Improving Diagnostic Robustness of Perfusion MRI in Brain Metastases: A Focus on 3D ROI Techniques and Automatic Thresholding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Dynamic Susceptibility Contrast (DSC) Sequences
2.3. Processing of Cerebral Perfusion Maps
2.4. Lesion ROI Definition Delineation
2.4.1. Reference Method
2.4.2. Automatic Thresholding
2.4.3. Healthy ROI Locations
2.5. Statistical Methods
3. Results
3.1. Population
3.2. Overall Performance of the Methods
- -
- IntelliSpace: whole tumor ROI + healthy ROI located the head of the caudate nucleus (All_ISP_NGC) and 5% tumor ROI + healthy ROI located in the centrum semiovale (5%_ISP_CSO)
- -
- Syngo.via: whole tumor ROI + healthy ROI located the head of the caudate nucleus (All_Syngo_NGC).
3.3. Sensitivity and Specificity Analysis
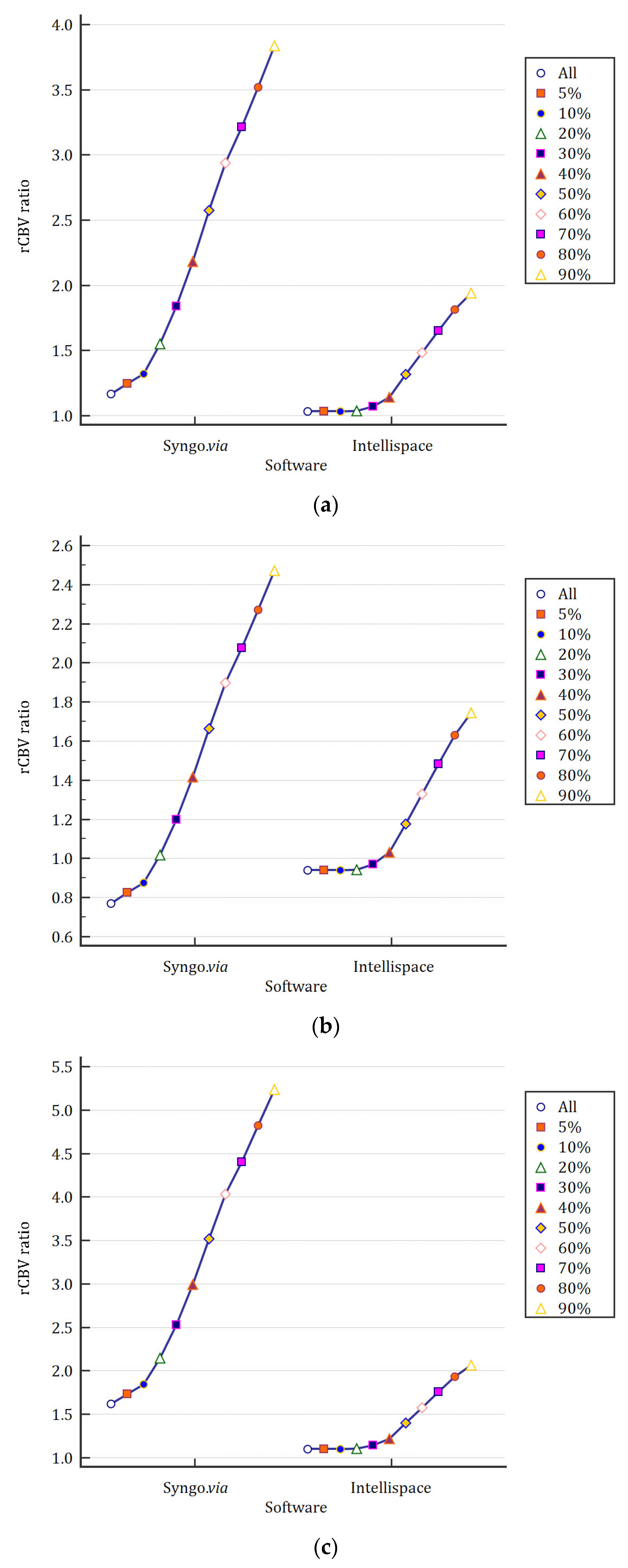
3.4. Impact of Automatic Thresholding on Mean rCBV Values
3.5. Robustness of rCBV Ratios Depending on Healthy ROI Placement and Software
3.6. Tumor Location Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, Radionecrosis, Inflammation or True Tumor Progression? Challenges Associated with Glioblastoma Response Assessment in an Evolving Therapeutic Landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.C.Y.; De Bruin, H.G.; Eijkenboom, W.; Sillevis Smitt, P.A.E.; Van Den Bent, M.J. Immediate Post-Radiotherapy Changes in Malignant Glioma Can Mimic Tumor Progression. Neurology 2004, 63, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Dhermain, F.; Vogin, G.; Reyns, N.; Metellus, P. Radionecrosis after Stereotactic Radiotherapy for Brain Metastases. Expert Rev. Neurother. 2016, 16, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical Features, Mechanisms, and Management of Pseudoprogression in Malignant Gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Khan, M.; Zhao, Z.; Arooj, S.; Liao, G. Bevacizumab for Radiation Necrosis Following Radiotherapy of Brain Metastatic Disease: A Systematic Review & Meta-Analysis. BMC Cancer 2021, 21, 167. [Google Scholar] [CrossRef]
- Katsura, M.; Sato, J.; Akahane, M.; Furuta, T.; Mori, H.; Abe, O. Recognizing Radiation-Induced Changes in the Central Nervous System: Where to Look and What to Look For. RadioGraphics 2021, 41, 224–248. [Google Scholar] [CrossRef]
- Chuang, M.-T.; Liu, Y.-S.; Tsai, Y.-S.; Chen, Y.-C.; Wang, C.-K. Differentiating Radiation-Induced Necrosis from Recurrent Brain Tumor Using MR Perfusion and Spectroscopy: A Meta-Analysis. PLoS ONE 2016, 11, e0141438. [Google Scholar] [CrossRef]
- Hojjati, M.; Badve, C.; Garg, V.; Tatsuoka, C.; Rogers, L.; Sloan, A.; Faulhaber, P.; Ros, P.R.; Wolansky, L.J. Role of FDG-PET/MRI, FDG-PET/CT, and Dynamic Susceptibility Contrast Perfusion MRI in Differentiating Radiation Necrosis from Tumor Recurrence in Glioblastomas. J. Neuroimaging 2018, 28, 118–125. [Google Scholar] [CrossRef]
- Mullins, M.E.; Barest, G.D.; Schaefer, P.W.; Hochberg, F.H.; Gonzalez, R.G.; Lev, M.H. Radiation Necrosis Versus Glioma Recurrence: Conventional MR Imaging Clues to Diagnosis. Am. J. Neuroradiol. 2005, 26, 1967–1972. [Google Scholar]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant Gliomas: MR Imaging Spectrum of Radiation Therapy- and Chemotherapy-Induced Necrosis of the Brain after Treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef]
- Dequesada, I.M.; Quisling, R.G.; Yachnis, A.; Friedman, W.A. Can Standard Magnetic Resonance Imaging Reliably Distinguish Recurrent Tumor from Radiation Necrosis after Radiosurgery for Brain Metastases? A Radiographic-Pathological Study. Neurosurgery 2008, 63, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Tomiguchi, S.; Shigematsu, Y.; Ikushima, I.; Kira, T.; Liang, L.; Ushio, Y.; Takahashi, M. Posttherapeutic Intraaxial Brain Tumor: The Value of Perfusion-Sensitive Contrast-Enhanced MR Imaging for Differentiating Tumor Recurrence from Nonneoplastic Contrast-Enhancing Tissue. Am. J. Neuroradiol. 2000, 21, 901–909. [Google Scholar]
- Morabito, R.; Alafaci, C.; Pergolizzi, S.; Pontoriero, A.; Iati’, G.; Bonanno, L.; Gaeta, M.; Salpietro, F.M.; Mormina, E.; Longo, M.; et al. DCE and DSC Perfusion MRI Diagnostic Accuracy in the Follow-up of Primary and Metastatic Intra-Axial Brain Tumors Treated by Radiosurgery with Cyberknife. Radiat. Oncol. 2019, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.; Ng, N.N.; Nagpal, S.; Pollom, E.L.; Soltys, S.; Hayden-Gephart, M.; Li, G.; Born and Michael, D.E. DSC Perfusion MRI–Derived Fractional Tumor Burden and Relative CBV Differentiate Tumor Progression and Radiation Necrosis in Brain Metastases Treated with Stereotactic Radiosurgery. Am. J. Neuroradiol. 2022, 43, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Frauenfelder, G.; Senese, R.; Zeccolini, F.; Schena, E.; Giurazza, F.; Jäger, H.R. Dynamic Susceptibility Contrast (DSC) Perfusion MRI in Differential Diagnosis between Radionecrosis and Neoangiogenesis in Cerebral Metastases Using rCBV, rCBF and K2. Radiol. Med. 2018, 123, 545–552. [Google Scholar] [CrossRef]
- Oei, M.T.H.; Meijer, F.J.A.; Mordang, J.-J.; Smit, E.J.; Idema, A.J.S.; Goraj, B.M.; Laue, H.O.A.; Prokop, M.; Manniesing, R. Observer Variability of Reference Tissue Selection for Relativecerebral Blood Volume Measurements in Glioma Patients. Eur. Radiol. 2018, 28, 3902–3911. [Google Scholar] [CrossRef]
- Wetzel, S.G.; Cha, S.; Johnson, G.; Lee, P.; Law, M.; Kasow, D.L.; Pierce, S.D.; Xue, X. Relative Cerebral Blood Volume Measurements in Intracranial Mass Lesions: Interobserver and Intraobserver Reproducibility Study. Radiology 2002, 224, 797–803. [Google Scholar] [CrossRef]
- Dijkstra, H.; Sijens, P.E.; Van Der Hoorn, A.; Van Laar, P.J. Inter-Observer Reproducibility of Quantitative Dynamic Susceptibility Contrast and Diffusion MRI Parameters in Histogram Analysis of Gliomas. Acta Radiol. 2020, 61, 76–84. [Google Scholar] [CrossRef]
- Jung, S.C.; Choi, S.H.; Yeom, J.A.; Kim, J.-H.; Ryoo, I.; Kim, S.C.; Shin, H.; Lee, A.L.; Yun, T.J.; Park, C.-K.; et al. Cerebral Blood Volume Analysis in Glioblastomas Using Dynamic Susceptibility Contrast-Enhanced Perfusion MRI: A Comparison of Manual and Semiautomatic Segmentation Methods. PLoS ONE 2013, 8, e69323. [Google Scholar] [CrossRef]
- Herings, S.D.A.; Van Den Elshout, R.; De Wit, R.; Mannil, M.; Ravesloot, C.; Scheenen, T.W.J.; Arens, A.; Van Der Kolk, A.; Meijer, F.J.A.; Henssen, D.J.H.A. How to Evaluate Perfusion Imaging in Post-Treatment Glioma: A Comparison of Three Different Analysis Methods. Neuroradiology 2024, 66, 1279–1289. [Google Scholar] [CrossRef]
- Law, M.; Cha, S.; Knopp, E.A.; Johnson, G.; Arnett, J.; Litt, A.W. High-Grade Gliomas and Solitary Metastases: Differentiation by Using Perfusion and Proton Spectroscopic MR Imaging. Radiology 2002, 222, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, W.H.T.; Govaerts, C.W.; Kramer, M.C.A.; Labrecque, J.A.; Smits, M.; Dirven, L.; Van Der Hoorn, A. Diagnostic Accuracy of MRI Techniques for Treatment Response Evaluation in Patients with Brain Metastasis: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2022, 177, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M.; for the American Society of Functional Neuroradiology MR Perfusion Standards and Practice Subcommittee of the ASFNR Clinical Practice Committee. ASFNR Recommendations for Clinical Performance of MR Dynamic Susceptibility Contrast Perfusion Imaging of the Brain. Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, A.-M.; Shetty, A.; Maitz, A.H.; Yan, D.; Doyle, D.; Richey, K.; Park, S.; Pieper, D.R.; Chen, P.Y.; et al. Differentiation between Intra-Axial Metastatic Tumor Progression and Radiation Injury Following Fractionated Radiation Therapy or Stereotactic Radiosurgery Using MR Spectroscopy, Perfusion MR Imaging or Volume Progression Modeling. Magn. Reson. Imaging 2011, 29, 993–1001. [Google Scholar] [CrossRef]
- Duan, C.; Li, N.; Liu, X.; Cui, J.; Wang, G.; Xu, W. Performance Comparison of 2D and 3D MRI Radiomics Features in Meningioma Grade Prediction: A Preliminary Study. Front. Oncol. 2023, 13, 1157379. [Google Scholar] [CrossRef]
- Witzmann, K.; Raschke, F.; Troost, E.G.C. MR Image Changes of Normal-Appearing Brain Tissue after Radiotherapy. Cancers 2021, 13, 1573. [Google Scholar] [CrossRef]
- Schmainda, K.M.; Prah, M.A.; Rand, S.D.; Liu, Y.; Logan, B.; Muzi, M.; Rane, S.D.; Da, X.; Yen, Y.-F.; Kalpathy-Cramer, J.; et al. Multisite Concordance of DSC-MRI Analysis for Brain Tumors: Results of a National Cancer Institute Quantitative Imaging Network Collaborative Project. Am. J. Neuroradiol. 2018, 39, 1008–1016. [Google Scholar] [CrossRef]
- Claassen, J.A.H.R.; Colier, W.N.J.M.; Jansen, R.W.M.M. Reproducibility of Cerebral Blood Volume Measurements by near Infrared Spectroscopy in 16 Healthy Elderly Subjects. Physiol. Meas. 2006, 27, 255–264. [Google Scholar] [CrossRef]
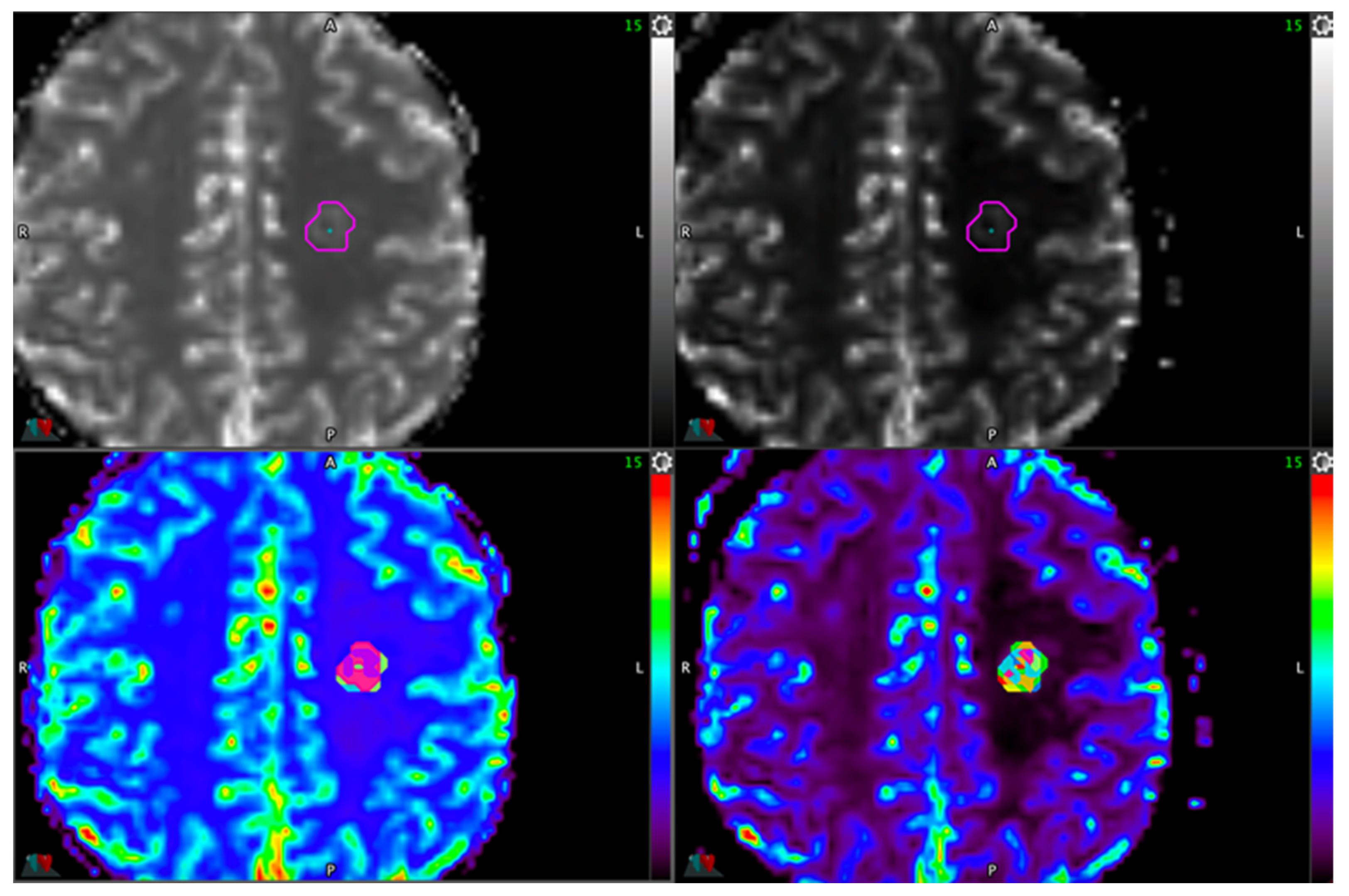
| Sotware | Lesion ROI Definition | Healthy ROI Definition | Combination Denomination | AUC | p | rCBV Ratio Threshold | Se | Sp | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| ISP | Manual | CL | Reference | 0.53 | 0.83 | >1.65 | 33.33 | 90.00 | 83.33 | 47.37 |
| ISP | Manual | CL | ISP_CL_Manual | 0.62 | 0.31 | ≤0.94 | 26.67 | 100.00 | 100.00 | 47.62 |
| NGC | ISP_NGC_Manual | 0.57 | 0.54 | >1.39 | 33.33 | 90.00 | 83.33 | 47.37 | ||
| CSO | ISP_CSO_Manual | 0.55 | 0.71 | >2.14 | 53.33 | 70.00 | 72.73 | 50.00 | ||
| Automatic | CL | ISP_CL_5 | 0.57 | 0.60 | ≤1.03 | 60.00 | 60.00 | 69.23 | 50.00 | |
| NGC | ISP_NGC_All | 0.65 | 0.21 | >1.01 | 40.00 | 90.00 | 85.71 | 50.00 | ||
| CSO | ISP_CSO_5 | 0.65 | 0.20 | >1.12 | 60.00 | 80.00 | 81.81 | 57.14 | ||
| Syngo | Manual | CL | Syngo_CL_Manual | 0.55 | 0.70 | ≤1.42 | 46.67 | 70.00 | 70.00 | 46.67 |
| NGC | Syngo_NGC_Manual | 0.59 | 0.47 | >1.61 | 46.67 | 80.00 | 77.78 | 50.00 | ||
| CSO | Syngo_CSO_Manual | 0.53 | 0.83 | ≤1.20 | 26.67 | 90.00 | 80.00 | 45.00 | ||
| Automatic | CL | Syngo_CL_5 | 0.53 | 0.84 | ≤1.21 | 46.67 | 30.00 | 50.00 | 27.27 | |
| NGC | Syngo_NGC_All | 0.65 | 0.18 | >0.75 | 53.33 | 80.00 | 80.00 | 53.33 | ||
| CSO | Syngo_CSO_5 | 0.61 | 0.38 | >1.88 | 46,67 | 80.00 | 77.78 | 50.00 |
| Lesion ROI Definition | Healthy ROI Definition | Combination Denomination | Cronbach’s alpha | ICC (CI95%) | ||
|---|---|---|---|---|---|---|
| Raw | Standardized | Single Measures | Average Measures | |||
| Manual | CL | CL_Manual | 0.56 | 0.57 | 0.39 (0.01–0.68) | 0.56 (0.01–0.81) |
| NGC | NGC_Manual | 0.62 | 0.62 | 0.45 (0.08–0.72) | 0.62 (0.15–0.83) | |
| CSO | CSO_Manual | 0.68 | 0.69 | 0.52 (0.16–0.75) | 0.68 (0.28–0.86) | |
| Automatic | CL | CL_5 | 0.47 | 0.93 | 0.31 (−0.09–0.62) | 0.47 (−0.20–0.77) |
| NGC | NGC_All | 0.60 | 0.92 | 0.43 (0.05–0.70) | 0.60 (0.10–0.83) | |
| CSO | CSO_5 | 0.39 | 0.91 | 0.24 (−0.16–0.57) | 0.39 (−0.39–0.73) | |
| Software | Lesion ROI Definition | Healthy ROI Definition | Combination Denomination | Supra-Tentorial Lesion | Sub-Tentorial Lesion |
|---|---|---|---|---|---|
| AUC (CI95%) | |||||
| ISP | Manual | CL | Reference | 0.52 (0.29–0.75) | 0.63 (0.19–0.94) |
| ISP | Manual | CL | ISP_CL_Manual | 0.68 (0.43–0.87) | 0.50 (0.12–0.88) |
| NGC | ISP_NGC_Manual | 0.52 (0.29–0.75) | 0.75 (0.29–0.98) | ||
| CSO | ISP_CSO_Manual | 0.51 (0.28–0.74) | 0.75 (0.29–0.98) | ||
| Automatic | CL | ISP_CL_5 | 0.64 (0.39–0.84) | 0.50 (0.12–0.88) | |
| NGC | ISP_NGC_All | 0.68 (0.43–0.87) | 0.50 (0.12–0.88) | ||
| CSO | ISP_CSO_5 | 0.65 (0.40–0.85) | 0.50 (0.12–0.88) | ||
| Syngo | Manual | CL | Syngo_CL_Manual | 0.50 (0.27–0.73) | 0.75 (0.29–0.98) |
| NGC | Syngo_NGC_Manual | 0.56 (0.32–0.78) | 0.75 (0.29–0.98) | ||
| CSO | Syngo_CSO_Manual | 0.50 (0.27–0.73) | 0.75 (0.29–0.98) | ||
| Automatic | CL | Syngo_CL_5 | 0.57 (0.33–0.79) | 0.63 (0.19–0.94) | |
| NGC | Syngo_NGC_All | 0.64 (0.39–0.84) | 0.75 (0.29–0.98) | ||
| CSO | Syngo_CSO_5 | 0.59 (0.35–0.81) | 0.63 (0.19–0.94) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzinska-Mistarz, S.; Dissaux, B.; Marchi, L.; Roux, A.-C.; Perrot, A.; Lucia, F.; Seizeur, R.; Pradier, O.; Dissaux, G.; Morjani, M.; et al. Improving Diagnostic Robustness of Perfusion MRI in Brain Metastases: A Focus on 3D ROI Techniques and Automatic Thresholding. Cancers 2025, 17, 2085. https://doi.org/10.3390/cancers17132085
Rudzinska-Mistarz S, Dissaux B, Marchi L, Roux A-C, Perrot A, Lucia F, Seizeur R, Pradier O, Dissaux G, Morjani M, et al. Improving Diagnostic Robustness of Perfusion MRI in Brain Metastases: A Focus on 3D ROI Techniques and Automatic Thresholding. Cancers. 2025; 17(13):2085. https://doi.org/10.3390/cancers17132085
Chicago/Turabian StyleRudzinska-Mistarz, Stéphanie, Brieg Dissaux, Laurie Marchi, Anne-Charlotte Roux, Alexis Perrot, François Lucia, Romuald Seizeur, Olivier Pradier, Gurvan Dissaux, Moncef Morjani, and et al. 2025. "Improving Diagnostic Robustness of Perfusion MRI in Brain Metastases: A Focus on 3D ROI Techniques and Automatic Thresholding" Cancers 17, no. 13: 2085. https://doi.org/10.3390/cancers17132085
APA StyleRudzinska-Mistarz, S., Dissaux, B., Marchi, L., Roux, A.-C., Perrot, A., Lucia, F., Seizeur, R., Pradier, O., Dissaux, G., Morjani, M., & Bourbonne, V. (2025). Improving Diagnostic Robustness of Perfusion MRI in Brain Metastases: A Focus on 3D ROI Techniques and Automatic Thresholding. Cancers, 17(13), 2085. https://doi.org/10.3390/cancers17132085






