraoui, E. HIV-1 Tat protein induces the production of IDO in human monocyte derived-dendritic cells through a direct mechanism: Effect on T cells proliferation. PLoS ONE 2013, 8, e74551. [Google Scholar] [CrossRef]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32ra36. [Google Scholar] [CrossRef]
- Terness, P.; Bauer, T.M.; Röse, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef]
- Judge, C.J.; Kostadinova, L.; Sherman, K.E.; Butt, A.A.; Falck-Ytter, Y.; Funderburg, N.T.; Landay, A.L.; Lederman, M.M.; Sieg, S.F.; Sandberg, J.K.; et al. CD56brightNK IL-7Rα expression negatively associates with HCV level, and IL-7-induced NK function is impaired during HCV and HIV infections. J. Leukoc. Biol. 2017, 102, 171–184. [Google Scholar] [CrossRef]
- Lucar, O.; Reeves, R.K.; Jost, S. A Natural Impact: NK Cells at the Intersection of Cancer and HIV Disease. Front. Immunol. 2019, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Cocker, A.T.H.; Liu, F.; Djaoud, Z.; Guethlein, L.A.; Parham, P. CD56-negative NK cells: Frequency in peripheral blood, expansion during HIV-1 infection, functional capacity, and KIR expression. Front. Immunol. 2022, 13, 992723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.; Lu, J.; Chen, Y.F.; Hu, H.Y.; Xu, X.Q.; Fu, G.F. Changes in NK Cell Subsets and Receptor Expressions in HIV-1 Infected Chronic Patients and HIV Controllers. Front. Immunol. 2021, 12, 792775. [Google Scholar] [CrossRef] [PubMed]
- Lurain, K.A.; Ramaswami, R.; Krug, L.T.; Whitby, D.; Ziegelbauer, J.M.; Wang, H.-W.; Yarchoan, R. HIV-associated cancers and lymphoproliferative disorders caused by Kaposi sarcoma herpesvirus and Epstein-Barr virus. Clin. Microbiol. Rev. 2024, 37, e0002223. [Google Scholar] [CrossRef]
- Shindiapina, P.; Ahmed, E.H.; Mozhenkova, A.; Abebe, T.; Baiocchi, R.A. Immunology of EBV-Related Lymphoproliferative Disease in HIV-Positive Individuals. Front. Oncol. 2020, 10, 1723. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A.; Caruso, A.; De Paoli, P.; Dolcetti, R. The impact of EBV and HIV infection on the microenvironmental niche underlying Hodgkin lymphoma pathogenesis. Int. J. Cancer 2017, 140, 1233–1245. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Banerjee, A.; Dass, D.; Mukherjee, S.; Kaul, M.; Harshithkumar, R.; Bagchi, P.; Mukherjee, A. The ‘Oma’s of the Gammas-Cancerogenesis by γ-Herpesviruses. Viruses 2024, 16, 1928. [Google Scholar] [CrossRef]
- Kalchschmidt, J.S.; Bashford-Rogers, R.; Paschos, K.; Gillman, A.C.; Styles, C.T.; Kellam, P.; Allday, M.J. Epstein-Barr virus nuclear protein EBNA3C directly induces expression of AID and somatic mutations in B cells. J. Exp. Med. 2016, 213, 921–928. [Google Scholar] [CrossRef]
- Shponka, V.; Reveles, C.Y.; Alam, S.; Jaramillo, M.; Maguire, A.; Rimsza, L.M.; Kendrick, S. Frequent expression of activation-induced cytidine deaminase in diffuse large B-cell lymphoma tissues from persons living with HIV. AIDS 2020, 34, 2025–2035. [Google Scholar] [CrossRef]
- Whitehurst, C.B.; Rizk, M.; Teklezghi, A.; Spagnuolo, R.A.; Pagano, J.S.; Wahl, A. HIV co-infection augments EBV-induced tumorigenesis in vivo. Front. Virol. 2022, 2, 861628. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Senanayake, S.; Macaranas, I.; Albano, P.M.; Mundo, L.; Fennell, E.; Leoncini, L.; Murray, P. MicroRNA and Other Non-Coding RNAs in Epstein-Barr Virus-Associated Cancers. Cancers 2021, 13, 3909. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, S.; Bastani, M.N. From virus to cancer: Epstein-Barr virus miRNA connection in Burkitt’s lymphoma. Infect. Agent. Cancer 2024, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L.; et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Chadburn, A.; Hyjek, E.M.; Tam, W.; Liu, Y.; Rengifo, T.; Cesarman, E.; Knowles, D.M. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV.; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD). Histopathology 2008, 53, 513–524. [Google Scholar] [CrossRef]
- Du, M.Q.; Liu, H.; Diss, T.C.; Ye, H.; Hamoudi, R.A.; Dupin, N.; Meignin, V.; Oksenhendler, E.; Boshoff, C.; Isaacson, P.G. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 2001, 97, 2130–2136. [Google Scholar] [CrossRef]
- Hu, D.; Wang, V.; Yang, M.; Abdullah, S.; Davis, D.A.; Uldrick, T.S.; Polizzotto, M.N.; Veeranna, R.P.; Pittaluga, S.; Tosato, G.; et al. Induction of Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Viral Interleukin-6 by X-Box Binding Protein 1. J. Virol. 2015, 90, 368–378. [Google Scholar] [CrossRef]
- Aoki, Y.; Yarchoan, R.; Wyvill, K.; Okamoto, S.; Little, R.F.; Tosato, G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood 2001, 97, 2173–2176. [Google Scholar] [CrossRef]
- Liu, C.; Okruzhnov, Y.; Li, H.; Nicholas, J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 2001, 75, 10933–10940. [Google Scholar] [CrossRef]
- Münz, C. Recent advances in animal models of lymphomagenesis caused by human γ-herpesviruses. Curr. Opin. Virol. 2025, 71, 101461. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Straista, M.; Caccuri, F.; Arnaut, N.; Caruso, A.; Slevin, M. Pathological Mechanisms Involved in HIV-Associated Lymphomagenesis: Novel Targeted Therapeutic Approaches. Cells 2025, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.M.; Richardson, J.T.; Buchacz, K.; Chmiel, J.S.; Durham, M.D.; Palella, F.J.; Wendrow, A.; Wood, K.; Young, B.; Brooks, J.T.; et al. Investigators Immune reconstitution inflammatory syndrome: Incidence implications for mortality. AIDS 2012, 26, 721–730. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Wang, X. Targeting the tumor microenvironment in B-cell lymphoma: Challenges and opportunities. J. Hematol. Oncol. 2021, 14, 125. [Google Scholar] [CrossRef]
- Alsharif, R.; Dunleavy, K. Burkitt Lymphoma and Other High-Grade B-Cell Lymphomas with or without MYC, BCL2, and/or BCL6 Rearrangements. Hematol. Oncol. Clin. North. Am. 2019, 33, 587–596. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Z.; Zeng, D.; Song, S.; Yang, Y.; Wang, A.; Xu, J.; Guo, W.; Wu, M.; Shi, Y.; et al. High expression of HIV-1 matrix protein p17 in both lymphoma and lymph node tissues of AIDS patients. Pathol. Res. Pract. 2022, 237, 154061. [Google Scholar] [CrossRef]
- Lazzi, S.; Bellan, C.; De Falco, G.; Cinti, C.; Ferrari, F.; Nyongo, A.; Claudio, P.P.; Tosi, G.M.; Vatti, R.; Gloghini, A.; et al. Expression of RB2/p130 tumor-suppressor gene in AIDS-related non-Hodgkin’s lymphomas: Implications for disease pathogenesis. Hum. Pathol. 2002, 33, 723–731. [Google Scholar] [CrossRef]
- Scala, G.; Ruocco, M.R.; Ambrosino, C.; Mallardo, M.; Giordano, V.; Baldassarre, F.; Dragonetti, E.; Quinto, I.; Venuta, S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J. Exp. Med. 1994, 179, 961–971. [Google Scholar] [CrossRef]
- Blazevic, V.; Heino, M.; Lagerstedt, A.; Ranki, A.; Krohn, K.J. Interleukin-10 gene expression induced by HIV-1 Tat and Rev in the cells of HIV-1 infected individuals. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, A.; Morettini, F.; Gazaneo, S.; Mundo, L.; Onnis, A.; Mannucci, S.; Rogena, E.A.; Bellan, C.; Leoncini, L.; De Falco, G. HIV-1 Tat induces DNMT over-expression through microRNA dysregulation in HIV-related non-Hodgkin lymphomas. Infect. Agents Cancer 2014, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Valyaeva, A.A.; Tikhomirova, M.A.; Potashnikova, D.M.; Bogomazova, A.N.; Snigiryova, G.P.; Penin, A.A.; Logacheva, M.D.; Arifulin, E.A.; Shmakova, A.A.; Germini, D.; et al. Ectopic expression of HIV-1 Tat modifies gene expression in cultured B cells: Implications for the development of B-cell lymphomas in HIV-1-infected patients. PeerJ 2022, 10, e13986. [Google Scholar] [CrossRef] [PubMed]
- Akbay, B.; Germini, D.; Bissenbaev, A.K.; Musinova, Y.R.; Sheval, E.V.; Vassetzky, Y.; Dokudovskaya, S. HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells. Int. J. Mol. Sci. 2021, 22, 1588. [Google Scholar] [CrossRef] [PubMed]
- Sall, F.B.; El Amine, R.; Markozashvili, D.; Tsfasman, T.; Oksenhendler, E.; Lipinski, M.; Vassetzky, Y.; Germini, D. HIV-1 Tat protein induces aberrant activation of AICDA in human B-lymphocytes from peripheral blood. J. Cell. Physiol. 2019, 234, 15678–15685. [Google Scholar] [CrossRef]
- Germini, D.; Tsfasman, T.; Klibi, M.; El-Amine, R.; Pichugin, A.; Iarovaia, O.V.; Bilhou-Nabera, C.; Subra, F.; Bou Saada, Y.; Sukhanova, A.; et al. HIV Tat induces a prolonged MYC relocalization next to IGH in circulating B-cells. Leukemia 2017, 31, 2515–2522. [Google Scholar] [CrossRef]
- Shmakova, A.; Hugot, C.; Kozhevnikova, Y.; Schwager Karpukhina, A.; Tsimailo, I.; Gérard, L.; Boutboul, D.; Oksenhendler, E.; Szewczyk-Roszczenko, O.; Roszczenko, P.; et al. Chronic HIV-1 Tat action induces HLA-DR downregulation in B cells: A mechanism for lymphoma immune escape in people living with HIV. J. Med. Virol. 2024, 96, e29423. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.K.; Sangiorgi, F.; Wu, L.Y.; Pattengale, P.K.; Hinton, D.R.; Gill, P.S.; Maxson, R. Expression of the human immunodeficiency virus-Tat gene in lymphoid tissues of transgenic mice is associated with B-cell lymphoma. Blood 1999, 94, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mdletshe, N.; Nel, A.; Shires, K.; Mowla, S. HIV Nef enhances the expression of oncogenic c-MYC and activation-induced cytidine deaminase in Burkitt lymphoma cells, promoting genomic instability. Infect. Agent. Cancer 2020, 15, 54. [Google Scholar] [CrossRef]
- Caccuri, F.; Iaria, M.L.; Campilongo, F.; Varney, K.; Rossi, A.; Mitola, S.; Schiarea, S.; Bugatti, A.; Mazzuca, P.; Giagulli, C.; et al. Cellular aspartyl proteases promote the unconventional secretion of biologically active HIV-1 matrix protein p17. Sci. Rep. 2016, 6, 38027. [Google Scholar] [CrossRef]
- Popovic, M.; Tenner-Racz, K.; Pelser, C.; Stellbrink, H.J.; van Lunzen, J.; Lewis, G.; Kalyanaraman, V.S.; Gallo, R.C.; Racz, P. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2005, 102, 14807–14812. [Google Scholar] [CrossRef]
- Curreli, S.; Krishnan, S.; Reitz, M.; Lunardi-Iskandar, Y.; Lafferty, M.K.; Garzino-Demo, A.; Zella, D.; Gallo, R.C.; Bryant, J. B cell lymphoma in HIV transgenic mice. Retrovirology 2013, 10, 92. [Google Scholar] [CrossRef]
- Dolcetti, R.; Giagulli, C.; He, W.; Selleri, M.; Caccuri, F.; Eyzaguirre, L.M.; Mazzuca, P.; Corbellini, S.; Campilongo, F.; Marsico, S.; et al. Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 14331–14336. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, C.; Marsico, S.; Magiera, A.K.; Bruno, R.; Caccuri, F.; Barone, I.; Fiorentini, S.; Andò, S.; Caruso, A. Opposite effects of HIV-1 p17 variants on PTEN activation and cell growth in B cells. PLoS ONE 2011, 6, e17831. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, C.; D’Ursi, P.; He, W.; Zorzan, S.; Caccuri, F.; Varney, K.; Orro, A.; Marsico, S.; Otjacques, B.; Laudanna, C.; et al. A single amino acid substitution confers B-cell clonogenic activity to the HIV-1 matrix protein p17. Sci. Rep. 2017, 7, 6555. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, C.; Caccuri, F.; Zorzan, S.; Bugatti, A.; Zani, A.; Filippini, F.; Manocha, E.; D’Ursi, P.; Orro, A.; Dolcetti, R.; et al. B-cell clonogenic activity of HIV-1 p17 variants is driven by PAR1-mediated EGF transactivation. Cancer Gene Ther. 2021, 28, 649–666. [Google Scholar] [CrossRef]
- Caccuri, F.; Messali, S.; Zani, A.; Campisi, G.; Giovanetti, M.; Zanussi, S.; Vaccher, E.; Fabris, S.; Bugatti, A.; Focà, E.; et al. HIV-1 mutants expressing B cell clonogenic matrix protein p17 variants are increasing their prevalence worldwide. Proc. Natl. Acad. Sci USA 2022, 119, e2122050119. [Google Scholar] [CrossRef]
- Martínez, L.E.; Comin-Anduix, B.; Güemes-Aragon, M.; Ibarrondo, J.; Detels, R.; Mimiaga, M.J.; Epeldegui, M. Characterization of unique B-cell populations in the circulation of people living with HIV prior to non-Hodgkin lymphoma diagnosis. Front. Immunol. 2024, 15, 1441994. [Google Scholar] [CrossRef]
- Martin, G.; Roy, J.; Barat, C.; Ouellet, M.; Gilbert, C.; Tremblay, M.J. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J. Virol. 2007, 81, 5872–5881. [Google Scholar] [CrossRef]
- Imbeault, M.; Ouellet, M.; Giguère, K.; Bertin, J.; Bélanger, D.; Martin, G.; Tremblay, M.J. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J. Virol. 2011, 85, 2189–2200. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bunting, S.; Feldhahn, N.; Bothmer, A.; Camps, J.; Deroubaix, S.; McBride, K.M.; Klein, I.A.; Stone, G.; Eisenreich, T.R.; et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol. Cell 2009, 36, 631–641. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Zhang, X.; Guo, C.; Zhang, Y. Virus-driven dysregulation of the BCR pathway: A potential mechanism for the high prevalence of HIV related B-cell lymphoma. Ann. Hematol. 2024, 103, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.M.; Cesarman, E.; Chadburn, A.; Frizzera, G.; Chen, J.; Rose, E.A.; Michler, R.E. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 1995, 85, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.W.S.; Chadburn, A.; Ferry, J.A. Post-transplant lymphoproliferative disorders. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.C., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., Orazi, A., et al., Eds.; IARC: Lyon, France, 2017; pp. 453–462. [Google Scholar]
- Natkunam, Y.; Goodlad, J.R.; Chadburn, A.; de Jong, D.; Gratzinger, D.; Chan, J.K.; Said, J.; Jaffe, E.S. EBV-Positive B-Cell Proliferations of Varied Malignant Potential: 2015 SH/EAHP Workshop Report-Part 1. Am. J. Clin. Pathol. 2017, 147, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Gratzinger, D.; Jaffe, E.S.; Chadburn, A.; Chan, J.K.; de Jong, D.; Goodlad, J.R.; Said, J.; Natkunam, Y. Primary/Congenital Immunodeficiency: 2015 SH/EAHP Workshop Report-Part 5. Am. J. Clin. Pathol. 2017, 147, 204–216. [Google Scholar] [CrossRef]
- Nador, R.G.; Chadburn, A.; Gundappa, G.; Cesarman, E.; Said, J.W.; Knowles, D.M. Human immunodeficiency virus (HIV)-associated polymorphic lymphoproliferative disorders. Am. J. Surg. Pathol. 2003, 27, 293–302. [Google Scholar] [CrossRef]
- Dojcinov, S.D.; Venkataraman, G.; Pittaluga, S.; Wlodarska, I.; Schrager, J.A.; Raffeld, M.; Hills, R.K.; Jaffe, E.S. Age-related EBV-associated lymphoproliferative disorders in the Western population: A spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011, 117, 4726–4735. [Google Scholar] [CrossRef]
- Dojcinov, S.D.; Venkataraman, G.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. EBV positive mucocutaneous ulcer—A study of 26 cases associated with various sources of immunosuppression. Am. J. Surg. Pathol. 2010, 34, 405–417. [Google Scholar] [CrossRef]
- Hart, M.; Thakral, B.; Yohe, S.; Balfour, H.H., Jr.; Singh, C.; Spears, M.; McKenna, R.W. EBV-positive mucocutaneous ulcer in organ transplant recipients: A localized indolent posttransplant lymphoproliferative disorder. Am. J. Surg. Pathol. 2014, 38, 1522–1529. [Google Scholar] [CrossRef]
- Pina-Oviedo, S.; Miranda, R.N.; Medeiros, L.J. Cancer Therapy-associated Lymphoproliferative Disorders: An Under-recognized Type of Immunodeficiency-associated Lymphoproliferative Disorder. Am. J. Surg. Pathol. 2018, 42, 116–129. [Google Scholar] [CrossRef]
- Carbone, A.; Chadburn, A.; Gloghini, A.; Vaccher, E.; Bower, M. Immune deficiency/dysregulation-associated lymphoproliferative disorders. Revised classification and management. Blood Rev. 2024, 64, 101167. [Google Scholar] [CrossRef]
- Novoa-Takara, L.; Perkins, S.L.; Qi, D.; Shidham, V.B.; Vesole, D.H.; Hariharan, S.; Luo, Y.; Ewton, A.; Chang, C.C. Histogenetic phenotypes of B cells in posttransplant lymphoproliferative disorders by immunohistochemical analysis correlate with transplant type: Solid organ vs hematopoietic stem cell transplantation. Am. J. Clin. Pathol. 2005, 123, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, Z.; Hassan, A.; Pathan, M.H.; White, G. Flow cytometric evaluation of posttransplant B-cell lymphoproliferative disorders. Arch. Pathol. Lab. Med. 2004, 128, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Rybicki, L.; Smith, M.D.; Abrahams, N.A.; Tubbs, R.R.; Pettay, J.; Farver, C.F.; Hsi, E.D. Posttransplant lymphoproliferative disorders in lung transplant patients: The Cleveland Clinic experience. Mod. Pathol. 2002, 15, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Arakawa, F.; Kiyasu, J.; Sato, K.; Miyoshi, H.; Niino, D.; Kimura, Y.; Takeuchi, M.; Yoshida, M.; Ishibashi, Y.; et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: Histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur. J. Haematol. 2013, 91, 20–28. [Google Scholar] [CrossRef]
- Bräuninger, A.; Spieker, T.; Mottok, A.; Baur, A.S.; Küppers, R.; Hansmann, M.L. Epstein-Barr virus (EBV)-positive lymphoproliferations in post-transplant patients show immunoglobulin V gene mutation patterns suggesting interference of EBV with normal B cell differentiation processes. Eur. J. Immunol. 2003, 33, 1593–1602. [Google Scholar] [CrossRef]
- Tokuhira, M.; Tamaru, J.I.; Kizaki, M. Clinical management for other iatrogenic immunodeficiency-associated lymphoproliferative disorders. J. Clin. Exp. Hematop. 2019, 59, 72–92. [Google Scholar] [CrossRef]
- Chadburn, A.; Gloghini, A.; Carbone, A. Classification of B-Cell Lymphomas and Immunodeficiency-Related Lymphoproliferations: What’s New? Hemato 2023, 4, 26–41. [Google Scholar] [CrossRef]
- Said, J.C.E.; Rosenwald, A.; Harris, N.L. Lymphomas associated with HIV infection. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.C., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., Orazi, A., et al., Eds.; IARC: Lyon, France, 2017; pp. 449–452. [Google Scholar]
- Chadburn, A.; Abdul-Nabi, A.M.; Teruya, B.S.; Lo, A.A. Lymphoid proliferations associated with human immunodeficiency virus infection. Arch. Pathol. Lab. Med. 2013, 137, 360–370. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. Part B: Biological Agents; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012; Volume 100. [Google Scholar]
- Carbone, A.; Borok, M.; Damania, B.; Gloghini, A.; Polizzotto, M.N.; Jayanthan, R.K.; Fajgenbaum, D.C.; Bower, M. Castleman disease. Nat. Rev. Dis. Primers 2021, 7, 84. [Google Scholar] [CrossRef]
- Chapman, J.R.; Bouska, A.C.; Zhang, W.; Alderuccio, J.P.; Lossos, I.S.; Rimsza, L.M.; Maguire, A.; Yi, S.; Chan, W.C.; Vega, F.; et al. EBV-positive HIV-associated diffuse large B cell lymphomas are characterized by JAK/STAT (STAT3) pathway mutations and unique clinicopathologic features. Br. J. Haematol. 2021, 194, 870–878. [Google Scholar] [CrossRef]
- Besson, C.; Lancar, R.; Prevot, S.; Algarte-Genin, M.; Delobel, P.; Bonnet, F.; Meyohas, M.C.; Partisani, M.; Oberic, L.; Gabarre, J.; et al. Outcomes for HIV-associated diffuse large B-cell lymphoma in the modern combined antiretroviral therapy era. AIDS 2017, 31, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.J.; Tapia, G.; Morgades, M.; Muncunill, J.; Muñoz-Marmol, A.M.; Montoto, S.; Gribben, J.G.; Calaminici, M.; Martinez, A.; Gonzalez-Farre, B.; et al. Using the Lymph2Cx assay for assessing cell-of-origin subtypes of HIV-related diffuse large B-cell lymphoma. Leuk. Lymphoma 2019, 60, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Liapis, K.; Clear, A.; Owen, A.; Coutinho, R.; Greaves, P.; Lee, A.M.; Montoto, S.; Calaminici, M.; Gribben, J.G. The microenvironment of AIDS-related diffuse large B-cell lymphoma provides insight into the pathophysiology and indicates possible therapeutic strategies. Blood 2013, 122, 424–433. [Google Scholar] [CrossRef]
- Baptista, M.J.; Tapia, G.; Muñoz-Marmol, A.M.; Muncunill, J.; Garcia, O.; Montoto, S.; Gribben, J.G.; Calaminici, M.; Martinez, A.; Veloza, L.; et al. Genetic and phenotypic characterisation of HIV-associated aggressive B-cell non-Hodgkin lymphomas, which do not occur specifically in this population: Diagnostic and prognostic implications. Histopathology 2022, 81, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Wiggill, T.; Mia, Z.; Patel, M. Tumour-associated macrophages in diffuse large B-cell lymphoma: The prognostic and therapeutic impact in a South African centre with high HIV seroprevalence. Immunol. Res. 2024, 72, 1393–1403. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Epeldegui, M.; Conti, D.V.; Guo, Y.; Cozen, W.; Penichet, M.L.; Martínez-Maza, O. Elevated numbers of PD-L1 expressing B cells are associated with the development of AIDS-NHL. Sci. Rep. 2019, 9, 9371. [Google Scholar] [CrossRef]
- Goodman, A.; Patel, S.P.; Kurzrock, R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol. 2017, 14, 203–220. [Google Scholar] [CrossRef]
- Kowalkowski, M.A.; Mims, M.P.; Amiran, E.S.; Lulla, P.; Chiao, E.Y. Effect of immune reconstitution on the incidence of HIV-related Hodgkin lymphoma. PLoS ONE 2013, 8, e77409. [Google Scholar] [CrossRef]
- Carroll, V.; Garzino-Demo, A. HIV-associated lymphoma in the era of combination antiretroviral therapy: Shifting the immunological landscape. Pathog. Dis. 2015, 73, ftv044. [Google Scholar] [CrossRef]
- Hleyhel, M.; Bouvier, A.M.; Belot, A.; Tattevin, P.; Pacanowski, J.; Genet, P.; De Castro, N.; Berger, J.L.; Dupont, C.; Lavolé, A.; et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: Results from a French cohort. AIDS 2014, 28, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Kowalkowski, M.A.; Mims, M.A.; Day, R.S.; Du, X.L.; Chan, W.; Chiao, E.Y. Longer duration of combination antiretroviral therapy reduces the risk of Hodgkin lymphoma: A cohort study of HIV-infected male veterans. Cancer Epidemiol. 2014, 38, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Said, J.W. Immunodeficiency-related Hodgkin lymphoma and its mimics. Adv. Anat. Pathol. 2007, 14, 189–194. [Google Scholar] [CrossRef]
- Carbone, A.; Spina, M.; Gloghini, A.; Tirelli, U. Classical Hodgkin’s lymphoma arising in different host’s conditions: Pathobiology parameters, therapeutic options, and outcome. Am. J. Hematol. 2011, 86, 170–179. [Google Scholar] [CrossRef]
- De Paoli, P.; Carbone, A. Microenvironmental abnormalities induced by viral cooperation: Impact on lymphomagenesis. Semin. Cancer Biol. 2015, 34, 70–80. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A.; Serraino, D.; Spina, M. HIV-associated Hodgkin lymphoma. Curr. Opin. HIV AIDS 2009, 4, 3–10. [Google Scholar] [CrossRef]
- Koulis, A.; Trivedi, P.; Ibrahim, H.; Bower, M.; Naresh, K.N. The role of the microenvironment in human immunodeficiency virus-associated classical Hodgkin lymphoma. Histopathology 2014, 65, 749–756. [Google Scholar] [CrossRef]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef]
- Alfano, M.; Locatelli, I.; D’Arrigo, C.; Mora, M.; Vozzi, G.; De Acutis, A.; Pece, R.; Tavella, S.; Costa, D.; Poggi, A.; et al. Lysyl-Oxidase Dependent Extracellular Matrix Stiffness in Hodgkin Lymphomas: Mechanical and Topographical Evidence. Cancers 2022, 14, 259. [Google Scholar] [CrossRef]
- Veldman, J.; Visser, L.; Huberts-Kregel, M.; Muller, N.; Hepkema, B.; van den Berg, A.; Diepstra, A. Rosetting T cells in Hodgkin lymphoma are activated by immunological synapse components HLA class II and CD58. Blood 2020, 136, 2437–2441. [Google Scholar] [CrossRef]
- Chantziou, A.; Brenna, C.; Ioannidou, K.; Chen, O.Y.; Korkolopoulou, P.; Antoniadou, A.; Psichogiou, M.; Papaioannou, M.; Tsirigotis, P.; Foukas, P.G.; et al. HIV infection is associated with compromised tumor microenvironment adaptive immune reactivity in Hodgkin lymphoma. Blood Adv. 2024, 8, 6215–6231. [Google Scholar] [CrossRef] [PubMed]
- Dukers, D.F.; Meij, P.; Vervoort, M.B.; Vos, W.; Scheper, R.J.; Meijer, C.J.; Bloemena, E.; Middeldorp, J.M. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J. Immunol. 2000, 165, 663–670. [Google Scholar] [CrossRef] [PubMed]
- de Jong, D.; Roemer, M.G.; Chan, J.K.; Goodlad, J.; Gratzinger, D.; Chadburn, A.; Jaffe, E.S.; Said, J.; Natkunam, Y. B-Cell and Classical Hodgkin Lymphomas Associated With Immunodeficiency: 2015 SH/EAHP Workshop Report-Part 2. Am. J. Clin. Pathol. 2017, 147, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.E.; Swerdlow, S.H.; Craig, F.E.; Surti, U.; Cook, J.R.; Nalesnik, M.A.; Lowe, C.; Wood, K.M.; Bacon, C.M. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: A distinct type of posttransplant lymphoproliferative disorder? Am. J. Surg. Pathol. 2011, 35, 807–815. [Google Scholar] [CrossRef]
- Gong, S.; Crane, G.M.; McCall, C.M.; Xiao, W.; Ganapathi, K.A.; Cuka, N.; Davies-Hill, T.; Xi, L.; Raffeld, M.; Pittaluga, S.; et al. Expanding the Spectrum of EBV-positive Marginal Zone Lymphomas: A Lesion Associated With Diverse Immunodeficiency Settings. Am. J. Surg. Pathol. 2018, 42, 1306–1316. [Google Scholar] [CrossRef]
- Guech-Ongey, M.; Simard, E.P.; Anderson, W.F.; Engels, E.A.; Bhatia, K.; Devesa, S.S.; Mbulaiteye, S.M. AIDS-related Burkitt lymphoma in the United States: What do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010, 116, 5600–5604. [Google Scholar] [CrossRef]
- Han, X.; Jemal, A.; Hulland, E.; Simard, E.P.; Nastoupil, L.; Ward, E.; Flowers, C.R. HIV Infection and Survival of Lymphoma Patients in the Era of Highly Active Antiretroviral Therapy. Cancer Epidemiol. Biomark. Prev. 2017, 26, 303–311. [Google Scholar] [CrossRef]
- Mbulaiteye, S.M.; Pullarkat, S.T.; Nathwani, B.N.; Weiss, L.M.; Rao, N.; Emmanuel, B.; Lynch, C.F.; Hernandez, B.; Neppalli, V.; Hawes, D.; et al. Epstein-Barr virus patterns in US Burkitt lymphoma tumors from the SEER residual tissue repository during 1979-2009. APMIS 2014, 122, 5–15. [Google Scholar] [CrossRef]
- Alves de Souza Rios, L.; Mapekula, L.; Mdletshe, N.; Chetty, D.; Mowla, S. HIV-1 Transactivator of Transcription (Tat) Co-operates With AP-1 Factors to Enhance c-MYC Transcription. Front. Cell Dev. Biol. 2021, 9, 693706. [Google Scholar] [CrossRef]
- D’Angelo, C.R. Diagnostic, Pathologic, and Therapeutic Considerations for Primary CNS Lymphoma. JCO Oncol. Pract. 2024, 20, 195–202. [Google Scholar] [CrossRef]
- Li, J.W.; Peng, H.L.; Zhou, X.Y.; Wang, J.J. Plasmablastic lymphoma: Current knowledge and future directions. Front. Immunol. 2024, 15, 1354604. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, Y.; Shu, X.; Li, Y.; Zhang, X.; Wang, C.; He, S.; Li, J.; Li, T.; Liu, T.; et al. Molecular mechanisms of viral oncogenesis in haematological malignancies: Perspectives from metabolic reprogramming, epigenetic regulation and immune microenvironment remodeling. Exp. Hematol. Oncol. 2025, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Chadburn, A.; Rubinstein, P.G. KSHV/HHV8-mediated hematologic diseases. Blood 2022, 139, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, V.; Scalzo, C.C.; Danese, C.; Vacca, K.; Pistilli, A.; Lo Coco, F. Human herpes virus-8 associated primary effusion lymphoma of the pleural cavity in HIV-negative elderly men. Eur. Respir. J. 1999, 14, 1231–1234. [Google Scholar] [CrossRef]
- Carbone, A.; De Paoli, P.; Gloghini, A.; Vaccher, E. KSHV-associated multicentric Castleman disease: A tangle of different entities requiring multitarget treatment strategies. Int. J. Cancer 2015, 137, 251–261. [Google Scholar] [CrossRef]
- Ramaswami, R.; Lurain, K.; Polizzotto, M.N.; Ekwede, I.; Waldon, K.; Steinberg, S.M.; Mangusan, R.; Widell, A.; Rupert, A.; George, J.; et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Adv. 2021, 5, 1660–1670. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef]
- Trickey, A.; McGinnis, K.; Gill, M.J.; Abgrall, S.; Berenguer, J.; Wyen, C.; Hessamfar, M.; Reiss, P.; Kusejko, K.; Silverberg, M.J.; et al. Longitudinal trends in causes of death among adults with HIV on antiretroviral therapy in Europe and North America from 1996 to 2020: A collaboration of cohort studies. Lancet HIV 2024, 11, e176–e185. [Google Scholar] [CrossRef]
- Tusch, E.; Ryom, L.; Pelchen-Matthews, A.; Mocroft, A.; Elbirt, D.; Oprea, C.; Günthard, H.F.; Staehelin, C.; Zangerle, R.; Suarez, I.; et al. Trends in Mortality in People With HIV From 1999 through 2020: A Multicohort Collaboration. Clin. Infect. Dis. 2024, 79, 1242–1257. [Google Scholar] [CrossRef]
- Castillo, J.; Perez, K.; Milani, C.; Dezube, B.J.; Pantanowitz, L. Peripheral T-cell lymphomas in HIV-infected individuals: A comprehensive review. J. HIV Ther. 2009, 14, 34–40. [Google Scholar] [PubMed]
- Perez, K.; Castillo, J.; Dezube, B.J.; Pantanowitz, L. Human Immunodeficiency Virus-associated anaplastic large cell lymphoma. Leuk. Lymphoma 2010, 51, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Arzoo, K.K.; Bu, X.; Espina, B.M.; Seneviratne, L.; Nathwani, B.; Levine, A.M. T-cell lymphoma in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2004, 36, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Biggar, R.J.; Chaturvedi, A.K.; Goedert, J.J.; Engels, E.A. AIDS-related cancer severity of immunosuppression in persons with AIDS. J. Natl. Cancer Inst. 2007, 99, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, D.; Taylor, G.P. Human T-lymphotropic virus/HIV co-infection: A clinical review. Curr. Opin. Infect. Dis. 2014, 27, 16–28. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Alves, P.D.S. Contribution of the Epstein-Barr virus to the oncogenesis of mature T-cell lymphoproliferative neoplasms. Front. Oncol. 2023, 13, 1240359. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Jakobsen, L.H.; Collins, G.P.; Cwynarski, K.; Bachanova, V.; Blum, K.A.; Boughan, K.M.; Bower, M.; Dalla Pria, A.; Danilov, A.; et al. Burkitt Lymphoma International Prognostic Index. J. Clin. Oncol. 2021, 39, 1129–1138. [Google Scholar] [CrossRef]
- Lipscomb, J.; Switchenko, J.M.; Flowers, C.R.; Gillespie, T.W.; Wortley, P.M.; Bayakly, A.R.; Almon, L.; Ward, K.C. Impact of multi-agent systemic therapy on all-cause and disease-specific survival for people living with HIV who are diagnosed with non-Hodgkin lymphoma: Population-based analyses from the state of Georgia. Leuk. Lymphoma 2023, 64, 151–160. [Google Scholar] [CrossRef]
- Atallah-Yunes, S.A.; Murphy, D.J.; Noy, A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020, 7, e594–e600. [Google Scholar] [CrossRef]
- Di Ciaccio, P.R.; Polizzotto, M.N.; Cwynarski, K.; Gerrie, A.S.; Burton, C.; Bower, M.; Kuruvilla, J.; Montoto, S.; McKay, P.; Fox, C.P.; et al. The influence of immunodeficiency, disease features, and patient characteristics on survival in plasmablastic lymphoma. Blood 2024, 143, 152–165. [Google Scholar] [CrossRef]
- Manyau, M.C.P.; Zambuko, B.; Chatambudza, M.; Zhou, D.T.; Manasa, J. Still Far to Go With Characterisation of Molecular and Genetic Features of Diffuse Large B-Cell Lymphoma in People Living With HIV: A Scoping Review. Oncol. Rev. 2024, 18, 1375291. [Google Scholar] [CrossRef]
- Lurain, K.; Ramaswami, R.; Oksenhendler, E.; Boutboul, D.; Dalla Pria, A.; Ulrich, L.; Shanmugasundaram, K.; Uldrick, T.S.; Bower, M.; Yarchoan, R.; et al. Primary Effusion Lymphoma Prognostic Score (PEL-PS): A Validated International Prognostic Score in HIV-Associated Primary Effusion Lymphoma. Am. J. Hematol. 2025, 100, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Coghill, A.E.; Shiels, M.S.; Suneja, G.; Engels, E.A. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J. Clin. Oncol. 2015, 33, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Royse, K.E.; Dong, Y.; Chang, E.; Raychaudhury, S.; Kramer, J.; White, D.L.; Chiao, E. Stable Incidence and Poor Survival for HIV-Related Burkitt Lymphoma Among the US Veteran Population During the Antiretroviral Era. J. Acquir. Immune Defic. Syndr. 2020, 84, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Horner, M.J.; Shiels, M.S.; Pfeiffer, R.M.; Engels, E.A. Deaths Attributable to Cancer in the US Human Immunodeficiency Virus Population During 2001-2015. Clin. Infect. Dis. 2021, 72, e224–e231. [Google Scholar] [CrossRef]
- McGee-Avila, J.K.; Suneja, G.; Engels, E.A.; Rositch, A.F.; Horner, M.J.; Luo, Q.; Shiels, M.S.; Islam, J.Y. Cancer Treatment Disparities in People With HIV in the United States, 2001-2019. J. Clin. Oncol. 2024, 42, 1810–1820. [Google Scholar] [CrossRef]
- Wieland, C.M.; Tuin, A.M.; Dort, E.J.; Hall, A.G.; Krishnan, M.; Velagapudi, M. Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV-A National Cancer Database (NCDB) Study. Cancers 2024, 16, 1397. [Google Scholar] [CrossRef]
- Noy, A. Optimizing treatment of HIV-associated lymphoma. Blood 2019, 134, 1385–1394. [Google Scholar] [CrossRef]
- Vaccher, E.; Gloghini, A.; Carbone, A. HIV-related lymphomas. Curr. Opin. Oncol. 2022, 34, 439–445. [Google Scholar] [CrossRef]
- Lurain, K. Treating Cancer in People With HIV. J. Clin. Oncol. 2023, 41, 3682–3688. [Google Scholar] [CrossRef]
- Barta, S.K.; Xue, X.; Wang, D.; Tamari, R.; Lee, J.Y.; Mounier, N.; Kaplan, L.D.; Ribera, J.M.; Spina, M.; Tirelli, U.; et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: A pooled analysis of 1546 patients. Blood 2013, 122, 3251–3262. [Google Scholar] [CrossRef]
- Tan, C.R.C.; Barta, S.K.; Lee, J.; Rudek, M.A.; Sparano, J.A.; Noy, A. Combination antiretroviral therapy accelerates immune recovery in patients with HIV-related lymphoma treated with EPOCH: A comparison within one prospective trial AMC034. Leuk. Lymphoma 2018, 59, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Olin, J.L.; Klibanov, O.; Chan, A.; Spooner, L.M. Managing Pharmacotherapy in People Living With HIV and Concomitant Malignancy. Ann. Pharmacother. 2019, 53, 812–832. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Schwenk, A.; Radford, M.; Myland, M.; Taylor, S.; Darley, A.; Barnes, J.; Fox, A.; Grimson, F.; Reeves, I.; et al. Polypharmacy and potential drug-drug interactions for people with HIV in the UK from the Climate-HIV database. HIV Med. 2020, 21, 471–480. [Google Scholar] [CrossRef]
- d’Arminio Monforte, A.; Bonnet, F.; Bucher, H.C.; Pourcher, V.; Pantazis, N.; Pelchen-Matthews, A.; Touloumi, G.; Wolf, E. What do the changing patterns of comorbidity burden in people living with HIV mean for long-term management? Perspectives from European HIV cohorts. HIV Med. 2020, 21 (Suppl. S2), 3–16. [Google Scholar] [CrossRef]
- Dalla Pria, A.; Merchant, S.; Bower, M. Oncological challenges for an ageing population living with HIV. AIDS 2017, 31 (Suppl. S2), S185–S189. [Google Scholar] [CrossRef]
- Mukhtar, F.; Ilozumba, M.; Utuama, O.; Cimenler, O. Change in Pattern of Secondary Cancers After Kaposi Sarcoma in the Era of Antiretroviral Therapy. JAMA Oncol. 2018, 4, 48–53. [Google Scholar] [CrossRef]
- Hessol, N.A.; Whittemore, H.; Vittinghoff, E.; Hsu, L.C.; Ma, D.; Scheer, S.; Schwarcz, S.K. Incidence of first and second primary cancers diagnosed among people with HIV, 1985–2013: A population-based, registry linkage study. Lancet HIV 2018, 5, e647–e655. [Google Scholar] [CrossRef]
- Mahale, P.; Ugoji, C.; Engels, E.A.; Shiels, M.S.; Peprah, S.; Morton, L.M. Cancer risk following lymphoid malignancies among HIV-infected people. AIDS 2020, 34, 1237–1245. [Google Scholar] [CrossRef]
- Abrahão, R.; Li, Q.W.; Malogolowkin, M.H.; Alvarez, E.M.; Ribeiro, R.C.; Wun, T.; Keegan, T.H.M. Chronic medical conditions and late effects following non-Hodgkin lymphoma in HIV-uninfected and HIV-infected adolescents and young adults: A population-based study. Br. J. Haematol. 2020, 190, 371–384. [Google Scholar] [CrossRef]
- Poizot-Martin, I.; Lions, C.; Delpierre, C.; Makinson, A.; Allavena, C.; Fresard, A.; Brégigeon, S.; Rojas Rojas, T.; Delobel, P.; Group The Dat’AIDS Study. Prevalence and Spectrum of Second Primary Malignancies among People Living with HIV in the French Dat’AIDS Cohort. Cancers 2022, 14, 401. [Google Scholar] [CrossRef]
- Schommers, P.; Hentrich, M.; Hoffmann, C.; Gillor, D.; Zoufaly, A.; Jensen, B.; Bogner, J.R.; Thoden, J.; Wasmuth, J.C.; Wolf, T.; et al. Survival of AIDS-related diffuse large B-cell lymphoma, Burkitt lymphoma, and plasmablastic lymphoma in the German HIV Lymphoma Cohort. Br. J. Haematol. 2015, 168, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Jaeger, U.; Sparano, J.A.; Talamini, R.; Simonelli, C.; Michieli, M.; Rossi, G.; Nigra, E.; Berretta, M.; Cattaneo, C.; et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: Pooled results from 3 phase 2 trials. Blood 2005, 105, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Boué, F.; Gabarre, J.; Gisselbrecht, C.; Reynes, J.; Cheret, A.; Bonnet, F.; Billaud, E.; Raphael, M.; Lancar, R.; Costagliola, D. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J. Clin. Oncol. 2006, 24, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Lee, J.Y.; Kaplan, L.D.; Levine, A.M.; Ramos, J.C.; Ambinder, R.F.; Wachsman, W.; Aboulafia, D.; Noy, A.; Henry, D.H.; et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010, 115, 3008–3016. [Google Scholar] [CrossRef]
- Dunleavy, K.; Little, R.F.; Pittaluga, S.; Grant, N.; Wayne, A.S.; Carrasquillo, J.A.; Steinberg, S.M.; Yarchoan, R.; Jaffe, E.S.; Wilson, W.H. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood 2010, 115, 3017–3024. [Google Scholar] [CrossRef]
- Ribera, J.M.; Morgades, M.; González-Barca, E.; Miralles, P.; López-Guillermo, A.; Gardella, S.; López, A.; Abella, E.; García, M.; Navarro, J.T.; et al. Long-term follow-up of patients with HIV-related diffuse large B-cell lymphomas treated in a phase II study with rituximab and CHOP. Br. J. Haematol. 2012, 157, 637–639. [Google Scholar] [CrossRef]
- Kaplan, L.D.; Lee, J.Y.; Ambinder, R.F.; Sparano, J.A.; Cesarman, E.; Chadburn, A.; Levine, A.M.; Scadden, D.T. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005, 106, 1538–1543. [Google Scholar] [CrossRef]
- Barta, S.K.; Lee, J.Y.; Kaplan, L.D.; Noy, A.; Sparano, J.A. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer 2012, 118, 3977–3983. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Wilson, W.H.; Jung, S.H.; Hsi, E.D.; Maurer, M.J.; Pederson, L.D.; Polley, M.C.; Pitcher, B.N.; Cheson, B.D.; Kahl, B.S.; et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J. Clin. Oncol. 2019, 37, 1790–1799. [Google Scholar] [CrossRef]
- Ramos, J.C.; Sparano, J.A.; Chadburn, A.; Reid, E.G.; Ambinder, R.F.; Siegel, E.R.; Moore, P.C.; Rubinstein, P.G.; Durand, C.M.; Cesarman, E.; et al. Impact of Myc in HIV-associated non-Hodgkin lymphomas treated with EPOCH and outcomes with vorinostat (AMC-075 trial). Blood 2020, 136, 1284–1297. [Google Scholar] [CrossRef]
- Pagani, C.; Rusconi, C.; Dalla Pria, A.; Ravano, E.; Schommers, P.; Bastos-Oreiro, M.; Verga, L.; Gini, G.; Spina, M.; Arcaini, L.; et al. MYC rearrangements in HIV-associated large B-cell lymphomas: EUROMYC, a European retrospective study. Blood Adv. 2024, 8, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.M.; Morton, L.M.; Shiels, M.S.; Clarke, C.A.; Engels, E.A. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: A population-based study. AIDS 2014, 28, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Noy, A.; Lee, J.Y.; Cesarman, E.; Ambinder, R.; Baiocchi, R.; Reid, E.; Ratner, L.; Wagner-Johnston, N.; Kaplan, L. AIDS Malignancy Consortium. AMC 048: Modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood 2015, 126, 160–166. [Google Scholar] [CrossRef]
- Alwan, F.; He, A.; Montoto, S.; Kassam, S.; Mee, M.; Burns, F.; Edwards, S.; Wilson, A.; Tenant-Flowers, M.; Marcus, R.; et al. Adding rituximab to CODOX-M/IVAC chemotherapy in the treatment of HIV-associated Burkitt lymphoma is safe when used with concurrent combination antiretroviral therapy. AIDS 2015, 29, 903–910. [Google Scholar] [CrossRef]
- Roschewski, M.; Dunleavy, K.; Abramson, J.S.; Powell, B.L.; Link, B.K.; Patel, P.; Bierman, P.J.; Jagadeesh, D.; Mitsuyasu, R.T.; Peace, D.; et al. Multicenter Study of Risk-Adapted Therapy With Dose-Adjusted EPOCH-R in Adults With Untreated Burkitt Lymphoma. J. Clin. Oncol. 2020, 38, 2519–2529. [Google Scholar] [CrossRef]
- Dunleavy, K.; Pittaluga, S.; Shovlin, M.; Steinberg, S.M.; Cole, D.; Grant, C.; Widemann, B.; Staudt, L.M.; Jaffe, E.S.; Little, R.F.; et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N. Engl. J. Med. 2013, 369, 1915–1925. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Olszewski, A.J.; Evens, A.M.; Collins, G.P.; Danilov, A.V.; Bower, M.; Jagadeesh, D.; Zhu, C.; Sperling, A.; Kim, S.H.; et al. HIV-associated Burkitt lymphoma: Outcomes from a US-UK collaborative analysis. Blood Adv. 2021, 5, 2852–2862. [Google Scholar] [CrossRef]
- Montoto, S.; Shaw, K.; Okosun, J.; Gandhi, S.; Fields, P.; Wilson, A.; Shanyinde, M.; Cwynarski, K.; Marcus, R.; de Vos, J.; et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J. Clin. Oncol. 2012, 30, 4111–4116. [Google Scholar] [CrossRef]
- Hentrich, M.; Berger, M.; Wyen, C.; Siehl, J.; Rockstroh, J.K.; Müller, M.; Fätkenheuer, G.; Seidel, E.; Nickelsen, M.; Wolf, T.; et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: Results of a prospective multicenter study. J. Clin. Oncol. 2012, 30, 4117–4123. [Google Scholar] [CrossRef]
- Rubinstein, P.G.; Moore, P.C.; Bimali, M.; Lee, J.Y.; Rudek, M.A.; Chadburn, A.; Ratner, L.; Henry, D.H.; Cesarman, E.; DeMarco, C.E.; et al. Brentuximab vedotin with AVD for stage II-IV HIV-related Hodgkin lymphoma (AMC 085): Phase 2 results from an open-label, single arm, multicentre phase 1/2 trial. Lancet Haematol. 2023, 10, e624–e632. [Google Scholar] [CrossRef] [PubMed]
- Lurain, K.; Polizzotto, M.N.; Aleman, K.; Bhutani, M.; Wyvill, K.M.; Gonçalves, P.H.; Ramaswami, R.; Marshall, V.A.; Miley, W.; Steinberg, S.M.; et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood 2019, 133, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, J.; Azar, N.; Autran, B.; Katlama, C.; Leblond, V. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated lymphoma. Lancet 2000, 355, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Re, A.; Cattaneo, C.; Michieli, M.; Casari, S.; Spina, M.; Rupolo, M.; Allione, B.; Nosari, A.; Schiantarelli, C.; Vigano, M.; et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J. Clin. Oncol. 2003, 21, 4423–4427. [Google Scholar] [CrossRef]
- Alvarnas, J.C.; Le Rademacher, J.; Wang, Y.; Little, R.F.; Akpek, G.; Ayala, E.; Devine, S.; Baiocchi, R.; Lozanski, G.; Kaplan, L.; et al. Autologous hematopoietic cell transplantation for HIV-related lymphoma: Results of the BMT CTN 0803/AMC 071 trial. Blood 2016, 128, 1050–1058. [Google Scholar] [CrossRef]
- Ambinder, R.F.; Wu, J.; Logan, B.; Durand, C.M.; Shields, R.; Popat, U.R.; Little, R.F.; McMahon, D.K.; Cyktor, J.; Mellors, J.W.; et al. Allogeneic Hematopoietic Cell Transplant for HIV Patients with Hematologic Malignancies: The BMT CTN-0903/AMC-080 Trial. Biol. Blood Marrow Transplant. 2019, 25, 2160–2166. [Google Scholar] [CrossRef]
- Allers, K.; Hütter, G.; Hofmann, J.; Loddenkemper, C.; Rieger, K.; Thiel, E.; Schneider, T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 2011, 117, 2791–2799. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Rust, B.J.; Kiem, H.P.; Uldrick, T.S. CAR T-cell therapy for cancer and HIV through novel approaches to HIV-associated haematological malignancies. Lancet Haematol. 2020, 7, e690–e696. [Google Scholar] [CrossRef]
- Carbone, A.; Gloghini, A.; Serraino, D.; Spina, M.; Tirelli, U.; Vaccher, E. Immunodeficiency-associated Hodgkin lymphoma. Expert Rev. Hematol. 2021, 14, 547–559. [Google Scholar] [CrossRef]
- Danilov, A.V.; Li, H.; Press, O.W.; Shapira, I.; Swinnen, L.J.; Noy, A.; Reid, E.; Smith, S.M.; Friedberg, J.W. Feasibility of interim positron emission tomography (PET)-adapted therapy in HIV-positive patients with advanced Hodgkin lymphoma (HL): A sub-analysis of SWOG S0816 Phase 2 trial. Leuk. Lymphoma 2017, 58, 461–465. [Google Scholar] [CrossRef]
- Serrao, A.; Canichella, M.; De Luca, M.L.; Tartaglia, G.; Annechini, G.; D’Elia, G.M.; Pulsoni, A. Nivolumab as a safe and effective treatment in an HIV patient with refractory Hodgkin lymphoma. Ann. Hematol. 2019, 98, 1505–1506. [Google Scholar] [CrossRef] [PubMed]
- Uldrick, T.S.; Gonçalves, P.H.; Abdul-Hay, M.; Claeys, A.J.; Emu, B.; Ernstoff, M.S.; Fling, S.P.; Fong, L.; Kaiser, J.C.; Lacroix, A.M.; et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019, 5, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cao, M.; Morán, T.; Dalmau, J.; Garcia-Corbacho, J.; Bracht, J.W.P.; Bernabe, R.; Juan, O.; de Castro, J.; Blanco, R.; Drozdowskyj, A.; et al. Assessment of the Feasibility and Safety of Durvalumab for Treatment of Solid Tumors in Patients With HIV-1 Infection: The Phase 2 DURVAST Study. JAMA Oncol. 2020, 6, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- El Zarif, T.; Nassar, A.H.; Adib, E.; Fitzgerald, B.G.; Huang, J.; Mouhieddine, T.H.; Rubinstein, P.G.; Nonato, T.; McKay, R.R.; Li, M.; et al. Safety and Activity of Immune Checkpoint Inhibitors in People Living With HIV and Cancer: A Real-World Report From the Cancer Therapy Using Checkpoint Inhibitors in People Living With HIV-International (CATCH-IT) Consortium. J. Clin. Oncol. 2023, 41, 3712–3723. [Google Scholar] [CrossRef]
- Herrera, A.F.; LeBlanc, M.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Parsons, S.K.; Ahmed, S.; et al. Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2024, 391, 1379–1389. [Google Scholar] [CrossRef]
- Langan, E.A.; Graetz, V.; Allerheiligen, J.; Zillikens, D.; Rupp, J.; Terheyden, P. Immune checkpoint inhibitors and tuberculosis: An old disease in a new context. Lancet Oncol. 2020, 21, e55–e65. [Google Scholar] [CrossRef]
- Anidi, I.U.; Sakai, S.; Brooks, K.; Fling, S.P.; Wagner, M.J.; Lurain, K.; Lindestam Arlehamn, C.S.; Sette, A.; Knox, K.S.; Brenchley, J.M.; et al. Exacerbation of CMV and Nontuberculous Mycobacterial Infections Following PD-1 Blockade for HIV-Associated Kaposi Sarcoma. Open Forum Infect. Dis. 2024, 11, ofae183. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, C.M.; Lurain, K.; Rous, C.; Weaver, L.; Raffeld, M.; Stetler-Stevenson, M.; Uldrick, T.S.; Filie, A.C.; Pittaluga, S.; et al. A novel approach for characterization of KSHV-associated multicentric Castleman disease from effusions. Br. J. Haematol. 2023, 200, 462–475. [Google Scholar] [CrossRef]
- Martin de Frémont, G.; Vanjak, A.; Sbihi, Z.; Knapp, S.; Garzaro, M.; Chbihi, M.; Fournier, B.; Poirot, J.; Dossier, A.; Silvestrini, M.A.; et al. Characteristics of circulating KSHV-infected viroblasts during active KSHV+ multicentric Castleman disease. Blood Adv. 2023, 7, 1682–1691. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Polizzotto, M.N.; Aleman, K.; Wyvill, K.M.; Marshall, V.; Whitby, D.; Wang, V.; Pittaluga, S.; O’Mahony, D.; Steinberg, S.M.; et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014, 124, 3544–3552. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, R.; Lurain, K.; Peer, C.J.; Serquiña, A.; Wang, V.; Widell, A.; Goncalves, P.; Steinberg, S.M.; Marshall, V.; George, J.; et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2020, 135, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Vaccher, E.; Gloghini, A.; Pria, A.D.; Bower, M. Management of KSHV/HHV8-Positive Multicentric Castleman Disease Concurrent With Other KSHV/HHV8-Positive Disorders: An Emergent Challenge in PLWH. J. Acquir. Immune Defic. Syndr. 2025, 99, e1–e2. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Adams, S.V.; Fromentin, R.; Roche, M.; Fling, S.P.; Gonçalves, P.H.; Lurain, K.; Ramaswami, R.; Wang, C.J.; Gorelick, R.J.; et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci. Transl. Med. 2022, 14, eabl3836. [Google Scholar] [CrossRef]
- Rasmussen, T.A.; Rajdev, L.; Rhodes, A.; Dantanarayana, A.; Tennakoon, S.; Chea, S.; Spelman, T.; Lensing, S.; Rutishauser, R.; Bakkour, S.; et al. Impact of Anti-PD-1 and Anti-CTLA-4 on the Human Immunodeficiency Virus (HIV) Reservoir in People Living With HIV With Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium 095 Study. Clin. Infect. Dis. 2021, 73, e1973–e1981. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.P. Immune checkpoint inhibitors in people living with HIV: What about anti-HIV effects? AIDS 2020, 34, 167–175. [Google Scholar] [CrossRef]
- Lavole, A.; Mazieres, J.; Schneider, S.; Brosseau, S.; Kiakouama, L.; Greillier, L.; Guihot, A.; Abbar, B.; Baron, M.; Makinson, A.; et al. Assessment of nivolumab in HIV-Infected patients with advanced non-small cell lung cancer after prior chemotherapy. The IFCT-1602 CHIVA2 phase 2 clinical trial. Lung Cancer 2021, 158, 146–150. [Google Scholar] [CrossRef]
- Assoumou, L.; Baldé, R.; Katlama, C.; Abbar, B.; Delobel, P.; Allegre, T.; Lavole, A.; Makinson, A.; Zaegel-Faucher, O.; Greillier, L.; et al. Safety and tolerability of immune checkpoint inhibitors in people with HIV infection and cancer: Insights from the national prospective real-world OncoVIHAC ANRS CO24 cohort study. J. Immunother. Cancer 2024, 12, e009728. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Lurain, K.; Zarif, T.E.; Ramaswami, R.; Nassar, A.H.; Adib, E.; Abdel-Wahab, N.; Chintapally, N.; Drolen, C.E.; Feldman, T.; Haykal, T.; et al. Real-World Multicenter Study of PD-1 Blockade in HIV-Associated Classical Hodgkin Lymphoma Across the United States. Clin. Lymphoma Myeloma Leuk. 2024, 24, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lurain, K.; Ramaswami, R.; Mangusan, R.; Widell, A.; Ekwede, I.; George, J.; Ambinder, R.; Cheever, M.; Gulley, J.L.; Goncalves, P.H.; et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin’s lymphoma. J. Immunother. Cancer 2021, 9, e002097. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Matta, H.; Tolani, B.; Triche TJr Chaudhary, P.M. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene 2016, 35, 1797–1810. [Google Scholar] [CrossRef]
- Davis, D.A.; Shrestha, P.; Aisabor, A.I.; Stream, A.; Galli, V.; Pise-Masison, C.A.; Tagawa, T.; Ziegelbauer, J.M.; Franchini, G.; Yarchoan, R. Pomalidomide increases immune surface marker expression and immune recognition of oncovirus-infected cells. Oncoimmunology 2018, 8, e1546544. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Y.; Zhou, F.; Chen, X.; Sheng, J.; Zhang, J. TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Front. Oncol. 2022, 12, 1091782. [Google Scholar] [CrossRef]
- Milunović, V.; Dragčević, D.; Bogeljić Patekar, M.; Mandac Smoljanović, I.; Gašparov, S. The Improving Outcomes in Relapsed-Refractory Diffuse Large B Cell Lymphoma: The Role of CAR T-Cell Therapy. Curr. Treat. Options Oncol. 2025, 26, 445–464. [Google Scholar] [CrossRef]
- Khodke, P.; Kumbhar, B.V. Engineered CAR-T cells: An immunotherapeutic approach for cancer treatment and beyond. Adv. Protein Chem. Struct. Biol. 2024, 140, 157–198. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.J.; Gopal, A.K.; Uldrick, T.S.; Menon, M.P. Exclusion of People Living with HIV in Aggressive B-Cell Non-Hodgkin Lymphoma Studies: A Cross-Sectional Analysis of Clinical Trials from 2014 to 2024. Cancer Investig. 2025, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hattenhauer, S.T.; Mispelbaum, R.; Hentrich, M.; Boesecke, C.; Monin, M.B. Enabling CAR T-cell therapies for HIV-positive lymphoma patients—A call for action. HIV Med. 2023, 24, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Chyra, Z.; Kotulová, J.; Celichowski, P.; Mihályová, J.; Charvátová, S.; Hájek, R. Impact of T cell characteristics on CAR-T cell therapy in hematological malignancies. Blood Cancer J. 2024, 14, 213. [Google Scholar] [CrossRef]
- Abbasi, A.; Peeke, S.; Shah, N.; Mustafa, J.; Khatun, F.; Lombardo, A.; Abreu, M.; Elkind, R.; Fehn, K.; de Castro, A.; et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J. Hematol. Oncol. 2020, 13, 1. [Google Scholar] [CrossRef]
- Villalba, J.A.; Maus, M.V.; Frigault, M.J.; Zaffini, R.; Gandhi, R.T.; Rosenberg, E.S.; Siedner, M.J. False-Positive Human Immunodeficiency Virus Test Results in Patients Receiving Lentivirus-Based Chimeric Antigen Receptor T-Cell Therapy: Case Report, Review of the Literature, and Proposed Recommendations. J. Infect. Dis. 2022, 225, 1933–1936. [Google Scholar] [CrossRef]
- Patel, K.K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From concept to cure: The evolution of CAR-T cell therapy. Mol. Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef]
- Urak, R.; Pahlavanneshan, S.; Gittins, B.; Nakamura, R.; Zaia, J.A.; Baird, J.H.; Clark, M.C.; Forman, S.J.; Wang, X. Simultaneous targeting of B-cell malignancies and human immunodeficiency virus with bispecific chimeric antigen receptor T cells. Haematologica 2024, 109, 3053–3058. [Google Scholar] [CrossRef]
- Ambinder, R.F.; Capoferri, A.A.; Durand, C.M. Haemopoietic cell transplantation in patients living with HIV. Lancet HIV 2020, 7, e652–e660. [Google Scholar] [CrossRef]
- Sáez-Cirión, A.; Mamez, A.C.; Avettand-Fenoel, V.; Nabergoj, M.; Passaes, C.; Thoueille, P.; Decosterd, L.; Hentzien, M.; Perdomo-Celis, F.; Salgado, M.; et al. Sustained HIV remission after allogeneic hematopoietic stem cell transplantation with wild-type CCR5 donor cells. Nat. Med. 2024, 30, 3544–3554. [Google Scholar] [CrossRef]
- McLaughlin, L.P.; Gottschalk, S.; Rooney, C.M.; Bollard, C.M. EBV-Directed T Cell Therapeutics for EBV-Associated Lymphomas. Methods Mol. Biol. 2017, 1532, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bollard, C.M.; Gottschalk, S.; Torrano, V.; Diouf, O.; Ku, S.; Hazrat, Y.; Carrum, G.; Ramos, C.; Fayad, L.; Shpall, E.J.; et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014, 32, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Icheva, V.; Kayser, S.; Wolff, D.; Tuve, S.; Kyzirakos, C.; Bethge, W.; Greil, J.; Albert, M.H.; Schwinger, W.; Nathrath, M.; et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol. 2013, 31, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bollard, C.M.; Tripic, T.; Cruz, C.R.; Dotti, G.; Gottschalk, S.; Torrano, V.; Dakhova, O.; Carrum, G.; Ramos, C.A.; Liu, H.; et al. Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. J. Clin. Oncol. 2018, 36, 1128–1139. [Google Scholar] [CrossRef]
- Khoury, R.; Grimley, M.S.; Nelson, A.S.; Leemhuis, T.; Cancelas, J.A.; Cook, E.; Wang, Y.; Heyenbruch, D.; Bollard, C.M.; Keller, M.D.; et al. Third-party virus-specific T cells for the treatment of double-stranded DNA viral reactivation and posttransplant lymphoproliferative disease after solid organ transplant. Am. J. Transplant. 2024, 24, 1634–1643. [Google Scholar] [CrossRef]
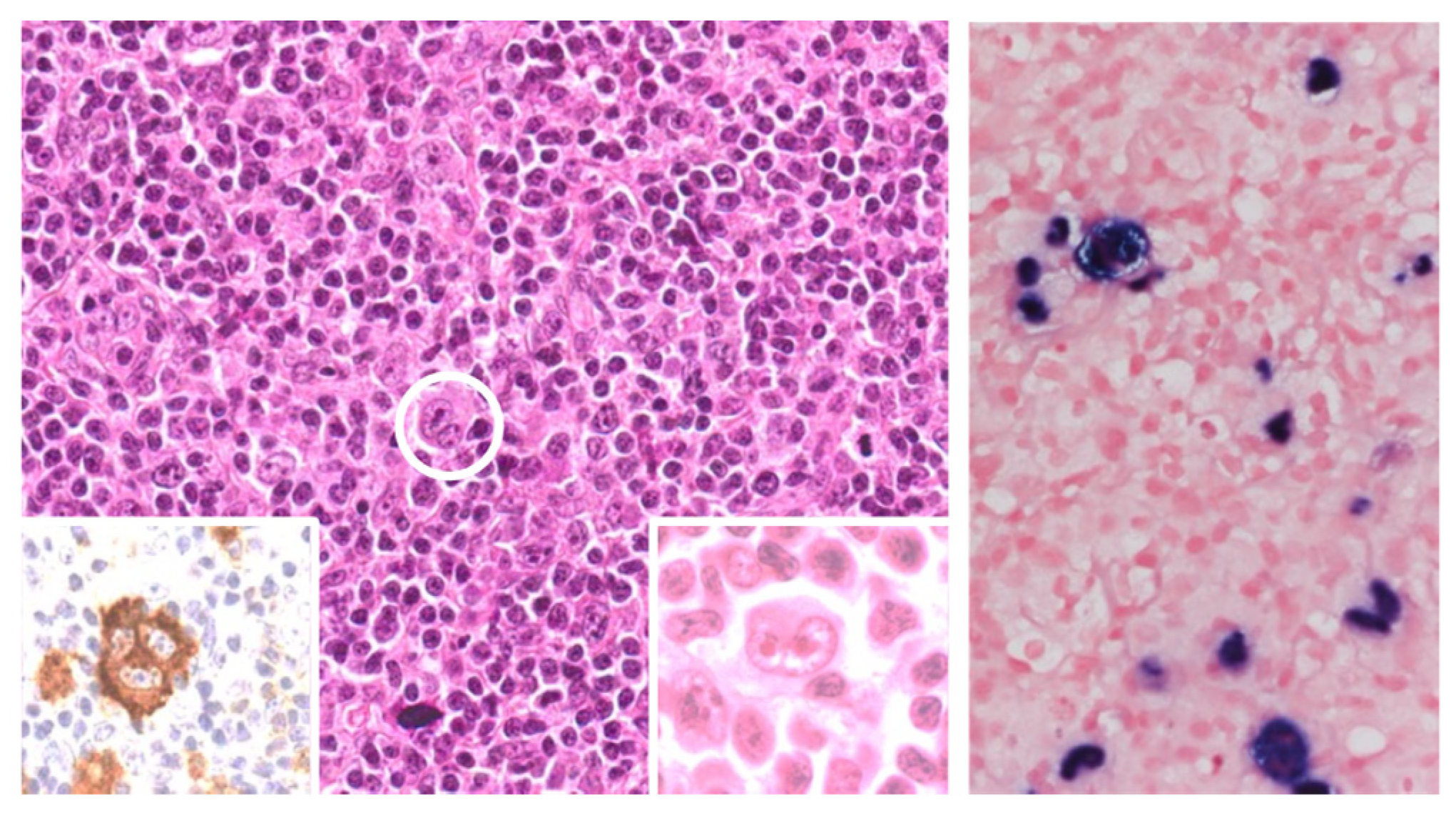
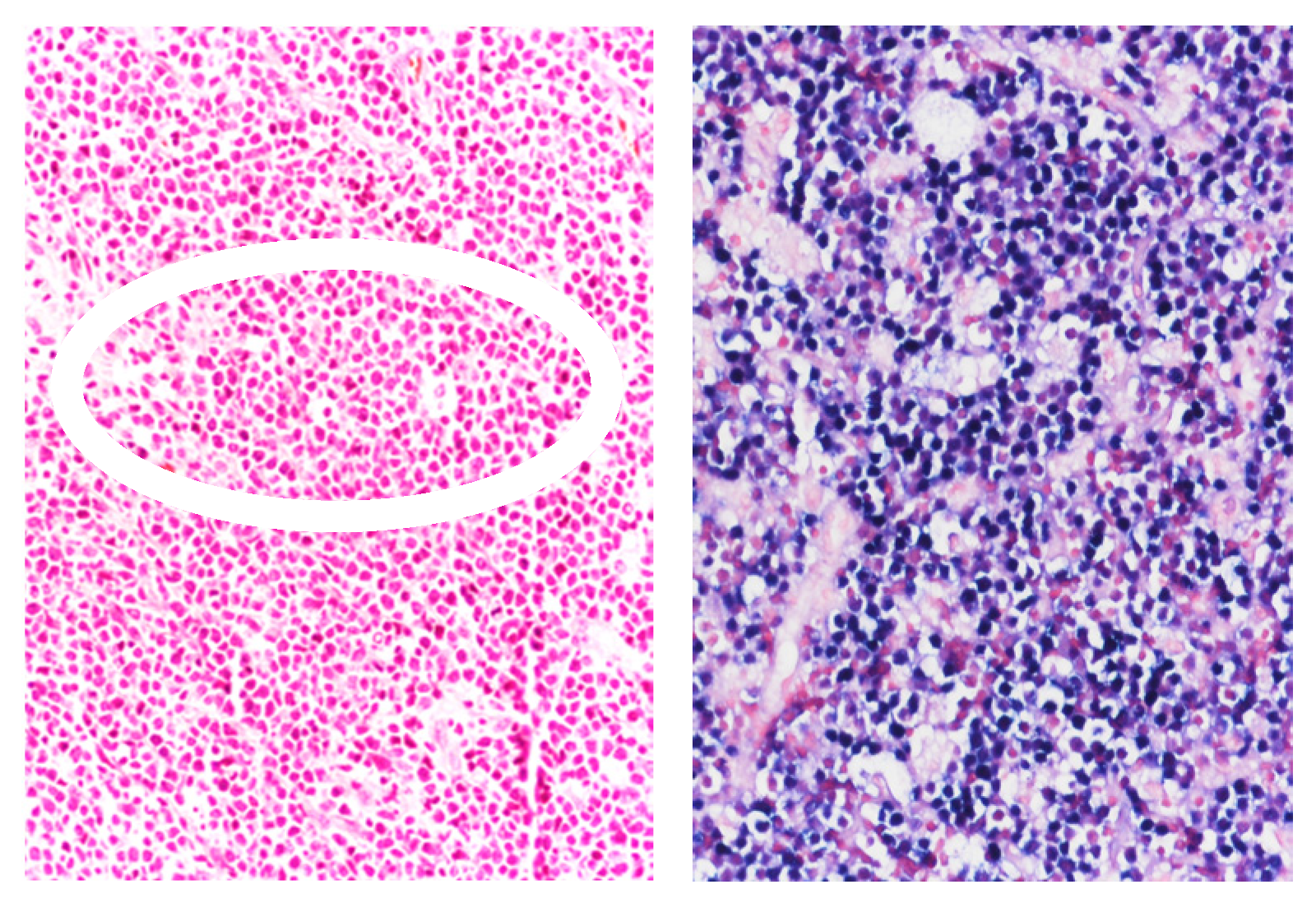
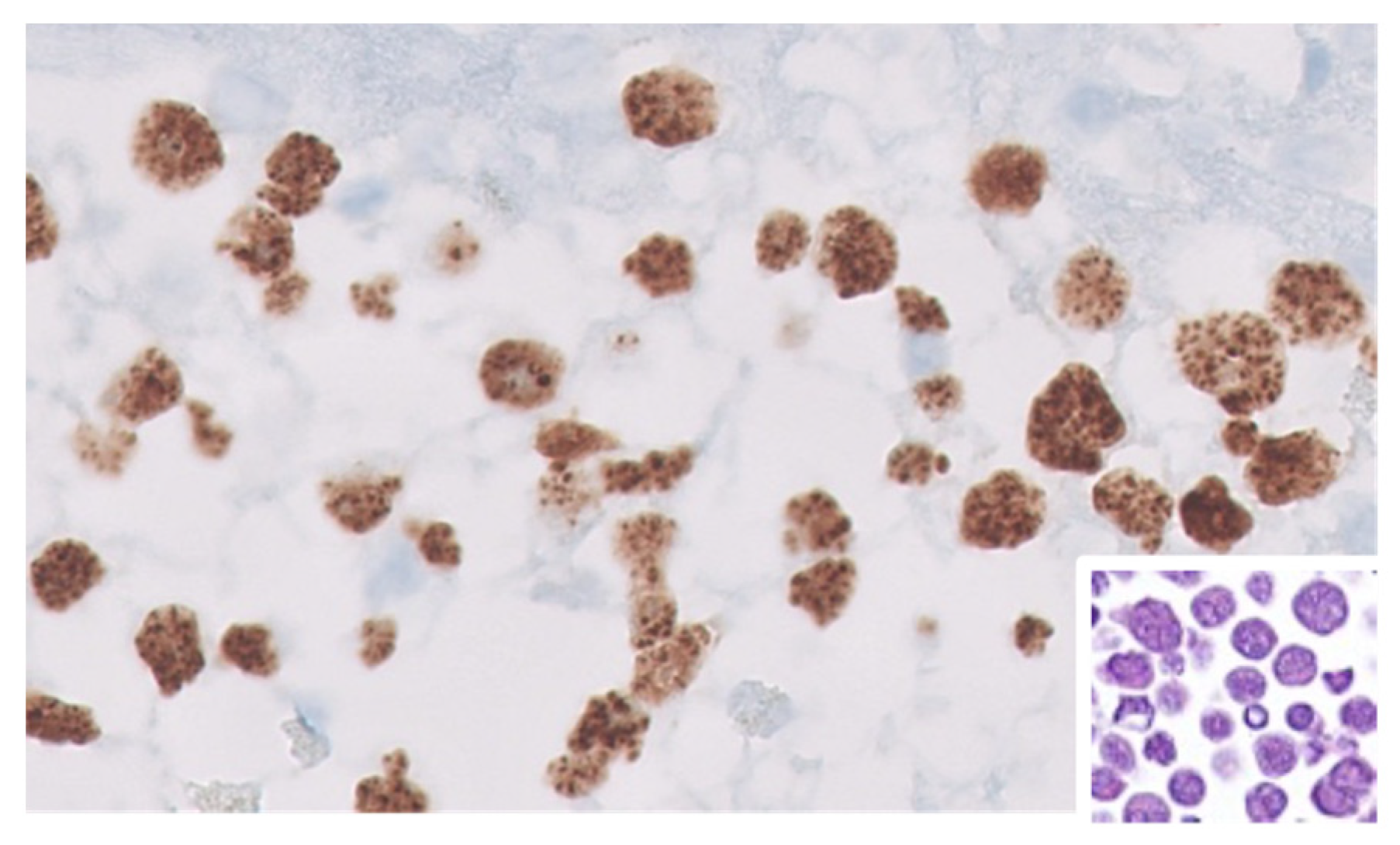
| Type of Lymphoproliferation | Viral Status (EBV and KSHV) |
|---|---|
| B-cell hyperplasias | EBV+; KSHV− |
| Polymorphic proliferations | EBV+ or − |
| Indolent B-cell lymphomas | EBV+/−; KSHV− |
| B-cell lymphomas * | |
| Hodgkin lymphoma | EBV+; KSHV− |
| Burkitt lymphoma | EBV−/+; KSHV− |
| DLBCL | EBV+; KSHV− |
| PEL and its solid variants | EBV+; KSHV+ |
| PBL of the oral cavity type | EBV+/−; KSHV− |
| MCD-associated LBCL | EBV−; KSHV+ |
| PBL associated with MCD | EBV−; KSHV+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolcetti, R.; Vaccher, E.; Carbone, A. Lymphoproliferations in People Living with HIV: Oncogenic Pathways, Diagnostic Challenges, and New Therapeutic Opportunities. Cancers 2025, 17, 2088. https://doi.org/10.3390/cancers17132088
Dolcetti R, Vaccher E, Carbone A. Lymphoproliferations in People Living with HIV: Oncogenic Pathways, Diagnostic Challenges, and New Therapeutic Opportunities. Cancers. 2025; 17(13):2088. https://doi.org/10.3390/cancers17132088
Chicago/Turabian StyleDolcetti, Riccardo, Emanuela Vaccher, and Antonino Carbone. 2025. "Lymphoproliferations in People Living with HIV: Oncogenic Pathways, Diagnostic Challenges, and New Therapeutic Opportunities" Cancers 17, no. 13: 2088. https://doi.org/10.3390/cancers17132088
APA StyleDolcetti, R., Vaccher, E., & Carbone, A. (2025). Lymphoproliferations in People Living with HIV: Oncogenic Pathways, Diagnostic Challenges, and New Therapeutic Opportunities. Cancers, 17(13), 2088. https://doi.org/10.3390/cancers17132088








