Supraclavicular Lymph Node Dissection in Breast Cancer with Synchronous Supraclavicular Metastases: A Systematic Review and Network Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
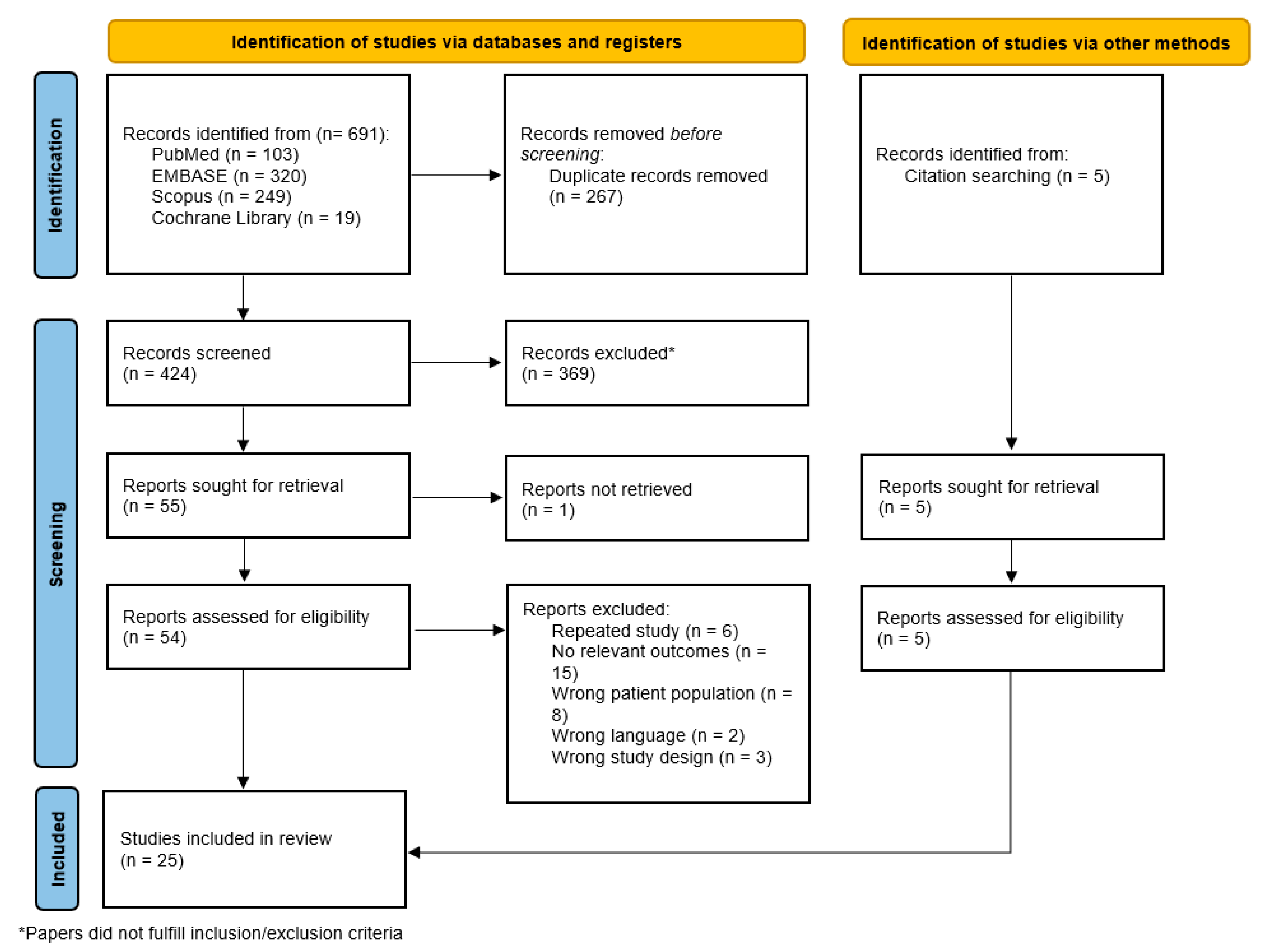
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analysis
2.4. Assessment of Heterogeneity and Inconsistency
2.5. Assessment of Quality of Evidence
2.6. Sensitivity Analysis
3. Results
3.1. Characteristics of Reviewed Studies
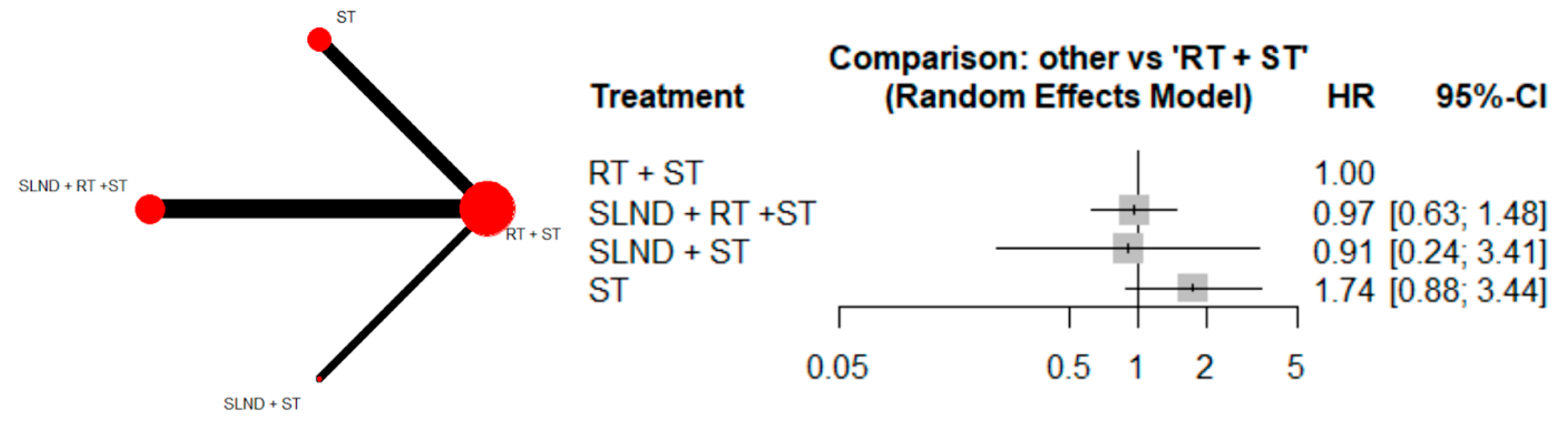
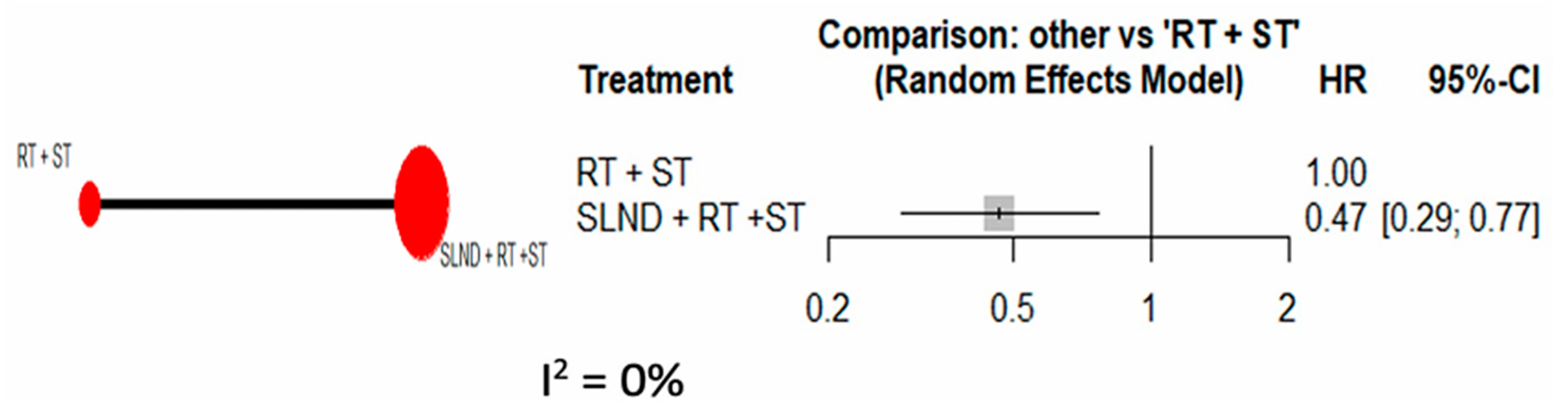
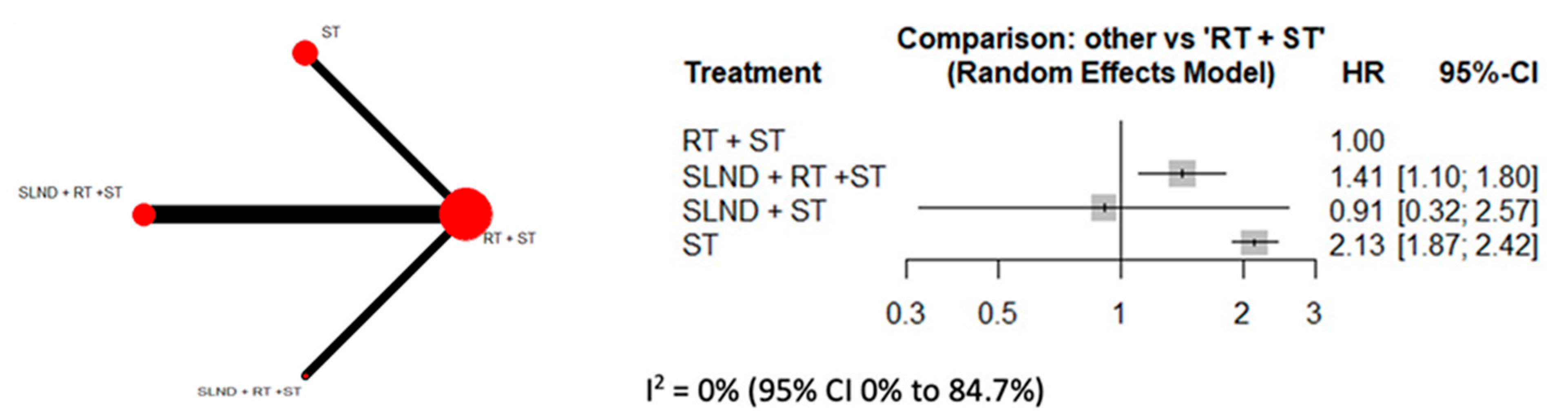
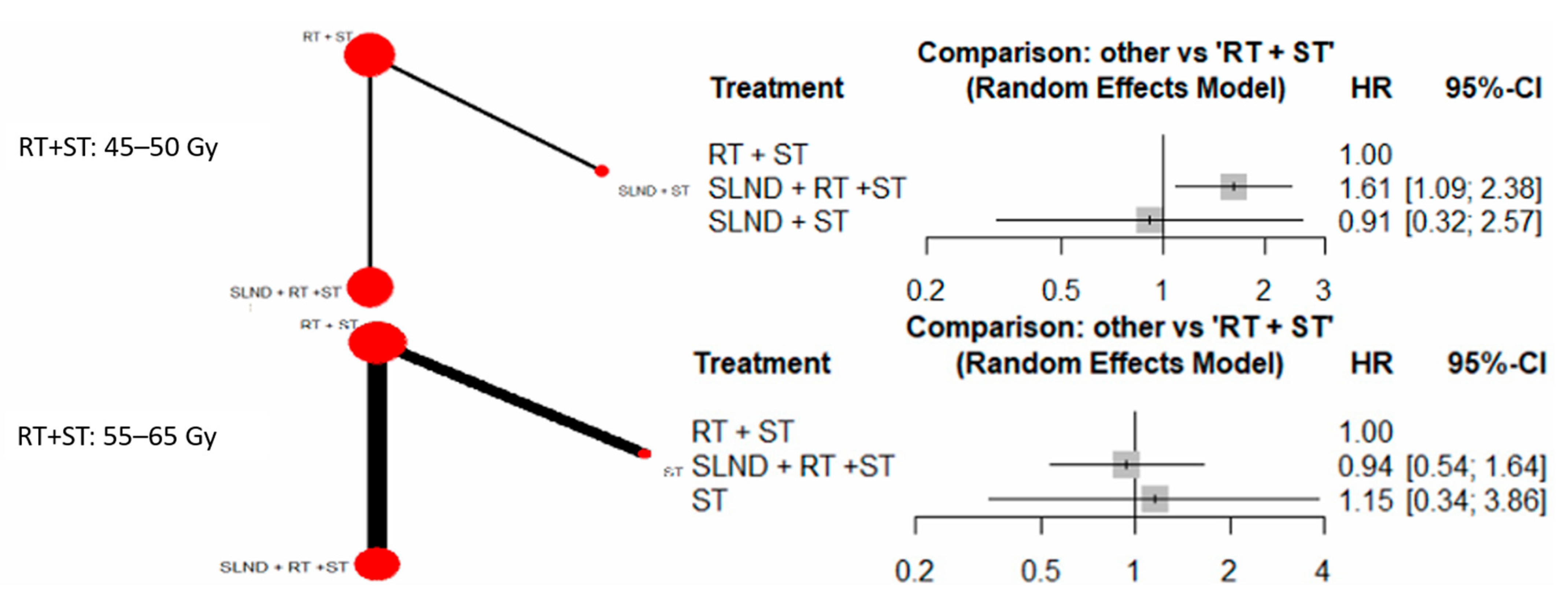
3.2. Primary Analysis
3.3. Sensitivity Analyses
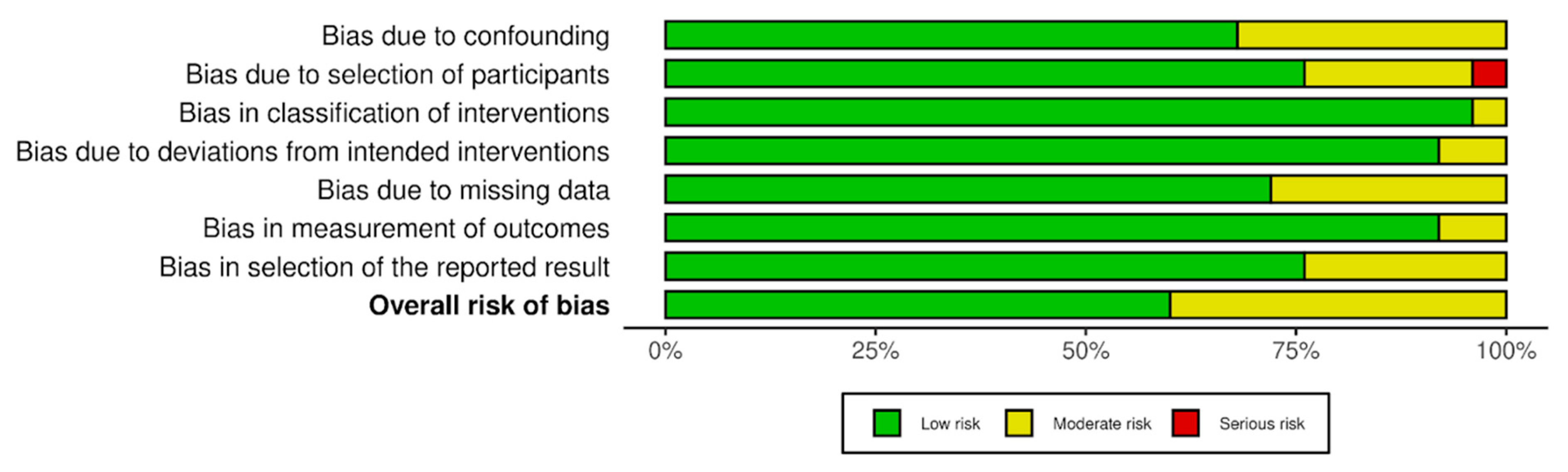
3.4. Risk-of-Bias Assessment
3.5. GRADE Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arzanova, E.; Mayrovitz, H.N. The Epidemiology of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Chen, S.C.; Chang, H.K.; Lin, Y.C.; Leung, W.M.; Tsai, C.S.; Cheung, Y.C.; Hsueh, S.; See, L.C.; Chen, M.F. Prognosis of breast cancer after supraclavicular lymph node metastasis: Not a distant metastasis. Ann. Surg. Oncol. 2006, 13, 1457–1465. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Qian, M.; Mao, X.; Shi, G.; Ma, G.; Yu, M.; Xie, H.; Ling, L.; Ding, Q.; et al. Comparison of Survival Outcomes Among Patients With Breast Cancer With Distant vs Ipsilateral Supraclavicular Lymph Node Metastases. JAMA Netw. Open 2021, 4, e211809. [Google Scholar] [CrossRef]
- Brito, R.A.; Valero, V.; Buzdar, A.U.; Booser, D.J.; Ames, F.; Strom, E.; Ross, M.; Theriault, R.L.; Frye, D.; Kau, S.W.; et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J. Clin. Oncol. 2001, 19, 628–633. [Google Scholar] [CrossRef]
- Singletary, S.E.; Allred, C.; Ashley, P.; Bassett, L.W.; Berry, D.; Bland, K.I.; Borgen, P.I.; Clark, G.; Edge, S.B.; Hayes, D.F.; et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J. Clin. Oncol. 2002, 20, 3628–3636. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Zhu, J.; Jiao, D.; Guo, X.; Qiao, J.; Ma, Y.; Zhang, J.; Chen, H.; Xiao, H.; Yang, Y.; Lu, Z.; et al. Predictive factors and prognostic value of pathologic complete response of ipsilateral supraclavicular lymph nodes in breast cancer after neoadjuvant chemotherapy. Ann. Transl. Med. 2019, 7, 666. [Google Scholar] [CrossRef]
- Zhang, L.; Mei, X.; Hu, Z.; Yu, B.; Zhang, C.; Li, Y.; Liu, K.; Ma, X.; Ma, J.; Chen, X.; et al. Adjuvant medial versus entire supraclavicular lymph node irradiation in high-risk early breast cancer (SUCLANODE): A protocol for a multicenter, randomized, open-label, phase 3 trial. BMC Cancer 2024, 24, 49. [Google Scholar] [CrossRef]
- Feng, K.; Xing, Z.; Dai, Q.; Cheng, H.; Wang, X. Role of aggressive locoregional surgery in treatment strategies for ipsilateral supraclavicular lymph node metastasis of breast cancer: A real-world cohort study. Front. Mol. Biosci. 2023, 10, 1248410. [Google Scholar] [CrossRef]
- Recht, A.; Edge, S.B.; Solin, L.J.; Robinson, D.S.; Estabrook, A.; Fine, R.E.; Fleming, G.F.; Formenti, S.; Hudis, C.; Kirshner, J.J.; et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J. Clin. Oncol. 2001, 19, 1539–1569. [Google Scholar] [CrossRef]
- Diao, K.; Andring, L.M.; Barcenas, C.H.; Singh, P.; Carisa Le-Petross, H.; Reed, V.K.; Reddy, J.P.; Bloom, E.S.; Ahmad, N.R.; Mayo, L.L.; et al. Contemporary Outcomes After Multimodality Therapy in Patients With Breast Cancer Presenting With Ipsilateral Supraclavicular Node Involvement. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 66–74. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.S.; Shin, K.H.; Kim, J.H.; Ahn, S.D.; Choi, D.H.; Park, W.; Lee, S.Y.; Chun, M.; Kim, J.H.; et al. Aggressive Surgical Excision of Supraclavicular Lymph Node Did Not Improve the Outcomes of Breast Cancer With Supraclavicular Lymph Node Involvement (KROG 16-14). Clin. Breast Cancer 2020, 20, 51–60. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Adamo, V.; Russi, E.; Santacaterina, A.; Maisano, R.; Numico, G.; Palazzolo, C.; Ferraù, F.; Settineri, N.; Altavilla, G.; et al. Prospective multicenter study of combined treatment with chemotherapy and radiotherapy in breast cancer women with the rare clinical scenario of ipsilateral supraclavicular node recurrence without distant metastases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 25–32. [Google Scholar] [CrossRef]
- Ai, X.; Wang, M.; Li, J.; Hu, Y.; Hou, L.; Zheng, X.; Yan, Y.; Pan, Q.; Jin, Y.; Liu, W.; et al. Supraclavicular lymph node dissection with radiotherapy versus radiotherapy alone for operable breast cancer with synchronous ipsilateral supraclavicular lymph node metastases: A real-world cohort study. Gland Surg. 2020, 9, 329–341. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Settineri, N.; Santacaterina, A.; Spadaro, P.; Maisano, R.; Caristi, N.; Adamo, V.; De Renzis, C.; Mesiti, M.; Cascinu, S. Ipsilateral supraclavicular lymph nodes metastases from breast cancer as only site of disseminated disease. Chemotherapy alone vs. induction chemotherapy to radical radiation therapy. Ann. Oncol. 2001, 12, 1091–1095. [Google Scholar] [CrossRef]
- Chang, X.Z.; Yin, J.; Sun, J.; Zhang, X.H.; Cao, X.C. A retrospective study of different local treatments in breast cancer patients with synchronous ipsilateral supraclavicular lymph node metastasis. J. Cancer Res. Ther. 2013, 9, S158–S161. [Google Scholar] [CrossRef]
- Cobb, A.N.; Diao, K.; Teshome, M.; Lucci, A.; Ueno, N.T.; Stauder, M.; Layman, R.M.; Kuerer, H.M.; Woodward, W.A.; Sun, S.X. Long-term Oncologic Outcomes in Patients with Inflammatory Breast Cancer with Supraclavicular Nodal Involvement. Ann. Surg. Oncol. 2022, 29, 6381–6392. [Google Scholar] [CrossRef]
- Deo, S.V.; Purkayastha, J.; Shukla, N.K.; Raina, V.; Asthana, S.; Das, D.K.; Rath, G.K. Intent of therapy in metastatic breast cancer with isolated ipsilateral supraclavicular lymph node spread—A therapeutic dilemma. J. Assoc. Physicians India 2003, 51, 272–275. [Google Scholar]
- Jung, J.; Kim, S.S.; Ahn, S.D.; Lee, S.W.; Ahn, S.H.; Son, B.H.; Lee, J.W.; Choi, E.K. Treatment Outcome of Breast Cancer with Pathologically Proven Synchronous Ipsilateral Supraclavicular Lymph Node Metastases. J. Breast Cancer 2015, 18, 167–172. [Google Scholar] [CrossRef]
- Liu, B.W.; Chen, L.X.; Ma, K.; Chi, J.R.; Yang, Z.J.; Yu, Y.; Cao, X.C. The role of surgery on locoregional treatment of patients with breast cancer newly diagnosed with ipsilateral supraclavicular lymph node metastasis. J. Cancer Res. Ther. 2022, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, J.; Guo, H.; Wang, C.; Tian, P.; Ma, Y.; Chen, X.; Luo, S. Impact of Ipsilateral Supraclavicular Lymph Node Dissection (ISLND) for Breast Cancer Patients and a Nomogram for Predicting Ipsilateral Supraclavicular Pathological Complete Response (ispCR). Ann. Surg. Oncol. 2021, 28, 5098–5109. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Mao, Y.; Wang, H. Combined Therapy Can Improve the Outcomes of Breast Cancer with Isolated Supraclavicular Lymph Node Involvement. Cancer Manag. Res. 2020, 12, 11857–11869. [Google Scholar] [CrossRef]
- Song, Y.C.; Kong, J.; Li, N.; Liu, X.L.; Li, X.H.; Zhu, L.Y.; Wang, Y.W.; Fang, H.; Jing, H.; Tang, Y.; et al. Comparison of supraclavicular surgery plus radiotherapy versus radiotherapy alone in breast cancer patients with synchronous ipsilateral supraclavicular lymph node metastasis: A multicenter retrospective study. Radiother. Oncol. 2023, 183, 109639. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Wang, Y.J.; Huang, T.; Niu, L.J.; Zhang, Q.; Liu, Z.Z. Comparison between surgery plus radiotherapy and radiotherapy alone in treating breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Gland Surg. 2020, 9, 1513–1520. [Google Scholar] [CrossRef]
- Tamirisa, N.P.; Ren, Y.; Campbell, B.M.; Thomas, S.M.; Fayanju, O.M.; Plichta, J.K.; Rosenberger, L.H.; Force, J.; Hyslop, T.; Hwang, E.S.; et al. Treatment Patterns and Outcomes of Women with Breast Cancer and Supraclavicular Nodal Metastases. Ann. Surg. Oncol. 2021, 28, 2146–2154. [Google Scholar] [CrossRef]
- Wu, S.G.; Sun, J.Y.; Zhou, J.; Li, F.Y.; Lin, Q.; Lin, H.X.; He, Z.Y. The value of radiotherapy in breast cancer patients with isolated ipsilateral supraclavicular lymph node metastasis without distant metastases at diagnosis: A retrospective analysis of Chinese patients. OncoTargets Ther. 2014, 7, 281–288. [Google Scholar]
- Xianfu, S.; Yingjie, W.; Tao, H. Retrospective analysis of survival of breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Eur. J. Cancer 2020, 138, S38. [Google Scholar] [CrossRef]
- Huang, E.H.; Strom, E.A.; Valero, V.; Fornage, B.; Perkins, G.H.; Oh, J.L.; Yu, T.K.; Tereffe, W.; Woodward, W.A.; Hunt, K.K.; et al. Locoregional treatment outcomes for breast cancer patients with ipsilateral supraclavicular metastases at diagnosis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 490–496. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, K.H.; Cho, K.H.; Park, I.H.; Lee, K.S.; Ro, J.; Jung, S.Y.; Lee, S.; Kim, S.W.; Kang, H.S.; et al. Outcomes of positron emission tomography-staged clinical N3 breast cancer treated with neoadjuvant chemotherapy, surgery, and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e689–e695. [Google Scholar] [CrossRef]
- Willner, J.; Kiricuta, I.; Kolbl, O.; Bohndorf, W. Supraclavicular lymph-node recurrence of breast-cancer. Oncol. Rep. 1994, 1, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Hessel, K.; Spornitz, B.; Mammen, J.M. Surgery in the treatment of breast cancer metastatic to ipsilateral supraclavicular lymph nodes. Ann. Surg. Oncol. 2017, 24, S73. [Google Scholar]
- Zhang, W.; Qi, X.M.; Chen, A.X.; Zhang, P.; Cao, X.C.; Xiao, C.H. The Role of Supraclavicular lymph node dissection in Breast Cancer Patients with Synchronous Ipsilateral Supraclavicular Lymph Node Metastasis. Zhonghua Zhong Liu Za Zhi 2017, 39, 374–379. [Google Scholar] [PubMed]
- Fan, Y.; Xu, B.; Liao, Y.; Yao, S.; Sun, Y. A retrospective study of metachronous and synchronous ipsilateral supraclavicular lymph node metastases in breast cancer patients. Breast 2010, 19, 365–369. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Tryfonidis, K.; Senkus, E.; Cardoso, M.J.; Cardoso, F. Management of locally advanced breast cancer-perspectives and future directions. Nat. Rev. Clin. Oncol. 2015, 12, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hou, L.; Chen, M.; Zhou, Y.; Liang, Y.; Wang, S.; Jiang, J.; Zhang, Y. Neoadjuvant Chemotherapy Creates Surgery Opportunities for Inoperable Locally Advanced Breast Cancer. Sci. Rep. 2017, 7, 44673. [Google Scholar] [CrossRef]
- Brackstone, M.; Palma, D.; Tuck, A.B.; Scott, L.; Potvin, K.; Vandenberg, T.; Perera, F.; D’Souza, D.; Taves, D.; Kornecki, A.; et al. Concurrent Neoadjuvant Chemotherapy and Radiation Therapy in Locally Advanced Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 769–776. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Kollmeier, M.; Cox, B.; Fidaleo, A.; Sperling, D.; Pei, X.; Carver, B.; Coleman, J.; Lovelock, M.; Hunt, M. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 125–129. [Google Scholar] [CrossRef]
- Harris, S.R. Brachial plexopathy after breast cancer: A persistent late effect of radiotherapy. Phys. Med. Rehabil. 2024, 16, 85–91. [Google Scholar] [CrossRef]
- Rudra, S.; Roy, A.; Brenneman, R.; Gabani, P.; Roach, M.C.; Ochoa, L.; Prather, H.; Appleton, C.; Margenthaler, J.; Peterson, L.L.; et al. Radiation-Induced Brachial Plexopathy in Patients with Breast Cancer Treated with Comprehensive Adjuvant Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100602. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Shen, S.C.; Yu, C.C.; Huang, T.S.; Lo, Y.F.; Chang, H.K.; Lin, Y.C.; Kuo, W.L.; Tsai, H.P.; Chou, H.H.; et al. Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis. Cancers 2021, 14, 164. [Google Scholar] [CrossRef]
- Seethala, R.R. Current state of neck dissection in the United States. Head Neck Pathol. 2009, 3, 238–245. [Google Scholar] [CrossRef]
- Noh, J.M.; Kim, K.H.; Park, W.; Suh, C.O.; Huh, S.J.; Choi, D.H.; Keum, K.C.; Kim, Y.B. Prognostic significance of nodal involvement region in clinical stage IIIc breast cancer patients who received primary systemic treatment, surgery, and radiotherapy. Breast 2015, 24, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Mersin, H.; Yildirim, E.; Bulut, H.; Berberoglu, U. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. Eur. J. Surg. Oncol. 2003, 29, 132–138. [Google Scholar] [CrossRef]
- Yan, M.; Kong, W.; Kerr, A.; Brundage, M. The radiation dose tolerance of the brachial plexus: A systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2019, 18, 23–31. [Google Scholar] [PubMed]
- Teymoortash, A.; Hoch, S.; Eivazi, B.; Werner, J.A. Postoperative morbidity after different types of selective neck dissection. Laryngoscope 2010, 120, 924–929. [Google Scholar] [CrossRef]
- Chia, J.L.L.; He, G.S.; Ngiam, K.Y.; Hartman, M.; Ng, Q.X.; Goh, S.S.N. Harnessing Artificial Intelligence to Enhance Global Breast Cancer Care: A Scoping Review of Applications, Outcomes, and Challenges. Cancers 2025, 17, 197. [Google Scholar] [CrossRef]
- ChiCtr. Supraclavicular Lymph Nodes Dissection Plus Supraclavicular Radiotherapy Versus Supraclavicular Radiotherapy Alone for Operable Breast Cancer with Supraclavicular Lymph Node Metastasis: A Randomized, Parallel Controlled, Single-center Clinical Trial. 2019. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900023098 (accessed on 2 June 2025).
- Nct. Clinical Significance of Supraclavicular Lymph Node Dissection for Breast Cancer. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03716245 (accessed on 2 June 2025).

| Study | Ai et al. (2020) [14] | Chang et al. (2013) [18] | Jung et al. (2015) [21] | Kim et al. (2020) [12] | Lv et al. (2021) [23] | Pergolizzi et al. (2001) [17] | Song et al. (2023) [25] | Sun et al. (2020) [26] | Tamirisa et al. (2021) [27] | Feng, et al. (2023) [9] | Fan et al. (2010) [35] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Characteristics | Country | China | China | Korea | Korea | China | Italy | China | China | USA | China | China |
| Study Design | RS | RS | RS | RS | RS | Non-Randomized CT | RS | RS | RS | RS | RS | |
| Median Age, Years | 48 | 47 | 49 | 49 | 50 | ST only: 58 RT + ST only: 55 | 51 | NR | 60 | 57 | 45 | |
| Age Range | 25–83 | 28–69 | 27–81 | 27–80 | 22–77 | ST only: 32–71 RT + ST only: 39–77 | 26–70 | NR | 50–69 | 24–75 | 24–74 | |
| Median Follow-up, Months | 36 | 47 | NR | 72 | 24 | 105 | SLND + RT + ST: 53.7 RT + ST: 63.5 | 75 | NR | 71 | 93 | |
| Locoregional Treatment Group | Total Population | 305 | 29 | 111 | 104 | 353 | 37 | 293 | 108 | 1827 | 250 | 33 |
| SLND + ST + RT | 146 | 0 | 73 | 57 | 307 | 0 | 85 | 84 | 0 | 65 | 0 | |
| RT + ST | 159 | 16 | 16 | 47 | 46 | 19 | 208 | 24 | 1362 | 185 | 26 | |
| SLND + ST | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| RT Only | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SLND only | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| ST Only | 0 | 0 | 4 | 0 | 0 | 18 | 0 | 0 | 465 | 0 | 7 | |
| No Treatment | 0 | 0 | Unknown: 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SLND | Levels | III, IV, Vb | IV, V | Vb | NR | Vb | NA | IV, Vb | III, IV, V | NA | I, II, III, IV, V | NA |
| Radiotherapy | Median Dosage (Gy) | NR | NR | 60 | 50 | NR | 60 | 60 | 60 | NR | 60 | 60 |
| Range | 46–50 | 45–50 | 45–66 | 45–64.8 | NR | NR | 50–70 | NR | NR | NR | NR | |
| Molecular Subtype | ER+, n | 198 | 19 | 53 | 105 | 202 | 11 | 188 | 60 | NR | 141 | NR |
| PR+ (%) | 166 | 19 | 53 | 66 | 176 | NR | 188 | 60 | NR | NR | NR | |
| HER2+ (%) | 83 | 10 | 48 | NR | 156 | NR | 113 | 48 | NR | 93 | NR | |
| Ki67 < 30 | NR | NR | NR | NR | 51 | NR | 87 | 22 | nr | NR | NR | |
| Ki67 > 30 | NR | NR | NR | 0 | 302 | NR | 188 | 86 | NR | NR | NR | |
| Primary Tumor Size | T0 (%) | 0 | 0 | NR | 56 | NR | 0 | NR | 0 | NR | 0 | 0 |
| T1 (%) | 57 | 2 | NR | 78 | NR | 3 | NR | 15 | NR | 47 | 9 | |
| T2 (%) | 195 | 11 | NR | 20 | NR | 18 | NR | 71 | NR | 116 | 11 | |
| T3 (%) | 29 | 10 | NR | 4 | NR | 6 | NR | NR | NR | 51 | 8 | |
| T4 (%) | 24 | 6 | NR | NR | 10 | NR | NR | NR | 36 | 5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.S.; Chia, J.L.L.; Santos, E.E.C.-D.L.; Chew, W.H.; Koh, W.Y.; Ng, Q.X.; Ow, S.; Goh, S.S.N. Supraclavicular Lymph Node Dissection in Breast Cancer with Synchronous Supraclavicular Metastases: A Systematic Review and Network Meta-Analysis. Cancers 2025, 17, 2081. https://doi.org/10.3390/cancers17132081
He GS, Chia JLL, Santos EEC-DL, Chew WH, Koh WY, Ng QX, Ow S, Goh SSN. Supraclavicular Lymph Node Dissection in Breast Cancer with Synchronous Supraclavicular Metastases: A Systematic Review and Network Meta-Analysis. Cancers. 2025; 17(13):2081. https://doi.org/10.3390/cancers17132081
Chicago/Turabian StyleHe, George Shiyao, Jolene Li Ling Chia, Emmeline Elaine Cua-De Los Santos, Wong Hung Chew, Wee Yao Koh, Qin Xiang Ng, Samuel Ow, and Serene Si Ning Goh. 2025. "Supraclavicular Lymph Node Dissection in Breast Cancer with Synchronous Supraclavicular Metastases: A Systematic Review and Network Meta-Analysis" Cancers 17, no. 13: 2081. https://doi.org/10.3390/cancers17132081
APA StyleHe, G. S., Chia, J. L. L., Santos, E. E. C.-D. L., Chew, W. H., Koh, W. Y., Ng, Q. X., Ow, S., & Goh, S. S. N. (2025). Supraclavicular Lymph Node Dissection in Breast Cancer with Synchronous Supraclavicular Metastases: A Systematic Review and Network Meta-Analysis. Cancers, 17(13), 2081. https://doi.org/10.3390/cancers17132081






