Liver Transplantation for Colorectal Metastases: Impact of a Standardised Protocol for Patient Selection on Transplant Outcomes
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. LITORALE Inclusion Criteria
2.2. Variables and Outcome Measures
2.3. Statistical Analysis
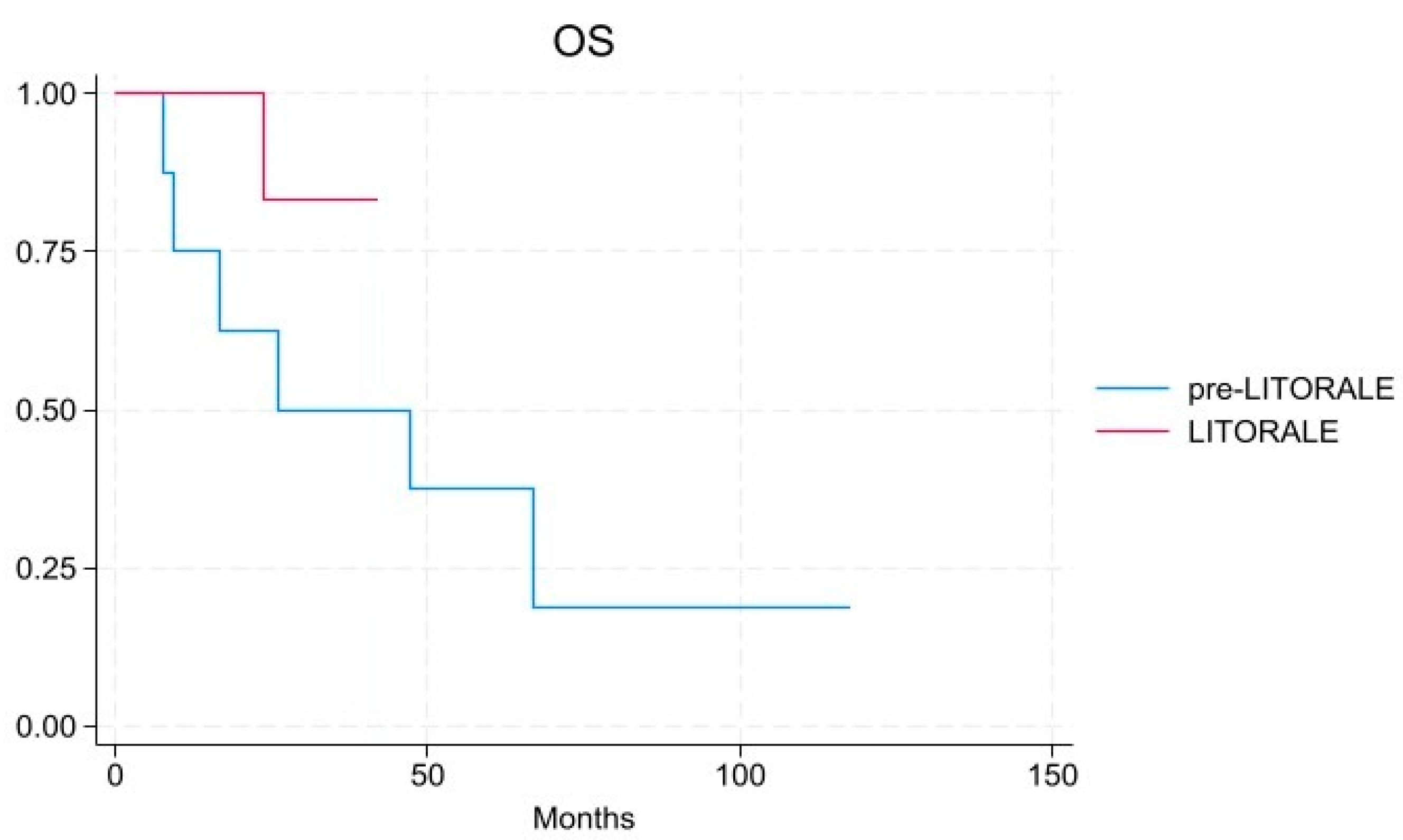
3. Results
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CEA | Carcinoembryonic antigen |
| CI | Confidence intervals |
| CRC | Colorectal cancer |
| CRLM | Colorectal liver metastases |
| DCD | Donors after cardiovascular determination of death |
| DND | Donors after neurological determination of death |
| ECD | Extended criteria donors |
| ECOG | Eastern cooperative oncology group |
| HR | Hazard ratio |
| ICIs | Immune checkpoint inhibitor |
| IQR | Interquartile range |
| LDLT | Living-donor liver transplantation |
| LT | Liver transplantation |
| OS | Overall survival |
| PET | Positron emission tomography |
| PR | Partial response |
| RECIST | Response evaluation criteria in solid tumours |
| SD | Stable disease |
| TBS | Tumour burden score |
| uCRLM | Unresectable colorectal liver metastases |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer, Global Cancer Observatory, All Cancers Factsheet. Available online: https://Gco.Iarc.Who.Int/Media/Globocan/Factsheets/Cancers/39-All-Cancers-Fact-Sheet.Pdf (accessed on 15 January 2025).
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024. JNCCN J. Natl. Compr. Cancer Netw. 2024, 22, e240029. [Google Scholar]
- Tomlinson, J.S.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kornprat, P.; Gonen, M.; Kemeny, N.; Brennan, M.F.; Blumgart, L.H.; D’Angelica, M. Actual 10-Year Survival After Resection of Colorectal Liver Metastases Defines Cure. J. Clin. Oncol. 2007, 25, 4575–4580. [Google Scholar] [CrossRef]
- Lanari, J.; Hagness, M.; Sartori, A.; Rosso, E.; Gringeri, E.; Dueland, S.; Cillo, U.; Line, P. Liver Transplantation versus Liver Resection for Colorectal Liver Metastasis: A Survival Benefit Analysis in Patients Stratified According to Tumor Burden Score. Transpl. Int. 2021, 34, 1722–1732. [Google Scholar] [CrossRef]
- Glinka, J.; Ardiles, V.; Pekolj, J.; Mattera, J.; Sanchez Clariá, R.; de Santibañes, E.; de Santibañes, M. Liver Transplantation for Non-Resectable Colorectal Liver Metastasis: Where We Are and Where We Are Going. Langenbecks Arch. Surg. 2020, 405, 255–264. [Google Scholar] [CrossRef]
- Foss, A.; Adam, R.; Dueland, S. Liver Transplantation for Colorectal Liver Metastases: Revisiting the Concept. Transpl. Int. 2010, 23, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Hoti, E.; Adam, R. Liver Transplantation for Primary and Metastatic Liver Cancers. Transpl. Int. 2008, 21, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Hagness, M.; Foss, A.; Line, P.-D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, Ø.; Gladhaug, I.P.; Egge, T.S.; et al. Liver Transplantation for Nonresectable Liver Metastases From Colorectal Cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.D. Survival Following Liver Transplantation for Patients with Nonresectable Liver-Only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef]
- Adam, R.; Piedvache, C.; Chiche, L.; Adam, J.P.; Salamé, E.; Bucur, P.; Cherqui, D.; Scatton, O.; Granger, V.; Ducreux, M.; et al. Liver Transplantation plus Chemotherapy versus Chemotherapy Alone in Patients with Permanently Unresectable Colorectal Liver Metastases (TransMet): Results from a Multicentre, Open-Label, Prospective, Randomised Controlled Trial. Lancet 2024, 404, 1107–1118. [Google Scholar] [CrossRef]
- Byrne, M.M.; Chávez-Villa, M.; Ruffolo, L.I.; Loria, A.; Endo, Y.; Niewiemski, A.; Jimenez-Soto, C.; Melaragno, J.I.; Ramaraju, G.A.; Farooq, P.D.; et al. The Rochester Protocol for Living Donor Liver Transplantation of Unresectable Colorectal Liver Metastasis: A 5-Year Report on Selection, Approval, and Outcomes. Am. J. Transplant. 2025, 25, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Sposito, C.; Pietrantonio, F.; Maspero, M.; Di Benedetto, F.; Vivarelli, M.; Tisone, G.; De Carlis, L.; Romagnoli, R.; Gruttadauria, S.; Colledan, M.; et al. Improving Outcome of Selected Patients With Non-Resectable Hepatic Metastases From Colorectal Cancer With Liver Transplantation: A Prospective Parallel Trial (COLT Trial). Clin. Color. Cancer 2023, 22, 250–255. [Google Scholar] [CrossRef]
- Reivell, V.; Hagman, H.; Haux, J.; Jorns, C.; Lindnér, P.; Taflin, H. SOULMATE: The Swedish Study of Liver Transplantation for Isolated Colorectal Cancer Liver Metastases Not Suitable for Operation or Ablation, Compared to Best Established Treatment—A Randomized Controlled Multicenter Trial. Trials 2022, 23, 831. [Google Scholar] [CrossRef]
- Cillo, U.; Burra, P.; Mazzaferro, V.; Belli, L.; Pinna, A.D.; Spada, M.; Nanni Costa, A.; Toniutto, P. A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model”. Am. J. Transplant. 2015, 15, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Neri, F.; Lazzarotto, T.; Bertuzzo, V.R.; Di Gioia, P.; Stacchini, G.; Morelli, M.C.; Ercolani, G.; Cescon, M.; Chiereghin, A.; et al. Immunosuppression Modifications Based on an Immune Response Assay. Transplantation 2015, 99, 1625–1632. [Google Scholar] [CrossRef]
- Hari, D.M.; Leung, A.M.; Lee, J.-H.; Sim, M.-S.; Vuong, B.; Chiu, C.G.; Bilchik, A.J. AJCC Cancer Staging Manual 7th Edition Criteria for Colon Cancer: Do the Complex Modifications Improve Prognostic Assessment? J. Am. Coll. Surg. 2013, 217, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Nickkholgh, A.; Weitz, J.; Encke, J.; Sauer, P.; Mehrabi, A.; Büchler, M.W.; Schmidt, J.; Schemmer, P. Utilization of extended donor criteria in liver transplantation: A comprehensive review of the literature. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S8), viii29–viii36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Brandi, G.; Ricci, A.D.; Rizzo, A.; Zanfi, C.; Tavolari, S.; Palloni, A.; De Lorenzo, S.; Ravaioli, M.; Cescon, M. Is Post-Transplant Chemotherapy Feasible in Liver Transplantation for Colorectal Cancer Liver Metastases? Cancer Commun. 2020, 40, 461–464. [Google Scholar] [CrossRef]
- Rezaee-Zavareh, M.S.; Yeo, Y.H.; Wang, T.; Guo, Z.; Tabrizian, P.; Ward, S.C.; Barakat, F.; Hassanein, T.I.; Shravan, D.; Veeral, A.; et al. Impact of Pre-Transplant Immune Checkpoint Inhibitor Use on Post-Transplant Outcomes in HCC: A Systematic Review and Individual Patient Data Meta-Analysis. J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Rudolph, M.; Shah, S.A.; Quillin, R.; Lemon, K.; Olowokure, O.; Latif, T.; Sohal, D. Immune Checkpoint Inhibitors in Liver Transplant: A Case Series. J. Gastrointest. Oncol. 2023, 14, 1141–1148. [Google Scholar] [CrossRef]
- De Stefano, N.; Patrono, D.; Colli, F.; Rizza, G.; Paraluppi, G.; Romagnoli, R. Liver Transplantation for Hepatocellular Carcinoma in the Era of Immune Checkpoint Inhibitors. Cancers 2024, 16, 2374. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Smedman, T.M.; Røsok, B.; Grut, H.; Syversveen, T.; Jørgensen, L.H.; Line, P. Treatment of Relapse and Survival Outcomes after Liver Transplantation in Patients with Colorectal Liver Metastases. Transpl. Int. 2021, 34, 2205–2213. [Google Scholar] [CrossRef]
- Ratti, F.; Cipriani, F.; Fiorentini, G.; Burgio, V.; Ronzoni, M.; Della Corte, A.; Cascinu, S.; De Cobelli, F.; Aldrighetti, L. Evolution of Surgical Treatment of Colorectal Liver Metastases in the Real World: Single Center Experience in 1212 Cases. Cancers 2021, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Milana, F.; Famularo, S.; Luberto, A.; Rimassa, L.; Scorsetti, M.; Comito, T.; Pressiani, T.; Franzese, C.; Poretti, D.; Di Tommaso, L.; et al. Multidisciplinary Tumor Board in the Management of Patients with Colorectal Liver Metastases: A Single-Center Review of 847 Patients. Cancers 2022, 14, 3952. [Google Scholar] [CrossRef]
- Raptis, D.A.; Elsheikh, Y.; Alnemary, Y.; Marquez, K.A.H.; Bzeizi, K.; Alghamdi, S.; Alabbad, S.; Alqahtani, S.A.; Troisi, R.I.; Boehnert, M.U.; et al. Robotic Living Donor Hepatectomy Is Associated with Superior Outcomes for Both the Donor and the Recipient Compared with Laparoscopic or Open—A Single-Center Prospective Registry Study of 3448 Cases. Am. J. Transplant. 2024, 24, 2080–2091. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Geller, D.A.; Ganesh, S.; Tohme, S.; Molinari, M.; Tevar, A.; Hughes, C.; Humar, A. Living Donor Liver Transplantation for Colorectal Cancer Liver Metastases: Midterm Outcomes at a Single Center in North America. Am. J. Transplant. 2024, 24, 681–687. [Google Scholar] [CrossRef]
- Cascales-Campos, P.A.; Ferreras, D.; Alconchel, F.; Febrero, B.; Royo-Villanova, M.; Martínez, M.; Rodríguez, J.M.; Fernández-Hernández, J.Á.; Ríos, A.; Pons, J.A.; et al. Controlled Donation after Circulatory Death up to 80 Years for Liver Transplantation: Pushing the Limit Again. Am. J. Transplant. 2020, 20, 204–212. [Google Scholar] [CrossRef]
- Haque, O.; Yuan, Q.; Uygun, K.; Markmann, J.F. Evolving Utilization of Donation after Circulatory Death Livers in Liver Transplantation: The Day of DCD Has Come. Clin. Transpl. 2021, 35, e14211. [Google Scholar] [CrossRef]
- Fernández-de la Varga, M.; del Pozo-del Valle, P.; Béjar-Serrano, S.; López-Andújar, R.; Berenguer, M.; Prieto, M.; Montalvá, E.; Aguilera, V. Good Post-Transplant Outcomes Using Liver Donors after Circulatory Death When Applying Strict Selection Criteria: A Propensity-Score Matched-Cohort Study. Ann. Hepatol. 2022, 27, 100724. [Google Scholar] [CrossRef] [PubMed]
- Fallani, G.; Stocco, A.; Siniscalchi, A.; Antonini, M.V.; Stella, A.P.; Amato, A.; Prosperi, E.; Turco, L.; Morelli, M.C.; Cescon, M.; et al. Beyond the Concepts of Elder and Marginal in DCD Liver Transplantation: A Prospective Observational Matched-Cohort Study in the Italian Clinical Setting. Transpl. Int. 2023, 36, 11697. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts From Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study With Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021, 105, 2385–2396. [Google Scholar] [CrossRef]
- Torri, F.; Balzano, E.; Melandro, F.; Maremmani, P.; Bertini, P.; Lo Pane, P.; Masini, M.; Rotondo, M.I.; Babboni, S.; Del Turco, S.; et al. Sequential Normothermic Regional Perfusion and End-Ischemic Ex Situ Machine Perfusion Allow the Safe Use of Very Old DCD Donors in Liver Transplantation. Transplantation 2024, 108, 1394–1402. [Google Scholar] [CrossRef]
- Ravaioli, M.; Fallani, G.; Cescon, M.; Prosperi, E.; De Pace, V.; Siniscalchi, A.; Sangiorgi, G.; Ferracin, M.; Ardizzoni, A.; Morelli, M.C.; et al. Heterotopic Auxiliary Segment 2–3 Liver Transplantation with Delayed Total Hepatectomy: New Strategies for Nonresectable Colorectal Liver Metastases. Surgery 2018, 164, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, S.; Settmacher, U.; Rauchfuß, F.; Balci, D.; Königsrainer, A.; Line, P.-D. RAPID Procedure for Colorectal Cancer Liver Metastasis. Int. J. Surg. 2020, 82, 93–96. [Google Scholar] [CrossRef]
- Nadalin, S.; Königsrainer, A.; Capobianco, I.; Settmacher, U.; Rauchfuss, F. Auxiliary Living Donor Liver Transplantation Combined with Two-Stage Hepatectomy for Unresectable Colorectal Liver Metastases. Curr. Opin. Organ. Transpl. 2019, 24, 651–658. [Google Scholar] [CrossRef]

| Inclusion Criteria |
| Age between 18 and 73 years. |
| Histologically confirmed colorectal adenocarcinoma previously treated with curative intent (pT4a, R0 resection). |
| uCRLM at diagnosis or due to recurrence after previous liver resection. |
| No signs of recurrence of the primary (PET, CT, colonoscopy). |
| No evidence of extrahepatic disease (PET and/or CT). |
| ECOG 0-1 |
| Neutrophils > 1.0 × 109/L (without G-CSF). |
| At least one line of chemotherapy, with SD or PR (mRECIST) for at least 3 months. |
| CEA < 80 µg/L, or ≥50% decrease from the highest previous CEA level. |
| Written informed consent and expected patient cooperation for treatment and follow-up. |
| No contraindications to liver transplant per institutional protocol. |
| Exclusion Criteria |
| Presence of other neoplasms. |
| Local recurrence of the primary tumour. |
| Presence of extrahepatic metastatic disease. |
| Patients not treated with neoadjuvant or adjuvant conventional therapy for the primary tumour. |
| Palliative resection of the primary tumour. |
| Variables | Study Population (n = 21) | Pre-LITORALE (n = 8) | LITORALE (n = 13) | p-Value |
|---|---|---|---|---|
| Age in years, median [IQR] | 53 [48–61] | 48 [43.1–54.5] | 60 [51–63.7] | 0.033 |
| Gender | 0.011 | |||
| Female, n (%) | 7 (33.3) | 0 | 7 (53.8) | |
| Male, n (%) | 14 (66.7) | 8 (100) | 6 (46.2) | |
| BMI in kg/m2, median [IQR] | 23.4 [21.6–27.8] | 22.8 [21.9–25.15] | 24.5 [21.6–28] | 0.447 |
| Previous liver resection, n (%) | 9 (42.9) | 2 (25) | 7 (53.8) | 0.195 |
| Primary tumour site | 0.675 | |||
| Right, n (%) | 3 (14.3) | 1 (12.5) | 2 (15.4) | |
| Left, n (%) | 10 (47.6) | 3 (37.5) | 7 (53.8) | |
| Rectum, n (%) | 8 (38.1) | 4 (50) | 4 (30.8) | |
| (y)pT stage | 0.701 | |||
| 0, n (%) | 1 (4.8) | 0 | 1 (7.7) | |
| 1, n (%) | 0 | 0 | 0 | |
| 2, n (%) | 2 (9.5) | 1 (12.5) | 1 (7.7) | |
| 3, n (%) | 17 (81.0) | 7 (87.5) | 10 (76.9) | |
| 4a, n (%) | 1 (4.8) | 0 | 1 (7.7) | |
| (y)pN stage | 0.586 | |||
| 0, n (%) | 5 (23.8) | 1 (12.5) | 4 (30.8) | |
| 1, n (%) | 10 (47.6) | 4 (50) | 6 (46.2) | |
| 2, n (%) | 6 (28.6) | 3 (37.5) | 3 (23.1) | |
| KRAS | 0.023 | |||
| wt, n (%) | 14 (66.7) | 8 (100) | 7 (53.8) | |
| mt, n (%) | 6 (33.3) | 0 | 6 (46.2) | |
| BRAF | - | |||
| wt, n (%) | 21 (100) | 8 (100) | 13 (100) | |
| mt, n (%) | 0 | 0 | 0 | |
| Synchronous CRLM, n (%) | 21 (100) | 8 (100) | 13 (100) | - |
| Neoadjuvant therapy prior to LT, n (%) | 21 (100) | 8 (100) | 13 (100) | - |
| Extrahepatic disease, n (%) | 1 (4.8) | 1 (12.5) | 0 | 0.191 |
| mRECIST | 0.112 | |||
| SD, n (%) | 14 (66.7) | 7 (87.5) | 7 (53.8) | |
| PR, n (%) | 7 (33.3) | 1 (12.5) | 6 (46.2) | |
| n. of metastases at diagnosis, n (%) | 0.011 | |||
| ≤5 | 7 (33.3) | 0 | 7 (53.8) | |
| 5 < x ≤ 10 | 6 (28.6) | 2 (25.0) | 4 (30.8) | |
| >10 | 8 (38.1) | 6 (75.0) | 2 (15.4) | |
| Largest lesion size at diagnosis in cm, median [IQR] | 4 [2.5–6] | 5.5 [3.65–8.8] | 3 [1.8–4.2] | 0.082 |
| Tumour burden score, median [IQR] | 9.03 [5.72–15.65] | 18.02 [13.13–24.97] | 6.32 [3.16–9.03] | 0.002 |
| Oslo score, n (%) | 0.164 | |||
| 0, n (%) | 10 (47.6) | 3 (37.5) | 7 (53.8) | |
| 1, n (%) | 9 (42.9) | 3 (37.5) | 6 (46.2) | |
| 2, n (%) | 2 (9.5) | 2 (25) | 0 | |
| CEA in µg/L, median [IQR] | 8.7 [2.8–17.6] | 12.5 [3.1–50.55] | 4.6 [2.8–13.6] | 0.365 |
| CEA ≥ 80 µg/L, n(%) | 2 (9.5) | 2 (25) | 0 | 0.058 |
| Variables | Study Population (n = 21) | Pre-LITORALE (n = 8) | LITORALE (n = 13) | p-Value |
|---|---|---|---|---|
| LT waiting time in days, median [IQR] | 37 [11–78] | 83.5 [14–176.5] | 34 [11–71] | 0.346 |
| Interval from primary resection and LT in days, median [IQR] | 389 [331–892] | 382.5 [231.5–522] | 389 [337–1032] | 0.192 |
| Donor type | 0.242 | |||
| DBD, n (%) | 7 (33.3) | 3 (37.5) | 4 (30.8) | |
| EC-DBD, n (%) | 8 (38.1) | 2 (25) | 6 (46.2) | |
| DCD, n (%) | 4 (19) | 1 (12.5) | 3 (23.1) | |
| LD, n (%) | 2 (9.5) | 2 (25) | 0 | |
| HOPE, n (%) | 11 (52.4) | 0 | 11 (84.6) | <0.001 |
| HOPE duration in minutes, median [IQR] | 0 | 134 [112.5–190] | - | |
| Veno-venous bypass, n (%) | 4 (19) | 2 (25) | 2 (15.4) | 0.586 |
| Caval reconstruction, n (%) | 0.209 | |||
| Conventional | 2 (9.5) | 0 | 2 (15.4) | |
| Piggyback | 17 (81) | 6 (75) | 11 (84.6) | |
| Latero-lateral | 1 (4.8) | 1 (12.5) | 0 | |
| RAVAS | 1 (4.8) | 1 (12.5) | 0 | |
| n°. of lesions at pathology, n (%) | 0.546 | |||
| ≤5 | 6 (28.6) | 2 (25) | 4 (30.8) | |
| 5 < x ≤ 10 | 3 (14.3) | 2 (25) | 1 (7.7) | |
| >10 | 12 (57.1) | 4 (50) | 8 (61.5) | |
| Largest lesion size at pathology, cm, median [IQR] | 3.5 [1.55–7.8] | 5.45 [2.75–9.25] | 2.75 [1.2–6.35] | 0.203 |
| Clavien–Dindo ≥ 3a, n (%) | 3 (14.3) | 2 (25) | 1 (7.7) | 0.271 |
| ICU stay, d, median [IQR] | 3 [2–5] | 4 [3–5] | 3 [2–4] | 0.205 |
| LOS, d, median [IQR] | 12 [10–20] | 15 [11.5–26.5] | 10 [10–12] | 0.096 |
| 90-days mortality, n (%) | 1 (4.8) | 0 | 1 (7.7) | 0.646 |
| LT-adjuvant CHT, n (%) | 17 (81) | 7 (87.5) | 10 (76.9) | 0.549 |
| Recurrence, n (%) | 13 (61.9) | 7 (87.5) | 6 (46.2) | 0.058 |
| Pattern of recurrence | 0.048 | |||
| No recurrence, n (%) | 8 (38.1) | 1 (12.5) | 7 (53.8) | |
| Single-site, n (%) | 8 (38.1) | 3 (37.5) | 5 (38.5) | |
| Multi-site, n (%) | 5 (23.1) | 4 (50) | 1 (7.7) | |
| Site of recurrence | ||||
| Liver-only, n (%) | 2/13 (15.4) | 1/7 (14.3) | 1/6 (16.7) | 0.906 |
| Lung-only, n (%) | 3/13 (23.1) | 0 | 3/6 (50) | 0.033 |
| Lymph nodes-only, n (%) | 1/13 (7.7) | 1/7 (14.3) | 0 | 0.335 |
| Bone-only, n (%) | 1/13 (7.7) | 1/7 (14.3) | 0 | 0.335 |
| Pelvic, n (%) | 1/13 (7.7) | 0 | 1/6 (16.7) | 0.261 |
| Multiorgan, n (%) | 5/13 (38.4) | 4/7 (57.1) | 1/6 (16.7) | 0.135 |
| Patient | LITORALE | Site of Recurrence | Treatment of Recurrence | |||
|---|---|---|---|---|---|---|
| Chemiotherapy | Radiotherapy | Surgery | Type of Surgery | |||
| 1 | 0 | Abdominal lymph node | Yes | Yes | Lymph node resection | |
| 2 | 0 | Liver | ||||
| 3 | 0 | Lung + brain | Yes | Yes | Yes | Lung resection |
| 4 | 0 | Lung + liver + adrenal gland | Yes | Yes | Yes | Lung resection |
| 5 | 0 | Bone | ||||
| 6 | 0 | Lung + liver | Yes | Lung resection | ||
| 7 | 0 | Lung + brain + liver + kidney + bone | Yes | |||
| 8 | 1 | Pelvic | Yes | Abdominoperineal rectal resection | ||
| 9 | 1 | Lung + bone | Yes | Yes | ||
| 10 | 1 | Liver | Yes | Liver resection | ||
| 11 | 1 | Lung | Yes | Yes | ||
| 12 | 1 | Lung | Yes | Yes | ||
| 13 | 1 | Lung | Yes | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, A.; Laurenzi, A.; Serenari, M.; Prosperi, E.; Fallani, G.; Bonatti, C.; Radi, G.; Prior, M.; Odaldi, F.; Zanfi, C.; et al. Liver Transplantation for Colorectal Metastases: Impact of a Standardised Protocol for Patient Selection on Transplant Outcomes. Cancers 2025, 17, 2046. https://doi.org/10.3390/cancers17122046
Stocco A, Laurenzi A, Serenari M, Prosperi E, Fallani G, Bonatti C, Radi G, Prior M, Odaldi F, Zanfi C, et al. Liver Transplantation for Colorectal Metastases: Impact of a Standardised Protocol for Patient Selection on Transplant Outcomes. Cancers. 2025; 17(12):2046. https://doi.org/10.3390/cancers17122046
Chicago/Turabian StyleStocco, Alberto, Andrea Laurenzi, Matteo Serenari, Enrico Prosperi, Guido Fallani, Chiara Bonatti, Giorgia Radi, Margherita Prior, Federica Odaldi, Chiara Zanfi, and et al. 2025. "Liver Transplantation for Colorectal Metastases: Impact of a Standardised Protocol for Patient Selection on Transplant Outcomes" Cancers 17, no. 12: 2046. https://doi.org/10.3390/cancers17122046
APA StyleStocco, A., Laurenzi, A., Serenari, M., Prosperi, E., Fallani, G., Bonatti, C., Radi, G., Prior, M., Odaldi, F., Zanfi, C., Mirici Cappa, F., Siniscalchi, A., Morelli, M. C., Ravaioli, M., & Cescon, M. (2025). Liver Transplantation for Colorectal Metastases: Impact of a Standardised Protocol for Patient Selection on Transplant Outcomes. Cancers, 17(12), 2046. https://doi.org/10.3390/cancers17122046








