miR-195-5p Suppresses KRT80 Expression Inducing Cell Cycle Arrest in Colon Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Paired CRC and Non-Tumor Tissues
2.2. Immunohistochemistry of KRT80 in FFPE Human Samples
2.3. Cell Cultures
2.4. RNA Extraction and RT-PCR of Cell Cultures, Human and Mouse Sections
2.5. Protein Extraction and Western Blot Analysis
2.6. Cell Cycle
2.7. Immunofluorescence in Mouse Samples
2.8. Bioinformatic and Statistical Analyses
3. Results
3.1. miR-195-5p as KRT80 Potential Regulator
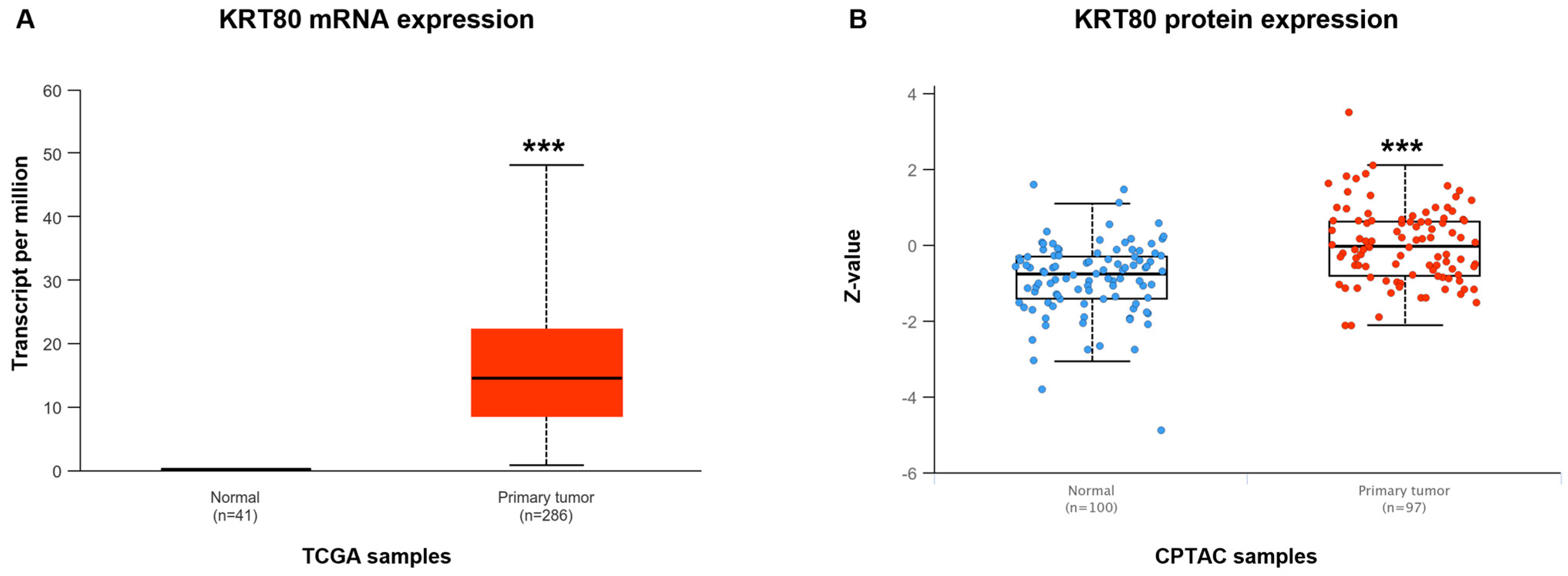
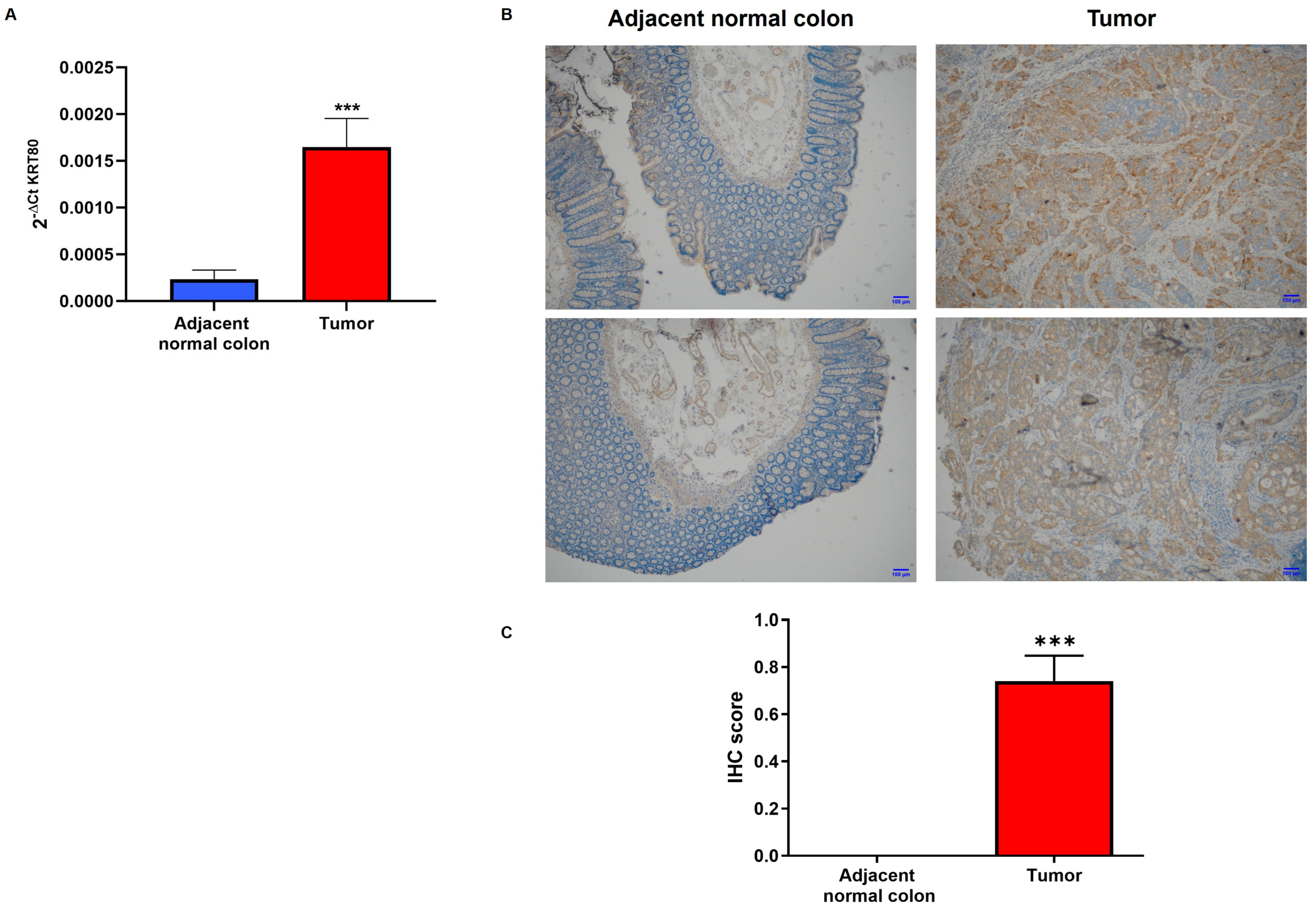
3.2. KRT80 Levels in Patients with Colon Cancer
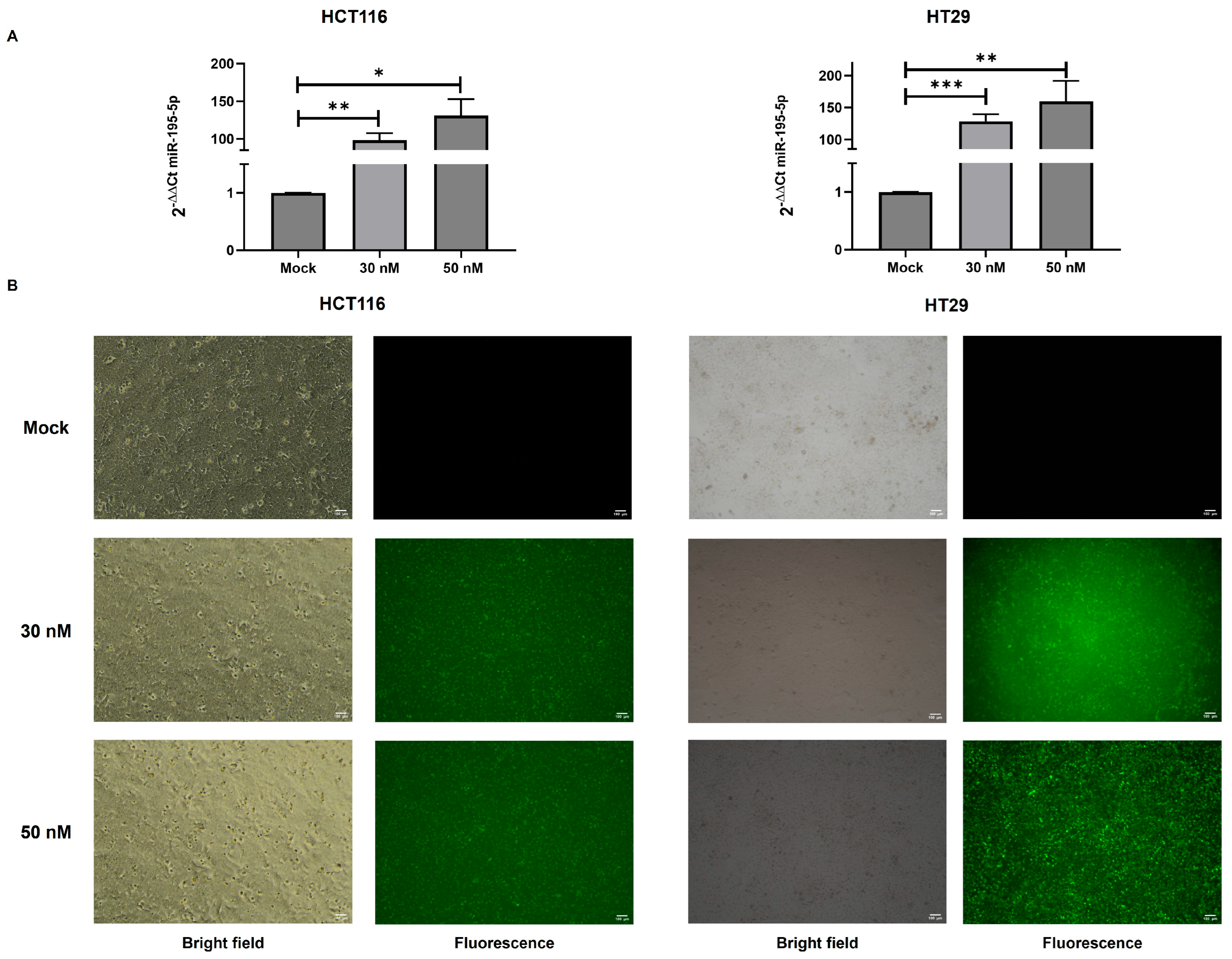
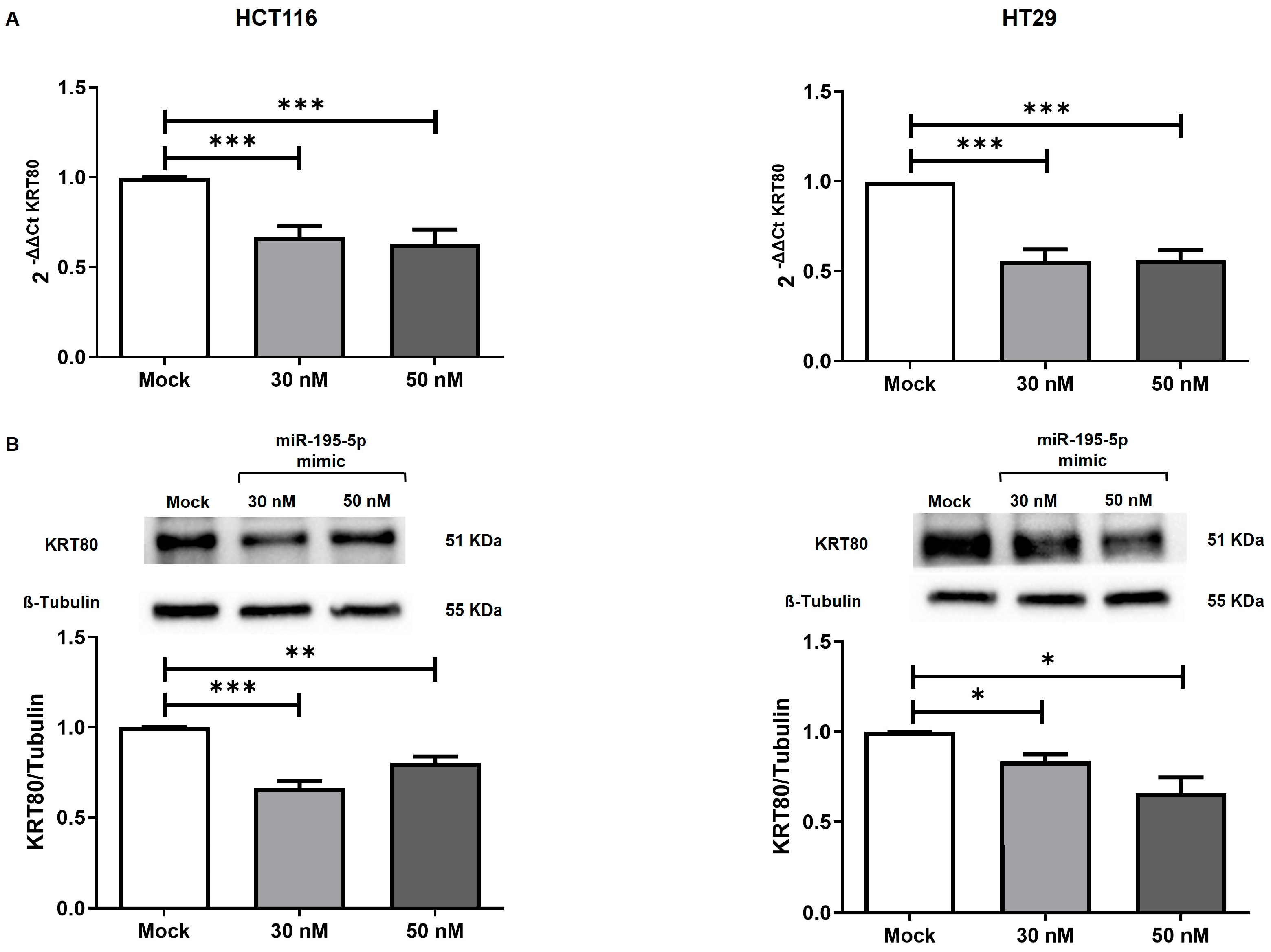
3.3. miR-195-5p Controls’ KRT80 Expression
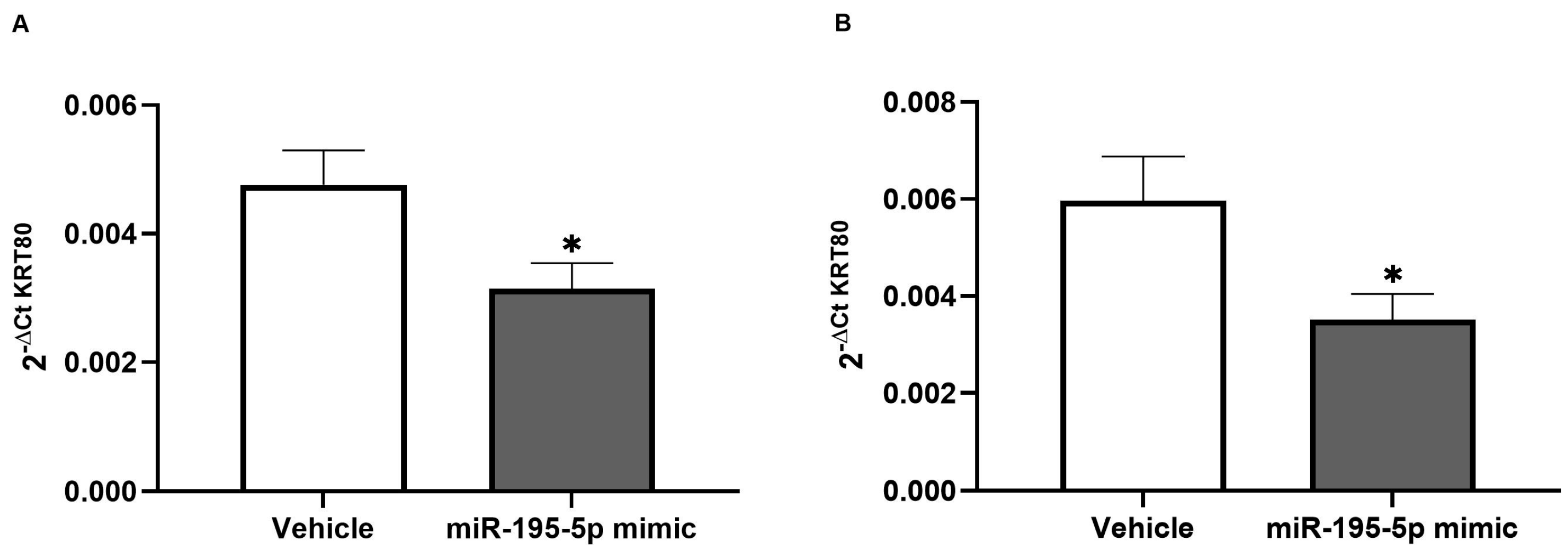
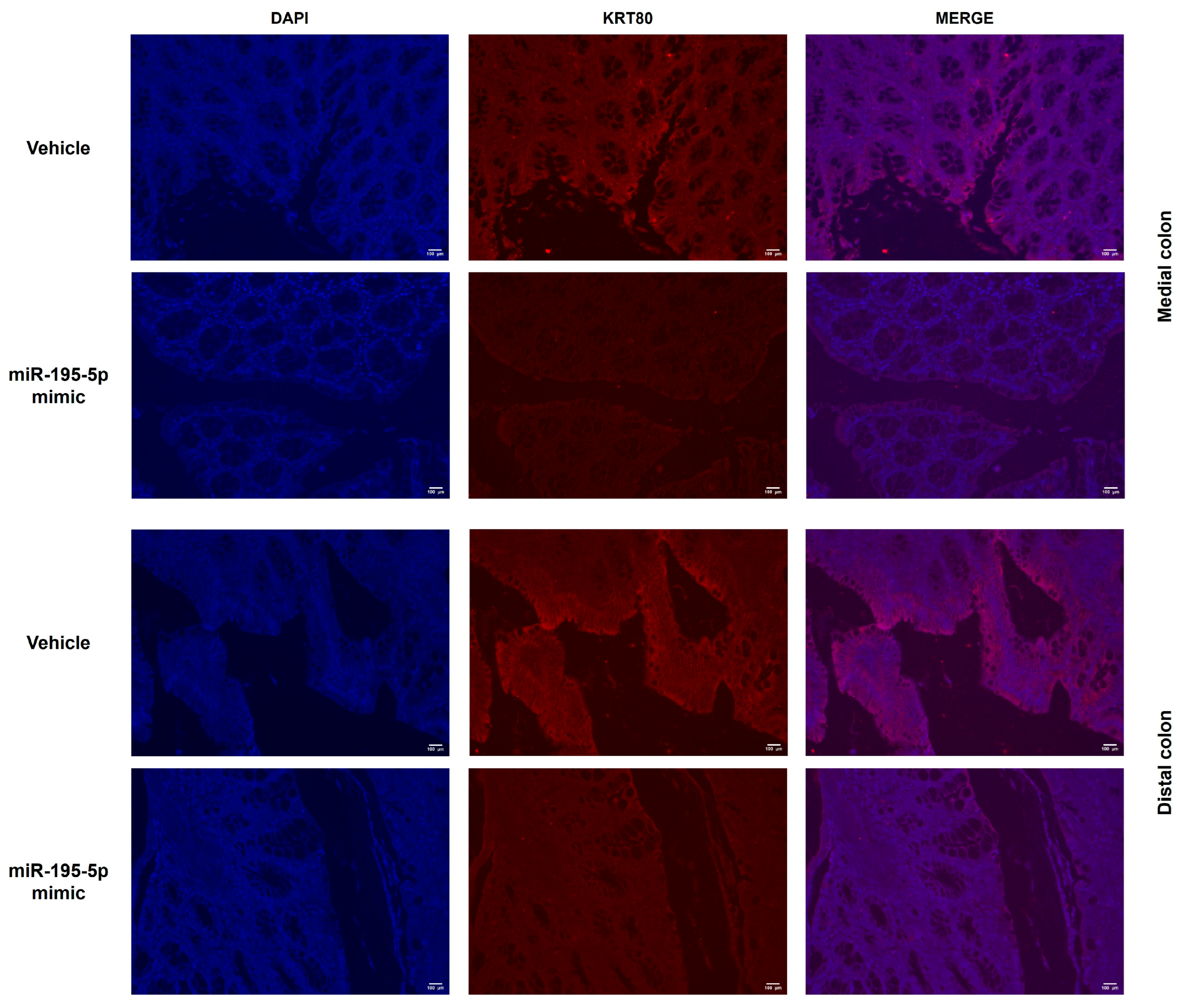
3.4. In Vivo Evaluation of miR-195-5p Effect on KRT80 Expression
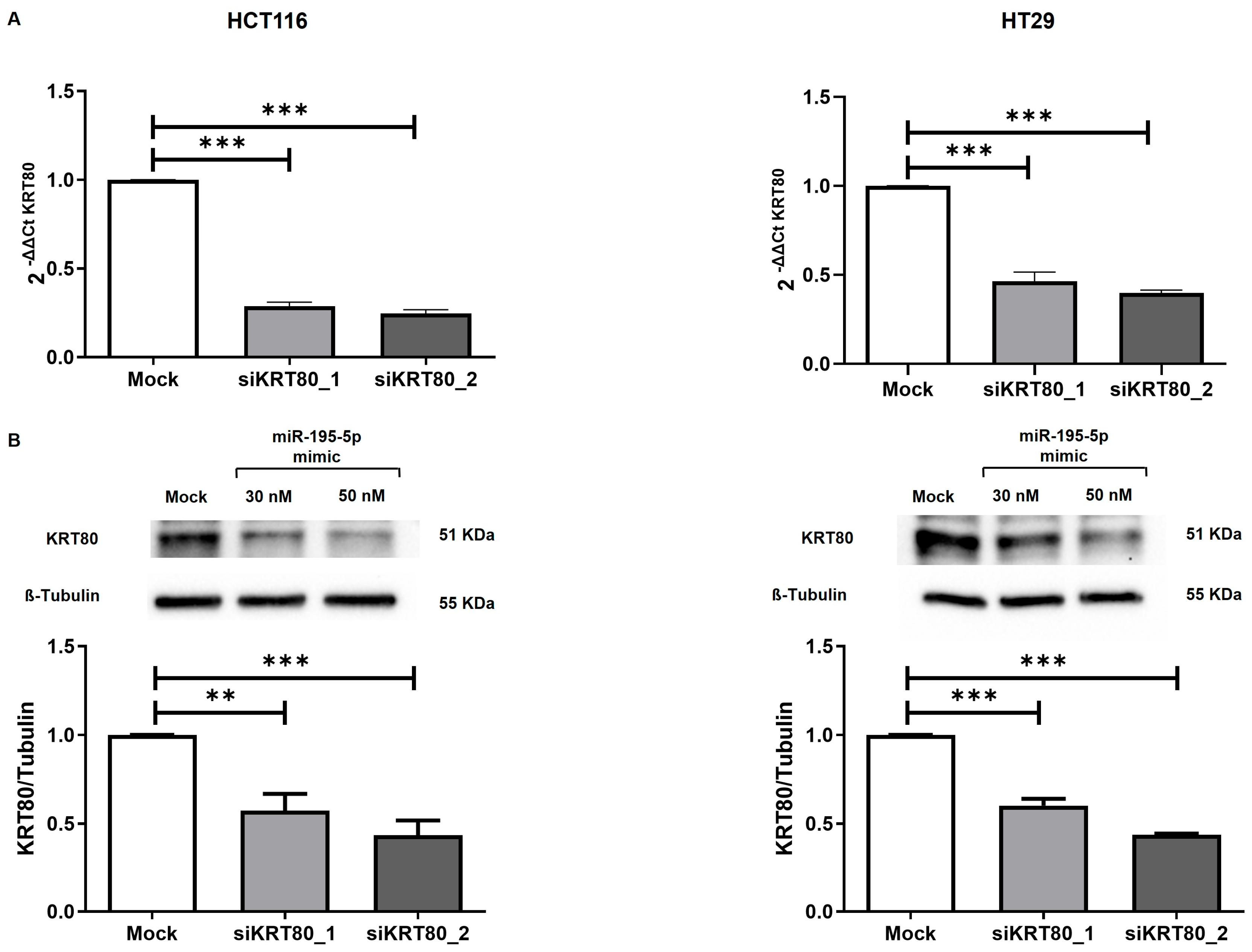
3.5. KRT80 Knockdown by Small Interfering RNAs (siRNAs) in CRC Cell Lines
3.6. Effect of KRT80 Downregulation by miR-195-5p or siRNA on CRC
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loschke, F.; Seltmann, K.; Bouameur, J.-E.; Magin, T.M. Regulation of keratin network organization. Curr. Opin. Cell Biol. 2015, 32, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Windoffer, R.; Beil, M.; Magin, T.M.; Leube, R.E. Cytoskeleton in motion: The dynamics of keratin intermediate filaments in epithelia. J. Cell Biol. 2011, 194, 669–678. [Google Scholar] [CrossRef]
- Di Russo, J.; Magin, T.M.; Leube, R.E. A keratin code defines the textile nature of epithelial tissue architecture. Curr. Opin. Cell Biol. 2023, 85, 102236. [Google Scholar] [CrossRef]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.-J.; Li, J.; Yin, N.-C.; Zhang, C.-Y.; Cui, Z.-G.; Zhang, L.; Zheng, H.-C. The facilitating effects of KRT80 on chemoresistance, lipogenesis, and invasion of esophageal cancer. Cancer Biol. Ther. 2024, 25, 2302162. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, G.; Yan, W.; Jiang, H. MiR-4268 suppresses gastric cancer genesis through inhibiting keratin 80. Cell Cycle 2022, 21, 2051–2064. [Google Scholar] [CrossRef]
- Liu, O.; Wang, C.; Wang, S.; Hu, Y.; Gou, R.; Dong, H.; Li, S.; Li, X.; Lin, B. Keratin 80 regulated by miR-206/ETS1 promotes tumor progression via the MEK/ERK pathway in ovarian cancer. J. Cancer 2021, 12, 6835. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cao, H.-X.; He, Y.-W.; Ouyang, J.-J.; Lv, M.; Li, Y.-X.; Lu, Y.-D. Increased keratin 80 expression predicts poor prognosis and promotes oxaliplatin resistance in gastric cancer. World J. Gastroenterol. 2025, 31, 103991. [Google Scholar] [CrossRef]
- Hua, R.; Yan, X.; He, J.; Wu, N.; Yu, W.; Yu, P.; Qin, L. KRT80 in hepatocellular carcinoma plays oncogenic role via epithelial–mesenchymal transition and PI3K/AKT pathway. IUBMB Life 2025, 77, e2925. [Google Scholar] [CrossRef]
- Huang, S.; Tong, W.; Yang, B.; Ma, L.; Zhang, J.; Wang, C.; Xu, L.; Mei, J. KRT80 Promotes Lung Adenocarcinoma Progression and Serves as a Substrate for VCP. J. Cancer 2024, 15, 2229. [Google Scholar] [CrossRef]
- Perone, Y.; Farrugia, A.J.; Rodríguez-Meira, A.; Győrffy, B.; Ion, C.; Uggetti, A.; Chronopoulos, A.; Marrazzo, P.; Faronato, M.; Shousha, S. SREBP1 drives Keratin-80-dependent cytoskeletal changes and invasive behavior in endocrine-resistant ERα breast cancer. Nat. Commun. 2019, 10, 2115. [Google Scholar] [CrossRef]
- Wada, M.; Goto, Y.; Tanaka, T.; Okada, R.; Moriya, S.; Idichi, T.; Noda, M.; Sasaki, K.; Kita, Y.; Kurahara, H. RNA sequencing-based microRNA expression signature in esophageal squamous cell carcinoma: Oncogenic targets by antitumor miR-143-5p and miR-143-3p regulation. J. Hum. Genet. 2020, 65, 1019–1034. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Liu, Y.; Liu, X.; Wang, R.; Liao, J.; Wu, S.; Fan, J.; Peng, Z.; Li, B. Keratin 80 promotes migration and invasion of colorectal carcinoma by interacting with PRKDC via activating the AKT pathway. Cell Death Dis. 2018, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging role of non-coding RNA in health and disease. Metab. Brain Dis. 2021, 36, 1119–1134. [Google Scholar] [CrossRef]
- Uzuner, E.; Ulu, G.T.; Gürler, S.B.; Baran, Y. The role of MiRNA in cancer: Pathogenesis, diagnosis, and treatment. Mirnomics MicroRNA Biol. Comput. Anal. 2022, 375–422. [Google Scholar] [CrossRef]
- Piccinno, E.; Scalavino, V.; Armentano, R.; Giannelli, G.; Serino, G. miR-195-5p as Regulator of γ-Catenin and Desmosome Junctions in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 17084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Zhou, S.; Mao, J.; Zhan, Z.; Duan, S. miRNA interplay: Mechanisms and therapeutic interventions in cancer. MedComm–Oncology 2024, 3, e93. [Google Scholar] [CrossRef]
- Huang, W.-C.; Jang, T.-H.; Tung, S.-L.; Yen, T.-C.; Chan, S.-H.; Wang, L.-H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 89. [Google Scholar] [CrossRef]
- Shi, H.-Q.; Li, X.; Chen, Z.; Dong, S.; Ye, C.; Hou, S.; Fan, D.-A.; Zhang, H.; Zhou, W.-C. KRT19 is regulated by miR-642a-5p and promotes pancreatic cancer progression through the Wnt/β-catenin pathway. Iscience 2024, 27, 110782. [Google Scholar] [CrossRef]
- Jang, T.-H.; Huang, W.-C.; Tung, S.-L.; Lin, S.-C.; Chen, P.-M.; Cho, C.-Y.; Yang, Y.-Y.; Yen, T.-C.; Lo, G.-H.; Chuang, S.-E. MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway. J. Biomed. Sci. 2022, 29, 42. [Google Scholar] [CrossRef]
- Piccinno, E.; Scalavino, V.; Labarile, N.; De Marinis, L.; Armentano, R.; Giannelli, G.; Serino, G. Identification of a Novel miR-195-5p/PNN Axis in Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 5980. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, E.; Scalavino, V.; Labarile, N.; Armentano, R.; Giannelli, G.; Serino, G. miR-195-5p Inhibits Colon Cancer Progression via KRT23 Regulation. Pharmaceutics 2024, 16, 1554. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Quillet, A.; Saad, C.; Ferry, G.; Anouar, Y.; Vergne, N.; Lecroq, T.; Dubessy, C. Improving bioinformatics prediction of microRNA targets by ranks aggregation. Front. Genet. 2020, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA–gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed]
- Vejnar, C.E.; Zdobnov, E.M. MiRmap: Comprehensive prediction of microRNA target repression strength. Nucleic Acids Res. 2012, 40, 11673–11683. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Piccinno, E.; Scalavino, V.; Labarile, N.; Bianco, G.; Savino, M.T.; Armentano, R.; Giannelli, G.; Serino, G. Downregulation of γ-Catenin by miR-195-5p Inhibits Colon Cancer Progression, Regulating Desmosome Function. Int. J. Mol. Sci. 2023, 25, 494. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 196. [Google Scholar]
- Wei, X.Y.; Zhao, J.; Tong, H.B.; Cheng, S.J.; He, N.; Song, F.X. Characters of KRT80 and its roles in neoplasms diseases. Cancer Med. 2023, 12, 13991–14003. [Google Scholar] [CrossRef]
- Scalavino, V.; Piccinno, E.; Labarile, N.; Armentano, R.; Giannelli, G.; Serino, G. Anti-Inflammatory Effects of miR-369-3p via PDE4B in Intestinal Inflammatory Response. Int. J. Mol. Sci. 2024, 25, 8463. [Google Scholar] [CrossRef] [PubMed]
- Scalavino, V.; Piccinno, E.; Giannelli, G.; Serino, G. miR-369-3p Ameliorates Inflammation and Apoptosis in Intestinal Epithelial Cells via the MEK/ERK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 4288. [Google Scholar] [CrossRef] [PubMed]
- Rama, K.; Rao, P.S.; Bitla, A.R. MicroRNAs in health and disease. J. Clin. Sci. Res. 2017, 6, 25–34. [Google Scholar]
- Azam, H.M.H.; Rößling, R.I.; Geithe, C.; Khan, M.M.; Dinter, F.; Hanack, K.; Prüß, H.; Husse, B.; Roggenbuck, D.; Schierack, P. MicroRNA biomarkers as next-generation diagnostic tools for neurodegenerative diseases: A comprehensive review. Front. Mol. Neurosci. 2024, 17, 1386735. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.M.; Ripon, M.A.R.; Islam, M.M.; Hossain, M.S. Regulatory roles of MicroRNAs in the pathogenesis of metabolic syndrome. Mol. Biotechnol. 2024, 66, 1599–1620. [Google Scholar] [CrossRef]
- Schetter, A.J.; Okayama, H.; Harris, C.C. The role of microRNAs in colorectal cancer. Cancer J. 2012, 18, 244–252. [Google Scholar] [CrossRef]
- Xuan, Y.; Yang, H.; Zhao, L.; Lau, W.B.; Lau, B.; Ren, N.; Hu, Y.; Yi, T.; Zhao, X.; Zhou, S. MicroRNAs in colorectal cancer: Small molecules with big functions. Cancer Lett. 2015, 360, 89–105. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.; Yang, P.; Yang, J.; Liang, J.; Chen, Y.; Wang, H.; Wei, G.; Ye, S.; Zhou, Y. MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectal cancer. Mol. Cell. Biochem. 2014, 388, 249–259. [Google Scholar] [CrossRef]
- Song, H.; Xu, Y.; Xu, T.; Fan, R.; Jiang, T.; Cao, M.; Shi, L.; Song, J. CircPIP5K1A activates KRT80 and PI3K/AKT pathway to promote gastric cancer development through sponging miR-671-5p. Biomed. Pharmacother. 2020, 126, 109941. [Google Scholar] [CrossRef]
- Gao, X.; Wang, G.; Zhang, M.; Zhang, X.; Zhang, S.; Long, H. LINC01485 contributes to colorectal cancer progression by targeting miR-383-5p/KRT80 axis. Environ. Toxicol. 2024, 39, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; De Franciscis, V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010, 28, 517–525. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhang, Y.; Hu, D.; Tang, L.; Zhou, B.; Yang, L. Landscape of small nucleic acid therapeutics: Moving from the bench to the clinic as next-generation medicines. Signal Transduct. Target. Ther. 2025, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Johari, B.; Rezaeejam, H.; Moradi, M.; Taghipour, Z.; Saltanatpour, Z.; Mortazavi, Y.; Nasehi, L. Increasing the colon cancer cells sensitivity toward radiation therapy via application of Oct4–Sox2 complex decoy oligodeoxynucleotides. Mol. Biol. Rep. 2020, 47, 6793–6805. [Google Scholar] [CrossRef]
- Saltanatpour, Z.; Johari, B.; Alizadeh, A.; Lotfinia, M.; Majidzadeh-A, K.; Nikbin, B.; Kadivar, M. Enrichment of cancer stem-like cells by the induction of epithelial-mesenchymal transition using lentiviral vector carrying E-cadherin shRNA in HT29 cell line. J. Cell. Physiol. 2019, 234, 22935–22946. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccinno, E.; Scalavino, V.; Labarile, N.; Bianco, G.; Armentano, R.; Giannelli, G.; Serino, G. miR-195-5p Suppresses KRT80 Expression Inducing Cell Cycle Arrest in Colon Cancer. Cancers 2025, 17, 2183. https://doi.org/10.3390/cancers17132183
Piccinno E, Scalavino V, Labarile N, Bianco G, Armentano R, Giannelli G, Serino G. miR-195-5p Suppresses KRT80 Expression Inducing Cell Cycle Arrest in Colon Cancer. Cancers. 2025; 17(13):2183. https://doi.org/10.3390/cancers17132183
Chicago/Turabian StylePiccinno, Emanuele, Viviana Scalavino, Nicoletta Labarile, Giusy Bianco, Raffaele Armentano, Gianluigi Giannelli, and Grazia Serino. 2025. "miR-195-5p Suppresses KRT80 Expression Inducing Cell Cycle Arrest in Colon Cancer" Cancers 17, no. 13: 2183. https://doi.org/10.3390/cancers17132183
APA StylePiccinno, E., Scalavino, V., Labarile, N., Bianco, G., Armentano, R., Giannelli, G., & Serino, G. (2025). miR-195-5p Suppresses KRT80 Expression Inducing Cell Cycle Arrest in Colon Cancer. Cancers, 17(13), 2183. https://doi.org/10.3390/cancers17132183








