Oncological and Functional Outcomes of Hemi-Ablation Versus Focal Ablation for Localized Prostate Cancer Using Irreversible Electroporation
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Treatment Protocol
2.4. Study Outcomes
2.5. Data Collection and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Early Oncological Control
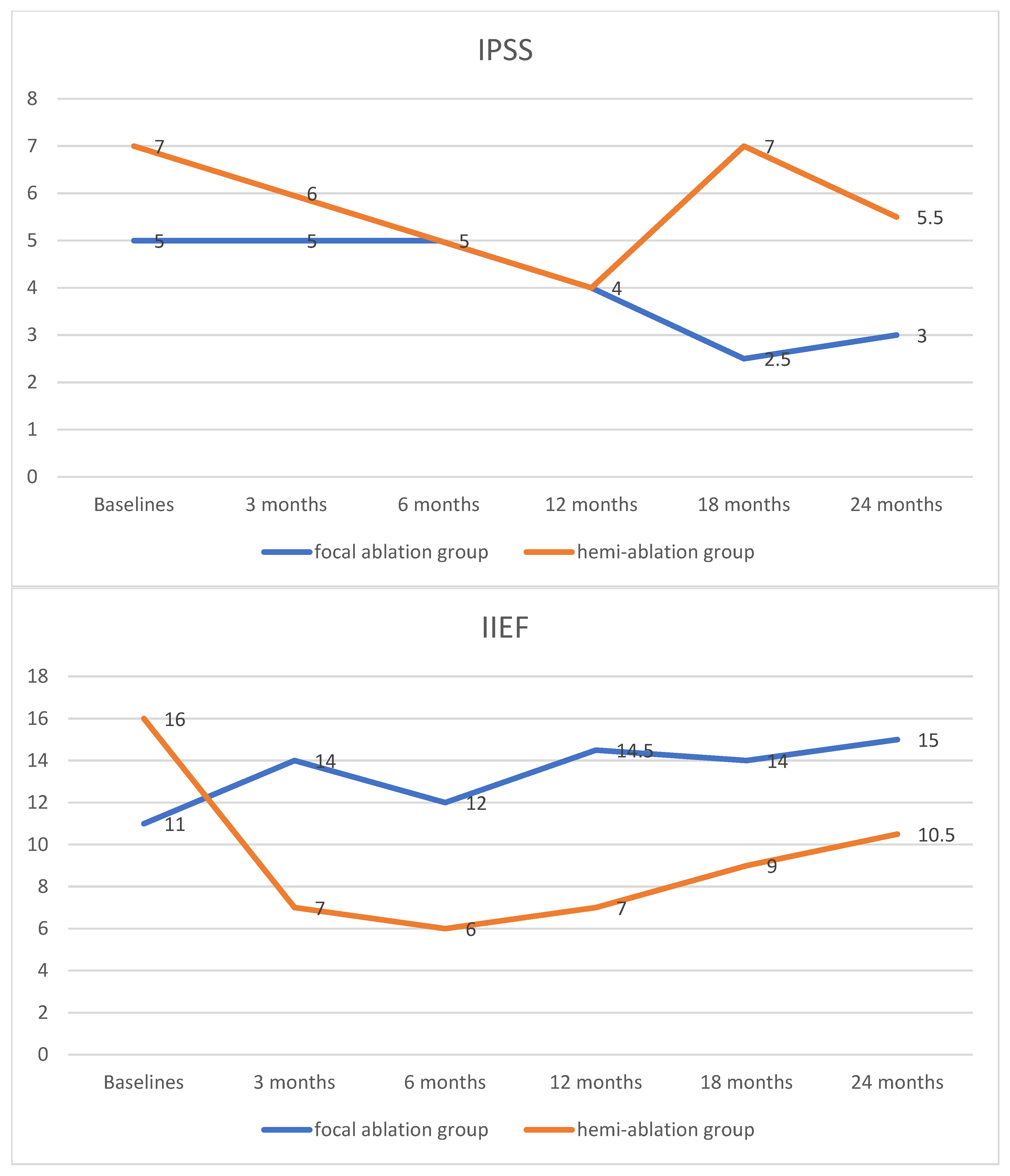
3.3. Functional Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bokhorst, L.P.; Valdagni, R.; Rannikko, A.; Kakehi, Y.; Pickles, T.; Bangma, C.H.; Roobol, M.J. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur. Urol. 2016, 70, 954–960. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.E.; Metcalfe, C.; Donovan, J.L.; Lane, J.A.; Davis, M.; Young, G.J.; Dutton, S.J.; Walsh, E.I.; Martin, R.M.; Peters, T.J.; et al. Ten-year Mortality, Disease Progression, and Treatment-related Side Effects in Men with Localised Prostate Cancer from the ProtecT Randomised Controlled Trial According to Treatment Received. Eur. Urol. 2020, 77, 320–330. [Google Scholar] [CrossRef]
- Resnick, M.J.; Koyama, T.; Fan, K.H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef]
- Lodeizen, O.; de Bruin, M.; Eggener, S.; Crouzet, S.; Ghai, S.; Varkarakis, I.; Katz, A.; Dominguez-Escrig, J.L.; Pahernik, S.; de Reijke, T.; et al. Ablation energies for focal treatment of prostate cancer. World J. Urol. 2019, 37, 409–418. [Google Scholar] [CrossRef]
- Blazevski, A.; Amin, A.; Scheltema, M.J.; Balakrishnan, A.; Haynes, A.M.; Barreto, D.; Cusick, T.; Thompson, J.; Stricker, P.D. Focal ablation of apical prostate cancer lesions with irreversible electroporation (IRE). World J. Urol. 2021, 39, 1107–1114. [Google Scholar] [CrossRef]
- Murray, K.S.; Ehdaie, B.; Musser, J.; Mashni, J.; Srimathveeravalli, G.; Durack, J.C.; Solomon, S.B.; Coleman, J.A. Pilot Study to Assess Safety and Clinical Outcomes of Irreversible Electroporation for Partial Gland Ablation in Men with Prostate Cancer. J. Urol. 2016, 196, 883–890. [Google Scholar] [CrossRef]
- Valerio, M.; Cerantola, Y.; Eggener, S.E.; Lepor, H.; Polascik, T.J.; Villers, A.; Emberton, M. New and Established Technology in Focal Ablation of the Prostate: A Systematic Review. Eur. Urol. 2017, 71, 17–34. [Google Scholar] [CrossRef]
- Zhang, K.; Teoh, J.; Zhu, G.; Ng, C.F.; Suberville, M.; Laguna, P.; de la Rosette, J. Irreversible Electroporation for the Focal Treatment of Prostate Cancer: A Systematic Review. World J. Men’s Health 2024, 43, 321–332. [Google Scholar] [CrossRef] [PubMed]
- de la Rosette, J.; Dominguez-Escrig, J.; Zhang, K.; Teoh, J.; Barret, E.; Ramon-Borja, J.C.; Muir, G.; Bohr, J.; de Reijke, T.; Ng, C.F.; et al. A Multicenter, Randomized, Single-blind, 2-Arm Intervention Study Evaluating the Adverse Events and Quality of Life After Irreversible Electroporation for the Ablation of Localized Low-intermediate Risk Prostate Cancer. J. Urol. 2023, 209, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Teoh, J.; Laguna, P.; Dominguez-Escrig, J.; Barret, E.; Ramon-Borja, J.C.; Muir, G.; Bohr, J.; de Reijke, T.M.; Pelechano Gómez, P.; et al. Effect of Focal vs. Extended Irreversible Electroporation for the Ablation of Localized Low- or Intermediate-Risk Prostate Cancer on Early Oncological Control: A Randomized Clinical Trial. JAMA Surg. 2023, 158, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Stricker, P.; Löhr, M.; Stehling, M.; Suberville, M.; Cussenot, O.; Lunelli, L.; Ng, C.F.; Teoh, J.; Laguna, P.; et al. A multi-center international study to evaluate the safety, functional and oncological outcomes of irreversible electroporation for the ablation of prostate cancer. Prostate Cancer Prostatic Dis. 2024, 27, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Blazevski, A.; Scheltema, M.J.; Yuen, B.; Masand, N.; Nguyen, T.V.; Delprado, W.; Shnier, R.; Haynes, A.M.; Cusick, T.; Thompson, J.; et al. Oncological and Quality-of-life Outcomes Following Focal Irreversible Electroporation as Primary Treatment for Localised Prostate Cancer: A Biopsy-monitored Prospective Cohort. Eur. Urol. Oncol. 2020, 3, 283–290. [Google Scholar] [CrossRef]
- Guenther, E.; Klein, N.; Zapf, S.; Weil, S.; Schlosser, C.; Rubinsky, B.; Stehling, M.K. Prostate cancer treatment with Irreversible Electroporation (IRE): Safety, efficacy and clinical experience in 471 treatments. PLoS ONE 2019, 14, e0215093. [Google Scholar] [CrossRef]
- Ting, F.; Tran, M.; Böhm, M.; Siriwardana, A.; Van Leeuwen, P.J.; Haynes, A.M.; Delprado, W.; Shnier, R.; Stricker, P.D. Focal irreversible electroporation for prostate cancer: Functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis. 2016, 19, 46–52. [Google Scholar] [CrossRef]
- Yaxley, W.J.; Gianduzzo, T.; Kua, B.; Oxford, R.; Yaxley, J.W. Focal therapy for prostate cancer with irreversible electroporation: Oncological and functional results of a single institution study. Investig. Clin. Urol. 2022, 63, 285–293. [Google Scholar] [CrossRef]
- van den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Böhm, M.; Haynes, A.M.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018, 121, 716–724. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Sonni, I.; Jariwala, N.; Juarez, R.; Reiter, R.E.; Raman, S.S.; Hope, T.A. The Role of PSMA PET/CT and PET/MRI in the Initial Staging of Prostate Cancer. Eur. Urol. Focus 2021, 7, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Gielchinsky, I.; Lev-Cohain, N. Focal Irreversible Electroporation for Localized Prostate Cancer—Oncological and Safety Outcomes Using mpMRI and Transperineal Biopsy Follow-Up. Res. Rep. Urol. 2023, 15, 27–35. [Google Scholar] [CrossRef]
- Shin, D.; Yoon, C.E.; Kwon, H.J.; Moon, H.W.; Park, Y.H.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Park, S.Y.; Ha, S.; et al. Irreversible electroporation for prostate cancer using PSMA PET-CT. Prostate Int. 2023, 11, 40–45. [Google Scholar] [CrossRef]
- Saha, A.; Bosma, J.S.; Twilt, J.J.; van Ginneken, B.; Bjartell, A.; Padhani, A.R.; Bonekamp, D.; Villeirs, G.; Salomon, G.; Giannarini, G.; et al. Artificial intelligence and radiologists in prostate cancer detection on MRI (PI-CAI): An international, paired, non-inferiority, confirmatory study. Lancet Oncol. 2024, 25, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, A.; Jia, A.Y.; Zaorsky, N.G.; Kashani, R.; Rao, S.; Shoag, J.E.; Vince, R.A., Jr.; Bittencourt, L.K.; Zuhour, R.; Price, A.T.; et al. Artificial intelligence applications in prostate cancer. Prostate Cancer Prostatic Dis. 2024, 27, 37–45. [Google Scholar] [CrossRef]
- Geboers, B.; Gondoputro, W.; Thompson, J.E.; Reesink, D.J.; van Riel, L.; Zhang, D.; Blazevski, A.; Doan, P.; Agrawal, S.; Matthews, J.; et al. Diagnostic Accuracy of Multiparametric Magnetic Resonance Imaging to Detect Residual Prostate Cancer Following Irreversible Electroporation-A Multicenter Validation Study. Eur. Urol. Focus 2022, 8, 1591–1598. [Google Scholar] [CrossRef]
| Focal Ablation Group (n = 40) (Median, IQR) | Hemi-Ablation Group (n = 66) (Median, IQR) | p-Value | |
|---|---|---|---|
| Age (years) | 68.5 (63.3–74.0) | 71.0 (66.0–75.0) | 0.138 |
| PSA (ng/mL) | 8.3 (5.6–11.6) | 6.8 (5.4–9.1) | 0.109 |
| Prostate volume (mL) | 42.0 (30.0–60.0) | 44.0 (30.3–58.8) | 0.537 |
| Biopsy cores | 21 (18–23) | 22 (18–23) | 0.963 |
| ISUP | 0.934 | ||
| 1 | 20 | 30 | |
| 2 | 16 | 30 | |
| 3 | 3 | 6 | |
| 4 | 1 | ||
| Number of electrodes | 4 (4–4.8) | 5 (5–5) | <0.001 |
| Duration of catheterization | 1 (0–2.8) | 11 (8–16.8) | <0.001 |
| Focal Ablation Group (n = 36) | Hemi-Ablation Group (n = 58) | p-Value | |
|---|---|---|---|
| Negative, No. (%) | 10 (27.8%) | 40 (69.0%) | <0.001 |
| Persistent tumor, No. (%) | 26 (72.2%) | 18 (31.0%) | |
| Persistent clinically significant tumor, No. (%) | 9 (25%) | 5 (8.6%) | 0.003 |
| Infield | 16 (44.4%) | 5 (8.6%) | |
| ISUP 1 | 5 | 3 | |
| ISUP 2 | 4 | 0 | |
| ISUP 3 | 0 | 1 | |
| ISUP 4 | 1 | 1 | |
| ISUP 5 | 1 | 0 | |
| N/A | 5 | 0 | |
| Outfield | 4 (11.1%) | 12 (20.7%) | |
| ISUP 1 | 2 | 8 | |
| ISUP 2 | 0 | 1 | |
| ISUP 3 | 1 | 1 | |
| N/A | 1 | 2 | |
| In- and outfield | 6 (16.7%) | 1 (1.7%) | |
| ISUP 1 | 2 | 0 | |
| ISUP 2 | 1 | 1 | |
| ISUP 5 | 1 | 0 | |
| N/A | 2 | 0 | |
| Retreatment | 21 (58.3%) | 9 (15.5%) | <0.001 |
| Radical prostatectomy | 7 | 1 | |
| Radiotherapy | 7 | 2 | |
| IRE | 1 | 6 | |
| ADT | 1 | 0 | |
| Radical prostatectomy +Radiotherapy | 3 | 0 | |
| IRE +Radiotherapy | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suberville, M.; Zhang, K.; Woillard, J.B.; Herafa, I.; Ducoux, D.; Nachef, R.; Teoh, J.; Zhu, G.; Ng, C.-F.; Laguna, P.; et al. Oncological and Functional Outcomes of Hemi-Ablation Versus Focal Ablation for Localized Prostate Cancer Using Irreversible Electroporation. Cancers 2025, 17, 2084. https://doi.org/10.3390/cancers17132084
Suberville M, Zhang K, Woillard JB, Herafa I, Ducoux D, Nachef R, Teoh J, Zhu G, Ng C-F, Laguna P, et al. Oncological and Functional Outcomes of Hemi-Ablation Versus Focal Ablation for Localized Prostate Cancer Using Irreversible Electroporation. Cancers. 2025; 17(13):2084. https://doi.org/10.3390/cancers17132084
Chicago/Turabian StyleSuberville, Michel, Kai Zhang, Jean Baptiste Woillard, Isabelle Herafa, Dorothée Ducoux, Rachid Nachef, Jeremy Teoh, Gang Zhu, Chi-Fai Ng, Pilar Laguna, and et al. 2025. "Oncological and Functional Outcomes of Hemi-Ablation Versus Focal Ablation for Localized Prostate Cancer Using Irreversible Electroporation" Cancers 17, no. 13: 2084. https://doi.org/10.3390/cancers17132084
APA StyleSuberville, M., Zhang, K., Woillard, J. B., Herafa, I., Ducoux, D., Nachef, R., Teoh, J., Zhu, G., Ng, C.-F., Laguna, P., & de la Rosette, J. (2025). Oncological and Functional Outcomes of Hemi-Ablation Versus Focal Ablation for Localized Prostate Cancer Using Irreversible Electroporation. Cancers, 17(13), 2084. https://doi.org/10.3390/cancers17132084








