The Evolving Landscape of GEP-NENs in the Era of Precision Oncology: Molecular Insights into Tumor Heterogeneity
Simple Summary
Abstract
1. Introduction
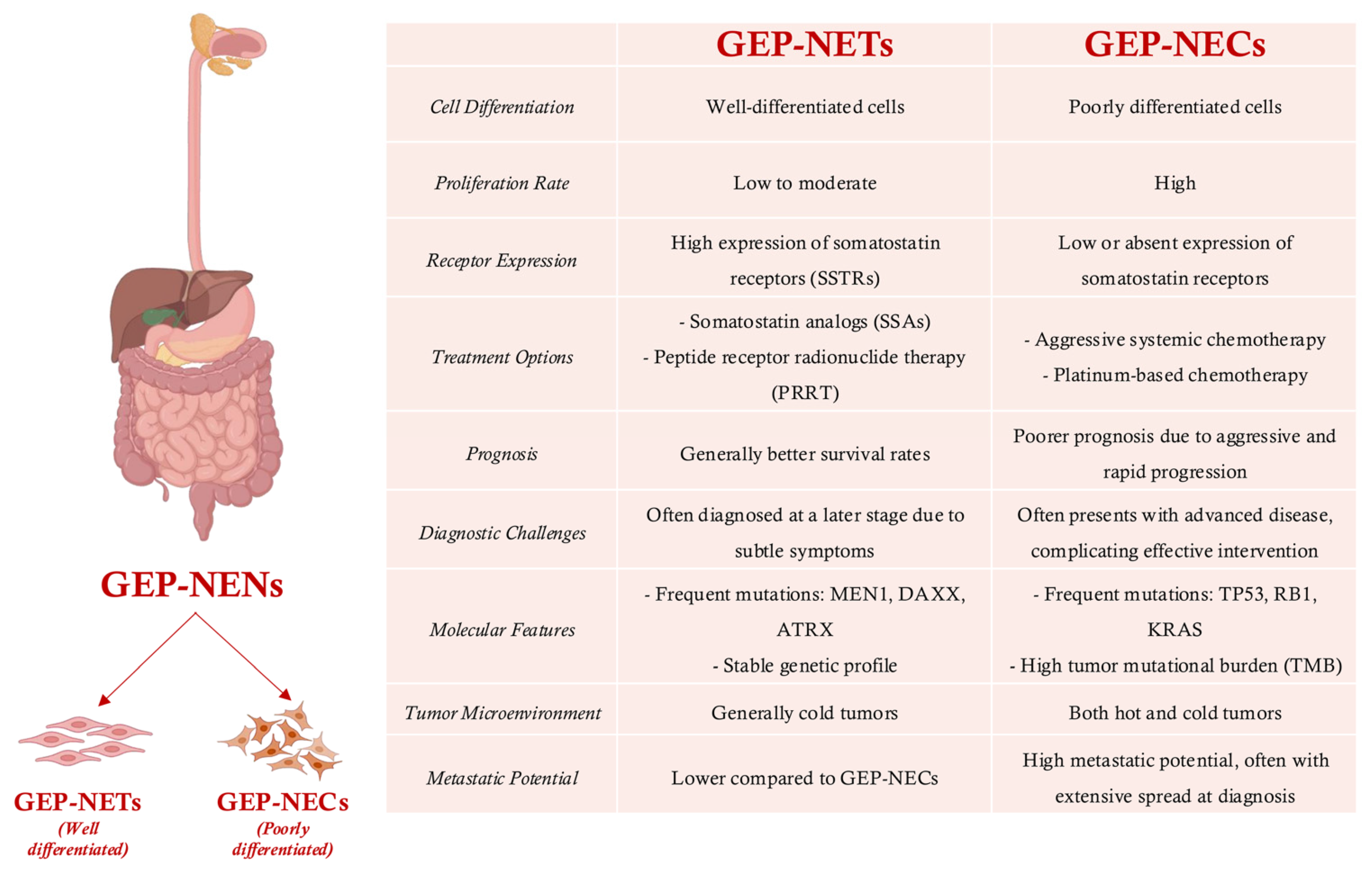
2. Advancing Grading and Classification of GEP-NENs: Molecular Insights, Challenges, and Technological Innovations
3. Tumor Heterogeneity and Therapy Response in GEP-NENs: Challenges and Emerging Solutions
4. Metastasis in GEP-NENs: Molecular Heterogeneity Drives Divergent Tumor Trajectories
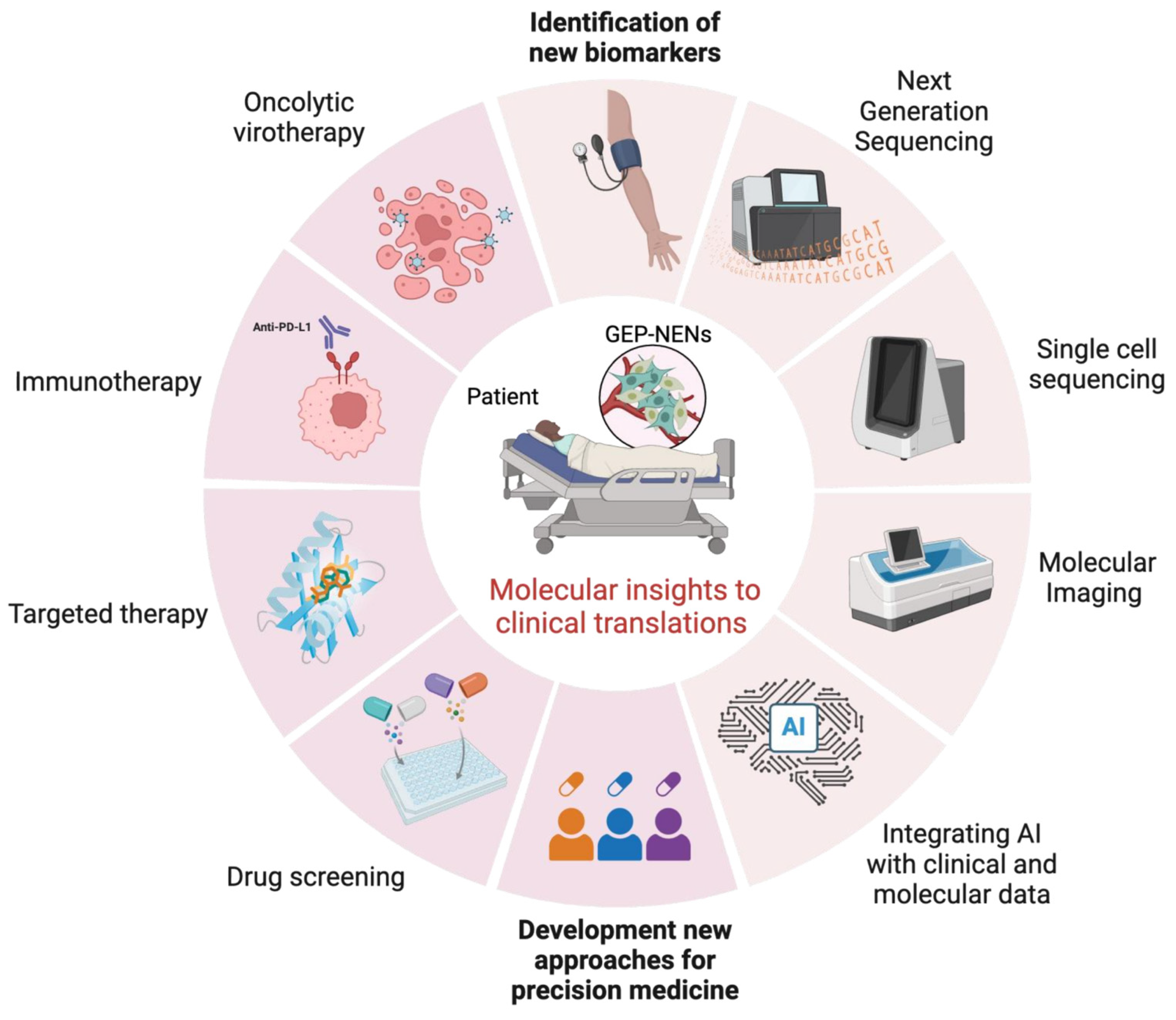
5. Integrating Molecular Insights into Clinical Practice for GEP-NENs: Bridging Diagnostic Gaps and Advancing Therapeutic Strategies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Fujii, M.; Sato, T. Gastroenteropancreatic Neuroendocrine Neoplasms: Genes, Therapies and Models. DMM Dis. Models Mech. 2018, 11, dmm029595. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wu, Z.H.; Lai, J.P. New Insights in Diagnosis and Treatment of Gastroenteropancreatic Neuroendocrine Neoplasms. World J. Gastroenterol. 2022, 28, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Krajewski, K.M.; Nishino, M.; Jagannathan, J.P.; Shinagare, A.B.; Tirumani, S.H.; Ramaiya, N.H. Update on the Management of Gastroenteropancreatic Neuroendocrine Tumors with Emphasis on the Role of Imaging. Am. J. Roentgenol. 2013, 201, 811–824. [Google Scholar] [CrossRef]
- Uri, I.; Grozinsky-Glasberg, S. Current Treatment Strategies for Patients with Advanced Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Clin. Diabetes Endocrinol. 2018, 4, 16. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef]
- Baldelli, R.; Barnabei, A.; Rizza, L.; Isidori, A.M.; Rota, F.; Di Giacinto, P.; Paoloni, A.; Torino, F.; Corsello, S.M.; Lenzi, A.; et al. Somatostatin Analogs Therapy in Gastroenteropancreatic Neuroendocrine Tumors: Current Aspects and New Perspectives. Front. Endocrinol. 2014, 5, 7. [Google Scholar] [CrossRef]
- Appetecchia, M.; Baldelli, R. Somatostatin Analogues in the Treatment of Gastroenteropancreatic Neuroendocrine Tumours, Current Aspects and New Perspectives. J. Exp. Clin. Cancer Res. 2010, 29, 19. [Google Scholar] [CrossRef]
- Sharma, R.; Earla, B.; Baidoo, K.E.; Zeiger, M.A.; Madigan, J.P.; Escorcia, F.E.; Sadowski, S.M. Upregulation of Somatostatin Receptor Type 2 Improves 177Lu-DOTATATE Therapy in Receptor-Deficient Pancreatic Neuroendocrine Tumor Model. Mol. Cancer Ther. 2023, 22, 1052–1062. [Google Scholar] [CrossRef]
- Mollazadegan, K.; Welin, S.; Crona, J. Systemic Treatment of Gastroenteropancreatic Neuroendocrine Carcinoma. Curr. Treat. Opt. Oncol. 2021, 22, 68. [Google Scholar] [CrossRef]
- Tissera, N.S.; Balconi, F.; García-Álvarez, A.; Cubero, J.H.; O’Connor, J.M.; Chacón, M.; Capdevila, J. Maintenance Therapy after First-Line Platinum-Based Chemotherapy in Gastroenteropancreatic Neuroendocrine Carcinomas: A Literature Review. Cancer Treat. Rev. 2025, 132, 102863. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, R.; Lehmann, K.; Winder, T.; Siebenhüner, A.R. What Have We Learnt from the Past—Would Treatment Decisions for GEP-NET Patients Differ between 2012 to 2016 by the New Recommendations in 2022? BMC Cancer 2023, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Q.; Han, C.; Zhen, D.; Lin, R. Variability of the Ki-67 Proliferation Index in Gastroenteropancreatic Neuroendocrine Neoplasms—A Single-Center Retrospective Study. BMC Endocr. Disord. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Luong, T.V.; Watkins, J.; Toumpanakis, C.; Caplin, M.E.; Meyer, T. A Comparison of Ki-67 and Mitotic Count as Prognostic Markers for Metastatic Pancreatic and Midgut Neuroendocrine Neoplasms. Br. J. Cancer 2013, 108, 1838–1845. [Google Scholar] [CrossRef]
- Díez, M.; Teulé, A.; Salazar, R. Gastroenteropancreatic Neuroendocrine Tumors: Diagnosis and Treatment. Ann. Gastroenterol. 2013, 26, 29–36. [Google Scholar]
- Herrera-Martínez, A.D.; Hofland, J.; Hofland, L.J.; Brabander, T.; Eskens, F.A.L.M.; Gálvez Moreno, M.A.; Luque, R.M.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Targeted Systemic Treatment of Neuroendocrine Tumors: Current Options and Future Perspectives. Drugs 2019, 79, 21–42. [Google Scholar] [CrossRef]
- Shi, M.; Fan, Z.; Xu, J.; Yang, J.; Li, Y.; Gao, C.; Su, P.; Wang, X.; Zhan, H. Gastroenteropancreatic Neuroendocrine Neoplasms G3: Novel Insights and Unmet Needs. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188637. [Google Scholar] [CrossRef]
- Couvelard, A.; Cazes, A.; Cros, J. Updates in Histopathological Classification and Tissue Biomarkers of Digestive Neuroendocrine Neoplasms: What the Clinician Should Know. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101795. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; De Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Díaz-López, S.; Jiménez-Castro, J.; Robles-Barraza, C.E.; de Miguel, C.A.; Chaves-Conde, M. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms in Gastroenteropancreatic Tract. World J. Gastrointest. Oncol. 2024, 16, 1166–1179. [Google Scholar] [CrossRef]
- La Rosa, S. Challenges in High-Grade Neuroendocrine Neoplasms and Mixed Neuroendocrine/Non-Neuroendocrine Neoplasms. Endocr. Pathol. 2021, 32, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G. Classification and Pathology of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Relat. Cancer 2011, 18, S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.R.; Aaltonen, L.A. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System; IARC Press: Lyon, France, 2000; Volume 18. [Google Scholar]
- Klimstra, D.S.; Modlin, I.R.; Coppola, D.; Lloyd, R.V.; Suster, S. The Pathologic Classification of Neuroendocrine Tumors: A Review of Nomenclature, Grading, and Staging Systems. Pancreas 2010, 39, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Tang, L.H.; Tomlinson, J.; Gonen, M.; Hochwald, S.N.; Brennan, M.F.; Klimstra, D.S.; Allen, P.J. Determining Prognosis in Patients with Pancreatic Endocrine Neoplasms: Can the WHO Classification System Be Simplified? J. Clin. Oncol. 2007, 25, 5609–5615. [Google Scholar] [CrossRef]
- Bosman, F.; Carneiro, F.; Hruban, R.; Theise, N. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; WHO—OMS; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1. [Google Scholar]
- Ramage, J.K.; Ahmed, A.; Ardill, J.; Bax, N.; Breen, D.J.; Caplin, M.E.; Corrie, P.; Davar, J.; Davies, A.H.; Lewington, V.; et al. Guidelines for the Management of Gastroenteropancreatic Neuroendocrine (Including Carcinoid) Tumours (NETs). Gut 2012, 61, 6–32. [Google Scholar] [CrossRef]
- Plöckinger, U.; Rindi, G.; Arnold, R.; Eriksson, B.; Krenning, E.P.; De Herder, W.W.; Goede, A.; Caplin, M.; Wiedenmann, B.; Öberg, K.; et al. Guidelines for the Diagnosis and Treatment of Neuroendocrine Gastrointestinal Tumours: A Consensus Statement on Behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology 2004, 80, 394–424. [Google Scholar] [CrossRef]
- Oberg, K.; Jelic, S. Neuroendocrine Gastroenteropancreatic Tumors: ESMO Clinical Recommendation for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2009, 20, iv150–iv153. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Couvelard, A.; Komminoth, P.; Körner, M.; Lopes, J.M.; McNicol, A.M.; Nilsson, O.; Perren, A.; Scarpa, A.; et al. TNM Staging of Midgut and Hindgut (Neuro) Endocrine Tumors: A Consensus Proposal Including a Grading System. Virchows Arch. 2007, 451, 757–762. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; De Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM Staging of Foregut (Neuro)Endocrine Tumors: A Consensus Proposal Including a Grading System. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef]
- Kulke, M.H.; Anthony, L.B.; Bushnell, D.L.; De Herder, W.W.; Goldsmith, S.J.; Klimstra, D.S.; Marx, S.J.; Pasieka, L.J.L. NANETS Treatment Guidelines Well-Differentiated Neuroendocrine Tumors of the Stomach and Pancreas. Pancreas 2010, 39, 735–752. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S. Diagnostic, Prognostic, and Predictive Role of Ki67 Proliferative Index in Neuroendocrine and Endocrine Neoplasms: Past, Present, and Future. Endocr. Pathol. 2023, 34, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, Y.; Law, C.; Singh, S.; Wright, F.C. Patient Experiences of Having a Neuroendocrine Tumour: A Qualitative Study. Eur. J. Oncol. Nurs. 2013, 17, 541–545. [Google Scholar] [CrossRef]
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.C.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver Metastases of Small Intestine Neuroendocrine Tumors: Ki-67 Heterogeneity and World Health Organization Grade Discordance with Primary Tumors. Am. J. Clin. Pathol. 2015, 143, 398–404. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Modlin, I.R.; Adsay, N.V.; Chetty, R.; Deshpande, V.; Gönen, M.; Jensen, R.T.; Kidd, M.; Kulke, M.H.; Lloyd, R.V.; et al. Pathology Reporting of Neuroendocrine Tumors: Application of the Delphic Consensus Process to the Development of a Minimum Pathology Data Set. Am. J. Surg. Pathol. 2010, 34, 300–313. [Google Scholar] [CrossRef]
- Capelli, P.; Fassan, M.; Scarpa, A. Pathology-Grading and Staging of GEP-NETs. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 705–717. [Google Scholar] [CrossRef]
- Mete, O.; Wenig, B.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head. Neck Pathol. 2022, 16, 123–142. [Google Scholar] [CrossRef]
- Vogele, D.; Schmidt, S.A.; Gnutzmann, D.; Thaiss, W.M.; Ettrich, T.J.; Kornmann, M.; Beer, M.; Juchems, M.S. Gastroenteropancreatic Neuroendocrine Tumors—Current Status and Advances in Diagnostic Imaging. Diagnostics 2023, 13, 2741. [Google Scholar] [CrossRef]
- Yin, L.; Wang, R.; Ma, X.; Jiang, K.; Hu, Y.; Zhao, X.; Zhang, L.; Wang, Z.; Long, T.; Lu, M.; et al. Exploring the Expression of DLL3 in Gastroenteropancreatic Neuroendocrine Neoplasms and Its Potential Diagnostic Value. Sci. Rep. 2025, 15, 3287. [Google Scholar] [CrossRef]
- KOHNO, S. Diagnosis and Surgical Treatment of Gastroenteropancreatic Neuroendocrine Neoplasms: A Literature Review. Cancer Diagn. Progn. 2022, 2, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.T.; Luo, Y.; Chan, T.; Peng, Z.; Chen, J.; Chen, M.; Li, Z.P. CT Evaluation of Gastroenteric Neuroendocrine Tumors: Relationship between CT Features and the Pathologic Classification. Am. J. Roentgenol. 2014, 202, W260–W266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Fan, Y.B.; Jing, R.; Getu, M.A.; Chen, W.Y.; Zhang, W.; Dong, H.X.; Dakal, T.C.; Hayat, A.; Cai, H.J.; et al. Gastroenteropancreatic Neuroendocrine Neoplasms: Current Development, Challenges, and Clinical Perspectives. Mil. Med. Res. 2024, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Sabella, G.; Simbolo, M.; Lagano, V.; Centonze, G.; Gentili, M.; Mangogna, A.; Coppa, J.; Munari, G.; Businello, G.; et al. Comprehensive Genomic and Transcriptomic Characterization of High-Grade Gastro-Entero-Pancreatic Neoplasms. Br. J. Cancer 2024, 131, 159–170. [Google Scholar] [CrossRef]
- Vesterinen, T.; Säilä, J.; Blom, S.; Pennanen, M.; Leijon, H.; Arola, J. Automated Assessment of Ki-67 Proliferation Index in Neuroendocrine Tumors by Deep Learning. APMIS 2022, 130, 11–20. [Google Scholar] [CrossRef]
- Poleé, I.N.; Hermans, B.C.M.; van der Zwan, J.M.; Bouwense, S.A.W.; Dercksen, M.W.; Eskens, F.A.L.M.; Havekes, B.; Hofland, J.; Kerkhofs, T.M.A.; Klümpen, H.J.; et al. Long-Term Survival in Patients with Gastroenteropancreatic Neuroendocrine Neoplasms: A Population-Based Study. Eur. J. Cancer 2022, 172, 252–263. [Google Scholar] [CrossRef]
- Yao, H.; Hu, G.; Jiang, C.; Fan, M.; Yuan, L.; Shi, H.; Lin, R. Epidemiologic Trends and Survival of Early-Onset Gastroenteropancreatic Neuroendocrine Neoplasms. Front. Endocrinol. 2023, 14, 1241724. [Google Scholar] [CrossRef]
- Li, X.; Fan, Y.; Tong, J.; Lou, M. Risk Factors, Prognostic Factors, and Nomograms for Distant Metastases in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Population-Based Study. Front. Endocrinol. 2024, 15, 1264952. [Google Scholar] [CrossRef]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive Genomic Profiling of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Clin. Cancer Res. 2020, 26, 5943–5951. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Horai, Y.; Mizukawa, M.; Nishina, H.; Nishikawa, S.; Ono, Y.; Takemoto, K.; Baba, N. Quantification of Histopathological Findings Using a Novel Image Analysis Platform. J. Toxicol. Pathol. 2019, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-G.; Park, S.; Schäffer, A.A.; Jiang, P.; Ruppin, E. Hallmarks of Artificial Intelligence Contributions to Precision Oncology. Nat. Cancer 2025, 6, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Montalto, M.C.; Edwards, R. And They Said It Couldn’t Be Done: Predicting Known Driver Mutations from H&E Slides. J. Pathol. Inform. 2019, 10, 17. [Google Scholar] [PubMed]
- Dimitriou, N.; Arandjelović, O.; Caie, P.D. Corrigendum: Deep Learning for Whole Slide Image Analysis: An Overview (Front. Med. (2019), 6, (264), 10.3389/Fmed.2019.00264). Front. Med. 2020, 7, 419. [Google Scholar]
- Fazio, N.; La Salvia, A. Precision Medicine in Gastroenteropancreatic Neuroendocrine Neoplasms: Where Are We in 2023? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101794. [Google Scholar] [CrossRef]
- Kumar-Sinha, C.; Chinnaiyan, A.M. Precision Oncology in the Age of Integrative Genomics. Nat. Biotechnol. 2018, 36, 46–60. [Google Scholar] [CrossRef]
- Sosinsky, A.; Ambrose, J.; Cross, W.; Turnbull, C.; Henderson, S.; Jones, L.; Hamblin, A.; Arumugam, P.; Chan, G.; Chubb, D.; et al. Insights for Precision Oncology from the Integration of Genomic and Clinical Data of 13,880 Tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 2024, 30, 279–289. [Google Scholar] [CrossRef]
- Poudel, B.H.; Koks, S. The Whole Transcriptome Analysis Using FFPE and Fresh Tissue Samples Identifies the Molecular Fingerprint of Osteosarcoma. Exp. Biol. Med. 2024, 249, 10161. [Google Scholar] [CrossRef]
- Iwahashi, N.; Umakoshi, H.; Ogata, M.; Fukumoto, T.; Kaneko, H.; Terada, E.; Katsuhara, S.; Uchida, N.; Sasaki, K.; Yokomoto-Umakoshi, M.; et al. Whole Transcriptome Profiling of Adrenocortical Tumors Using Formalin-Fixed Paraffin-Embedded Samples. Front. Endocrinol. 2022, 13, 808331. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.H.; Liu, X.; Xu, H.; Chen, C.; Zhou, X.; Liu, Y.; Ma, X. Application of Radiomics Analysis Based on CT Combined with Machine Learning in Diagnostic of Pancreatic Neuroendocrine Tumors Patient’s Pathological Grades. Front. Oncol. 2021, 10, 521831. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Katz, S.; Kontos, D.; Roshkovan, L. State of the Art: Radiomics and Radiomics-Related Artificial Intelligence on the Road to Clinical Translation. BJR|Open 2023, 6, tzad004. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Yu, Q. Intra-Tumor Heterogeneity of Cancer Cells and Its Implications for Cancer Treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor Heterogeneity Reshapes the Tumor Microenvironment to Influence Drug Resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef]
- Grillo, F.; Albertelli, M.; Brisigotti, M.P.; Borra, T.; Boschetti, M.; Fiocca, R.; Ferone, D.; Mastracci, L. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology 2016, 103, 452–459. [Google Scholar] [CrossRef]
- Varghese, D.G.; Del Rivero, J.; Bergsland, E. Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2023, 15, 3712. [Google Scholar] [CrossRef]
- Phan, A.T.; Kunz, P.L.; Reidy-Lagunes, D.L. New and Emerging Treatment Options for Gastroenteropancreatic Neuroendocrine Tumors. Clin. Adv. Hematol. Oncol. 2015, 13, 1–18. [Google Scholar]
- Chan, J.A.; Kulke, M.H. Progress in the Treatment of Neuroendocrine Tumors. Curr. Oncol. Rep. 2009, 11, 193–199. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blazević, A.; Minczeles, N.S.; Feelders, R.A.; De Herder, W.W.; Hofland, J. Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e3665–e3672. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Arevalo, S.; Ibáñez-Costa, A.; Blázquez-Encinas, R.; Branco, M.R.; Vázquez-Borrego, M.C.; Herrera-Martínez, A.D.; Venegas-Moreno, E.; Serrano-Blanch, R.; Arjona-Sánchez, Á.; Gálvez-Moreno, M.A.; et al. Epigenetic and Post-Transcriptional Regulation of Somatostatin Receptor Subtype 5 (SST5) in Pituitary and Pancreatic Neuroendocrine Tumors. Mol. Oncol. 2022, 16, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Klomp, M.J.; Dalm, S.U.; de Jong, M.; Feelders, R.A.; Hofland, J.; Hofland, L.J. Epigenetic Regulation of Somatostatin and Somatostatin Receptors in Neuroendocrine Tumors and Other Types of Cancer. Rev. Endocr. Metab. Disord. 2021, 22, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Klieser, E.; Urbas, R.; Stättner, S.; Primavesi, F.; Jäger, T.; Dinnewitzer, A.; Mayr, C.; Kiesslich, T.; Holzmann, K.; Di Fazio, P.; et al. Comprehensive Immunohistochemical Analysis of Histone Deacetylases in Pancreatic Neuroendocrine Tumors: HDAC5 as a Predictor of Poor Clinical Outcome. Hum. Pathol. 2017, 65, 41–52. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Nikolaienko, O.; Deng, W.; Lothe, I.M.B.; Couvelard, A.; Hjortland, G.O.; Sundlöv, A.; Svensson, J.; et al. The Molecular Characteristics of High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Relat. Cancer 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Klomp, M.J.; Refardt, J.; van Koetsveld, P.M.; Campana, C.; Dalm, S.U.; Dogan, F.; van Velthuysen, M.L.F.; Feelders, R.A.; de Herder, W.W.; Hofland, J.; et al. Epigenetic Regulation of SST2 Expression in Small Intestinal Neuroendocrine Tumors. Front. Endocrinol. 2023, 14, 1184436. [Google Scholar] [CrossRef]
- Capozzi, M.; Von Arx, C.; De Divitiis, C.; Ottaiano, A.; Tatangelo, F.; Romano, I.M.; Tafuto, S. Antiangiogenic Therapy in Pancreatic Neuroendocrine Tumors. Anticancer Res. 2016, 36, 5025–5030. [Google Scholar] [CrossRef]
- Svejda, B.; Kidd, M.; Kazberouk, A.; Lawrence, B.; Pfragner, R.; Modlin, I.M. Limitations in Small Intestinal Neuroendocrine Tumor Therapy by MTor Kinase Inhibition Reflect Growth Factor-Mediated PI3K Feedback Loop Activation via ERK1/2 and AKT. Cancer 2011, 117, 4141–4154. [Google Scholar] [CrossRef]
- Zitzmann, K.; von Rüden, J.; Brand, S.; Göke, B.; Lichtl, J.; Spöttl, G.; Auernhammer, C.J. Compensatory Activation of Akt in Response to MTOR and Raf Inhibitors—A Rationale for Dual-Targeted Therapy Approaches in Neuroendocrine Tumor Disease. Cancer Lett. 2010, 295, 100–109. [Google Scholar] [CrossRef]
- Shi, C.; Morse, M.A. Mechanisms of Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2022, 14, 6114. [Google Scholar] [CrossRef]
- Passacantilli, I.; Capurso, G.; Archibugi, L.; Calabretta, S.; Caldarola, S.; Loreni, F.; Fave, G.D.; Sette, C. Combined Therapy with RAD001 e BEZ235 Overcomes Resistance of PET Immortalized Cell Lines to MTOR Inhibition. Oncotarget 2014, 5, 5381–5391. [Google Scholar] [CrossRef] [PubMed]
- Avniel-Polak, S.; Leibowitz, G.; Riahi, Y.; Glaser, B.; Gross, D.J.; Grozinsky-Glasberg, S. Abrogation of Autophagy by Chloroquine Alone or in Combination with MTOR Inhibitors Induces Apoptosis in Neuroendocrine Tumor Cells. Neuroendocrinology 2016, 103, 724–737. [Google Scholar] [CrossRef]
- Song, Y.L.; Weng, J.H.; Zhao, D.C.; Zhang, J.L.; Chen, Y.J.; Xu, B.H. SQSTM1/P62 Is a Prognostic Molecular Marker and Potential Therapeutic Target for Pancreatic Neuroendocrine Tumours. Endocrine 2024, 85, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Chander, G.; Martinez, M.; Ward, C.; Khan, S.R.; Naik, M.; Barwick, T.; Aboagye, E.; Sharma, R. Study Protocol of LANTana: A Phase Ib Study to Investigate Epigenetic Modification of Somatostatin Receptor-2 with ASTX727 to Improve Therapeutic Outcome with [177Lu]Lu-DOTA-TATE in Patients with Metastatic Neuroendocrine Tumours, UK. BMJ Open 2023, 13, e075221. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in Patients with Somatostatin-Receptor-Positive Solid Tumors: 5-Year Follow-up of Hematological and Renal Toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Chan, H.S.; De Blois, E.; Morgenstern, A.; Bruchertseifer, F.; De Jong, M.; Breeman, W.; Konijnenberg, M. In Vitro Comparison Of213Bi- And177Lu-Radiation for Peptide Receptor Radionuclide Therapy. PLoS ONE 2017, 12, e0181473. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of Ipilimumab and Nivolumab in Patients with High-Grade Neuroendocrine Neoplasms. ESMO Open 2022, 7, 100364. [Google Scholar] [CrossRef]
- Fine, R.L.; Gulati, A.P.; Krantz, B.A.; Moss, R.A.; Schreibman, S.; Tsushima, D.A.; Mowatt, K.B.; Dinnen, R.D.; Mao, Y.; Stevens, P.D.; et al. Capecitabine and Temozolomide (CAPTEM) for Metastatic, Well-Differentiated Neuroendocrine Cancers: The Pancreas Center at Columbia University Experience. Cancer Chemother. Pharmacol. 2013, 71, 663–670. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.T.; Helm, J.; Kvols, L. First-Line Chemotherapy with Capecitabine and Temozolomide in Patients with Metastatic Pancreatic Endocrine Carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef]
- Kress, O.; Wagner, H.J.; Wied, M.; Klose, K.J.; Arnold, R.; Alfke, H. Transarterial Chemoembolization of Advanced Liver Metastases of Neuroendocrine Tumors—A Retrospective Single-Center Analysis. Digestion 2003, 68, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.H.; Charnsangavej, C.; Ajani, J.; Samaan, N.A.; Richli, W.; Wallace, S. The Carcinoid Syndrome: Palliation by Hepatic Artery Embolization. Am. J. Roentgenol. 1986, 147, 149–154. [Google Scholar] [CrossRef]
- Puppala, S. Technical Update on Transcatheter Arterial Chemoembolization. Hepatoma Res. 2019, 5, 44. [Google Scholar] [CrossRef][Green Version]
- Therasse, E.; Breittmayer, F.; Roche, A.; De Baere, T.; Indushekar, S.; Ducreux, M.; Lasser, P.; Elias, D.; Rougier, P. Transcatheter Chemoembolization of Progressive Carcinoid Liver Metastasis. Radiology 1993, 189, 541–547. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Kaltsas, G.; Kaltsatou, M.; Lev-Cohain, N.; Klimov, A.; Vergadis, V.; Uri, I.; Bloom, A.I.; Gross, D.J. Hepatic Intra-Arterial Therapies in Metastatic Neuroendocrine Tumors: Lessons from Clinical Practice. Endocrine 2018, 60, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.S.; Dezarn, W.A.; McNeillie, P.; Coldwell, D.; Nutting, C.; Carter, D.; Murthy, R.; Rose, S.; Warner, R.R.P.; Liu, D.; et al. Radioembolization for Unresectable Neuroendocrine Hepatic Metastases Using Resin 90Y-Microspheres: Early Results in 148 Patients. Am. J. Clin. Oncol. Cancer Clin. Trials 2008, 31, 271–279. [Google Scholar] [CrossRef]
- Cives, M.; Pelle, E.; Strosberg, J. Emerging Treatment Options for Gastroenteropancreatic Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 3655. [Google Scholar] [CrossRef]
- Perez, K.; Chan, J. Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Surg. Pathol. Clin. 2019, 12, 1045–1053. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus Long-Acting Octreotide versus High-dose Long-Acting Octreotide for the Treatment of Newly Diagnosed, Advanced Grade 2–3, Well-Differentiated, Gastroenteropancreatic Neuroendocrine Tumours (NETTER-2): An Open-Label, Randomised, Phase 3 Study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Z.; Liu, Y.; Guo, L.; Teng, L.; Guo, L.; Liang, L.; Wang, J.; Gao, J.; Li, R.; et al. Genomic Characterization Reveals Distinct Mutation Landscapes and Therapeutic Implications in Neuroendocrine Carcinomas of the Gastrointestinal Tract. Cancer Commun. 2022, 42, 1367–1386. [Google Scholar] [CrossRef]
- Reccia, I.; Pai, M.; Kumar, J.; Spalding, D.; Frilling, A. Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2023, 15, 1861. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.J.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and Prognostic Factors for Treatment and Survival in 305 Patients with Advanced Gastrointestinal Poorly Differentiated Neuroendocrine Carcinoma: The NORDIC NEC Study. J. Clin. Oncol. 2012, 30, 4015. [Google Scholar] [CrossRef]
- Elvebakken, H.; Hjortland, G.O.; Garresori, H.; Andresen, P.A.; Janssen, E.A.M.; Vintermyr, O.K.; Lothe, I.M.B.; Sorbye, H. Impact of KRAS and BRAF Mutations on Treatment Efficacy and Survival in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J. Neuroendocrinol. 2023, 35, e13256. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Oprea, G.; Botrus, G.; Gbolahan, O.B.; Lesinski, G.B.; Avadhani, V.; Zakka, K.M.; Alese, O.B. Investigation into the Immune Microenvironment of Gastroenteropancreatic High-Grade Neuroendocrine Carcinoma. J. Clin. Oncol. 2023, 41, e16244. [Google Scholar] [CrossRef]
- McDonald, A.; Avadhani, V.; Oprea-Ilies, G.; Zakka, K.; Lesinski, G.B.; Gbolahan, O.B.; Alese, O. A Pilot Study of the Immune Microenvironment of GI Neuroendocrine Carcinoma. Endocr. Relat. Cancer 2024, 31, e240046. [Google Scholar] [CrossRef]
- Rinke, A.; Gress, T.M. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion 2017, 95, 109–114. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-Cell Profiling of Tumor Heterogeneity and the Microenvironment in Advanced Non-Small Cell Lung Cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- SInha, S.; Vegesna, R.; Dhruba, S.R.; Wu, W.; Kerr, D.L.; Stroganov, O.V.; Grishagin, I.; Aldape, K.D.; Blakely, C.M.; Jiang, P.; et al. Predicting Patient Treatment Response and Resistance via Single-Cell Transcriptomics of Their Tumors. J. Clin. Oncol. 2022, 40, e20540. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Liu, C.; Yang, J.; Lin, Q.; Zheng, S.; Chen, C.; Zhou, Q.; Chen, R. Single-Cell Rna Sequencing Reveals Spatiotemporal Heterogeneity and Malignant Progression in Pancreatic Neuroendocrine Tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef]
- Someach, E.; Halder, D.; Spitzer, A.; Barbolin, C.; Tyler, M.; Halperin, R.; Biton, M.; Tirosh, A.; Tirosh, I. Subtypes and Proliferation Patterns of Small Intestine Neuroendocrine Tumors Revealed by Single Cell RNA Sequencing. 2024. Available online: https://elifesciences.org/reviewed-preprints/101153v1 (accessed on 29 April 2025).
- Yan, T.; Cui, H.; Zhou, Y.; Yang, B.; Kong, P.; Zhang, Y.; Liu, Y.; Wang, B.; Cheng, Y.; Li, J.; et al. Multi-Region Sequencing Unveils Novel Actionable Targets and Spatial Heterogeneity in Esophageal Squamous Cell Carcinoma. Nat. Commun. 2019, 10, 1670. [Google Scholar] [CrossRef]
- Amirouchene-Angelozzi, N.; Swanton, C.; Bardelli, A. Tumor Evolution as a Therapeutic Target. Cancer Discov. 2017, 7, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Laisné, M.; Lupien, M.; Vallot, C. Epigenomic Heterogeneity as a Source of Tumour Evolution. Nat. Rev. Cancer 2024, 25, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S.; et al. Spatial Transcriptomics Reveals Distinct and Conserved Tumor Core and Edge Architectures That Predict Survival and Targeted Therapy Response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Sawyer, T.W.; Witten, B.L.; Song, H.; Else, T.; Merchant, J.L. Spatial Profiling Reveals Tissue-Specific Neuro-Immune Interactions in Gastroenteropancreatic Neuroendocrine Tumors. J. Pathol. 2024, 262, 362–376. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Ren, Y.; Liu, S.; Ba, Y.; Zuo, A.; Luo, P.; Cheng, Q.; Xu, H.; Han, X. Multi-Stage Mechanisms of Tumor Metastasis and Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 270. [Google Scholar] [CrossRef]
- Lauricella, E.; Mandriani, B.; Cavallo, F.; Pezzicoli, G.; Chaoul, N.; Porta, C.; Cives, M. Angiogenesis in NENs, with a Focus on Gastroenteropancreatic NENs: From Biology to Current and Future Therapeutic Implications. Front. Oncol. 2022, 12, 957068. [Google Scholar] [CrossRef]
- Kuiper, P.; Hawinkels, L.J.A.C.; de Jonge-Muller, E.S.M.; Biemond, I.; Lamers, C.B.H.W.; Verspaget, H.W. Angiogenic Markers Endoglin and Vascular Endothelial Growth Factor in Gastroenteropancreatic Neuroendocrine Tumors. World J. Gastroenterol. 2011, 17, 219–225. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, B.; Chen, H. Gastroenteropancreatic Neuroendocrine Neoplasms: Epidemiology, Genetics, and Treatment. Front. Endocrinol. 2024, 15, 1424839. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the Mesenchymal-Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Yonemori, K.; Kurahara, H.; Maemura, K.; Mataki, Y.; Sakoda, M.; Iino, S.; Ueno, S.; Shinchi, H.; Natsugoe, S. Impact of Snail and E-Cadherin Expression in Pancreatic Neuroendocrine Tumors. Oncol. Lett. 2017, 14, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, E.; Campione, S.; Pignatiello, S.; Borrelli, G.; De Dominicis, G.; De Rosa, N.; De Caro, M.D.B. Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features. Diagnostics 2020, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF Serum Values Are Associated with Locoregional Spread of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Tabernero, J. A Shining Light in the Darkness for the Treatment of Pancreatic Neuroendocrine Tumors. Cancer Discov. 2011, 1, 213–221. [Google Scholar] [CrossRef]
- Corbo, V.; Beghelli, S.; Bersani, S.; Antonello, D.; Talamini, G.; Brunelli, M.; Capelli, P.; Falconi, M.; Scarpa, A. Pancreatic Endocrine Tumours: Mutational and Immunohistochemical Survey of Protein Kinases Reveals Alterations in Targetable Kinases in Cancer Cell Lines and Rare Primaries. Ann. Oncol. 2012, 23, 127–134. [Google Scholar] [CrossRef]
- van Riet, J.; van de Werken, H.J.G.; Cuppen, E.; Eskens, F.A.L.M.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The Genomic Landscape of 85 Advanced Neuroendocrine Neoplasms Reveals Subtype-Heterogeneity and Potential Therapeutic Targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef]
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic Heterogeneity of Primary Lesion and Metastasis in Small Intestine Neuroendocrine Tumors. Sci. Rep. 2018, 8, 3811. [Google Scholar] [CrossRef]
- Wong, H.L.; Yang, K.C.; Shen, Y.; Zhao, E.Y.; Loree, J.M.; Kennecke, H.F.; Kalloger, S.E.; Karasinska, J.M.; Lim, H.J.; Mungall, A.J.; et al. Molecular Characterization of Metastatic Pancreatic Neuroendocrine Tumors (PNETs) Using Whole-Genome and Transcriptome Sequencing. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002329. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, C.; Cheng, Z.; Chen, R.; Li, Y.; Su, F.; Shi, Y.; Tan, H. Molecular and Functional Heterogeneity of Primary Pancreatic Neuroendocrine Tumors and Metastases. Neuroendocrinology 2023, 113, 943–956. [Google Scholar] [CrossRef]
- Roy, S.; LaFramboise, W.A.; Liu, T.C.; Cao, D.; Luvison, A.; Miller, C.; Lyons, M.A.; O’Sullivan, R.J.; Zureikat, A.H.; Hogg, M.E.; et al. Loss of Chromatin-Remodeling Proteins and/or CDKN2A Associates with Metastasis of Pancreatic Neuroendocrine Tumors and Reduced Patient Survival Times. Gastroenterology 2018, 154, 2060–2063.e8. [Google Scholar] [CrossRef]
- Hofving, T.; Elias, E.; Rehammar, A.; Inge, L.; Altiparmak, G.; Persson, M.; Kristiansson, E.; Johansson, M.E.; Nilsson, O.; Arvidsson, Y. SMAD4 Haploinsufficiency in Small Intestinal Neuroendocrine Tumors. BMC Cancer 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Samsom, K.G.; Levy, S.; van Veenendaal, L.M.; Roepman, P.; Kodach, L.L.; Steeghs, N.; Valk, G.D.; Wouter Dercksen, M.; Kuhlmann, K.F.D.; Verbeek, W.H.M.; et al. Driver Mutations Occur Frequently in Metastases of Well-Differentiated Small Intestine Neuroendocrine Tumours. Histopathology 2021, 78, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, J.; Tan, Y.; Sun, J.; Zhou, M. Single-Cell and Bulk Transcriptome Analysis Reveals Tumor Cell Heterogeneity and Underlying Molecular Program in Uveal Melanoma. J. Transl. Med. 2024, 22, 1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Zheng, S.; Yao, Y.; Wang, S.; Wang, X.; Yin, E.; Zeng, Q.; Zhang, C.; Zhang, G.; et al. Molecular Subtypes of Neuroendocrine Carcinomas: A Cross-Tissue Classification Framework Based on Five Transcriptional Regulators. Cancer Cell 2024, 42, 1106–1125.e8. [Google Scholar] [CrossRef]
- Patte, C.; Pommier, R.M.; Ferrari, A.; Chung, F.F.-L.; Ouzounova, M.; Moullé, P.; Richaud, M.; Khoueiry, R.; Hervieu, M.; Breusa, S.; et al. Comprehensive Molecular Portrait Reveals Genetic Diversity and Distinct Molecular Subtypes of Small Intestinal Neuroendocrine Tumors. Nat. Commun. 2025, 16, 2197. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Díaz-Pérez, J.A.; Martínez del Prado, M.P.; Alonso Orduña, V.; Sevilla-García, I.; Villabona-Artero, C.; Beguiristain-Gómez, A.; Llanos-Muñoz, M.; et al. Incidence, Patterns of Care and Prognostic Factors for Outcome of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef]
- Meng, Q.; Halfdanarson, T.R.; Bornhorst, J.; Jann, H.; Shaheen, S.; Zhang, S.R.; Halperin, D.M. Chromogranin A as Surveillance Biomarker in Patients with CARcinoids (CASPAR). Endocr. Abstr. 2023, 41, e16242. [Google Scholar] [CrossRef]
- Seregni, E.; Ferrari, L.; Bajetta, E.; Martinetti, A.; Bombardieri, E. Clinical Significance of Blood Chromogranin A Measurement in Neuroendocrine Tumours. Ann. Oncol. 2001, 12, S69–S72. [Google Scholar] [CrossRef]
- Chou, W.C.; Hung, Y.S.; Hsu, J.T.; Chen, J.S.; Lu, C.H.; Hwang, T.L.; Rau, K.M.; Yeh, K.Y.; Chen, T.C.; Sun, C.F. Chromogranin a Is a Reliable Biomarker for Gastroenteropancreatic Neuroendocrine Tumors in an Asian Population of Patients. Neuroendocrinology 2012, 95, 344–350. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One Hundred Years after “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Korse, C.M.; Taal, B.G.; Van Velthuysen, M.L.F.; Visser, O. Incidence and Survival of Neuroendocrine Tumours in the Netherlands According to Histological Grade: Experience of Two Decades of Cancer Registry. Eur. J. Cancer 2013, 49, 1975–1983. [Google Scholar] [CrossRef]
- Dureja, S.; McDonnell, M.; Van Genechten, D.; Bouvier, C.; Kolarova, T.; O’Toole, D.; Singh, H.; Chen, J.; Howe, J.; Singh, S.; et al. Global Challenges in Access to Diagnostics and Treatment for Neuroendocrine Tumor (NET) Patients. J. Neuroendocrinol. 2023, 35, e13310. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef]
- Modlin, I.M.; Drozdov, I.; Kidd, M. The Identification of Gut Neuroendocrine Tumor Disease by Multiple Synchronous Transcript Analysis in Blood. PLoS ONE 2013, 8, e63364. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Drozdov, I.A.; Chirindel, A.; Nicolas, G.; Imagawa, D.; Gulati, A.; Tsuchikawa, T.; Prasad, V.; Halim, A.B.; Strosberg, J. NETest® 2.0—A Decade of Innovation in Neuroendocrine Tumor Diagnostics. J. Neuroendocrinol. 2025, 37, e70002. [Google Scholar] [CrossRef]
- Rossi, R.E.; La Salvia, A. NETest and Gastro-Entero-Pancreatic Neuroendocrine Tumors: Still Far from Routine Clinical Application? A Systematic Review. Genes 2025, 16, 161. [Google Scholar] [CrossRef]
- Hijioka, S.; Hosoda, W.; Matsuo, K.; Ueno, M.; Furukawa, M.; Yoshitomi, H.; Kobayashi, N.; Ikeda, M.; Ito, T.; Nakamori, S.; et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin. Cancer Res. 2017, 23, 4625–4632. [Google Scholar] [CrossRef]
- Mounajjed, T.; Henry, M.; Zhang, L. Ki-67 Proliferative Index in Gastroenteropancreatic Neuroendocrine Neoplasms. Pathol. Case Rev. 2014, 19, 256–260. [Google Scholar] [CrossRef]
- Tao, Z.; Xue, R.; Wei, Z.; Qin, L.; Bai, R.; Liu, N.; Wang, J.; Wang, C. The Assessment of Ki-67 for Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasm Patients: A Systematic Review and Meta-Analysis. Transl. Cancer Res. 2023, 12, 1980–1991. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming Cold Tumors: A Combination Strategy of Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef] [PubMed]
- Popa Ilie, I.R.; Georgescu, C.E. Immunotherapy in Gastroenteropancreatic Neuroendocrine Neoplasia. Neuroendocrinology 2023, 113, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Kojima, M.; Suzuki, T.; Sugimoto, M.; Kobayashi, S.; Takahashi, S.; Konishi, M.; Gotohda, N.; Ikeda, M.; Nakatsura, T.; et al. Profiling the Tumour Immune Microenvironment in Pancreatic Neuroendocrine Neoplasms with Multispectral Imaging Indicates Distinct Subpopulation Characteristics Concordant with WHO 2017 Classification. Sci. Rep. 2018, 8, 13166. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, P.; Rychcik-Pazyrska, P.; Stec, R. Defining Tumor Microenvironment as a Possible Target for Effective GEP-NENs Immunotherapy—A Systematic Review. Cancers 2023, 15, 5232. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.; Bhosale, P.; Mahvash, A.; Estrella, J.S.; Rubin, L.; Morani, A.C.; Knafl, M.; et al. Assessment of Clinical Response Following Atezolizumab and Bevacizumab Treatment in Patients with Neuroendocrine Tumors a Nonrandomized Clinical Trial. JAMA Oncol. 2022, 8, 904–909. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Han, G.R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine Learning in Point-of-Care Testing: Innovations, Challenges, and Opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Zehra, T.; Parwani, A.; Abdul-Ghafar, J.; Ahmad, Z. A Suggested Way Forward for Adoption of AI-Enabled Digital Pathology in Low Resource Organizations in the Developing World. Diagn. Pathol. 2023, 18, 68. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, S.B.; Shanker, R.M.; Madigan, J.P.; Sadowski, S.M. The Evolving Landscape of GEP-NENs in the Era of Precision Oncology: Molecular Insights into Tumor Heterogeneity. Cancers 2025, 17, 2080. https://doi.org/10.3390/cancers17132080
Mukherjee SB, Shanker RM, Madigan JP, Sadowski SM. The Evolving Landscape of GEP-NENs in the Era of Precision Oncology: Molecular Insights into Tumor Heterogeneity. Cancers. 2025; 17(13):2080. https://doi.org/10.3390/cancers17132080
Chicago/Turabian StyleMukherjee, Sunanda Biswas, Rachyl M. Shanker, James P. Madigan, and Samira M. Sadowski. 2025. "The Evolving Landscape of GEP-NENs in the Era of Precision Oncology: Molecular Insights into Tumor Heterogeneity" Cancers 17, no. 13: 2080. https://doi.org/10.3390/cancers17132080
APA StyleMukherjee, S. B., Shanker, R. M., Madigan, J. P., & Sadowski, S. M. (2025). The Evolving Landscape of GEP-NENs in the Era of Precision Oncology: Molecular Insights into Tumor Heterogeneity. Cancers, 17(13), 2080. https://doi.org/10.3390/cancers17132080





