An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization
Simple Summary
Abstract
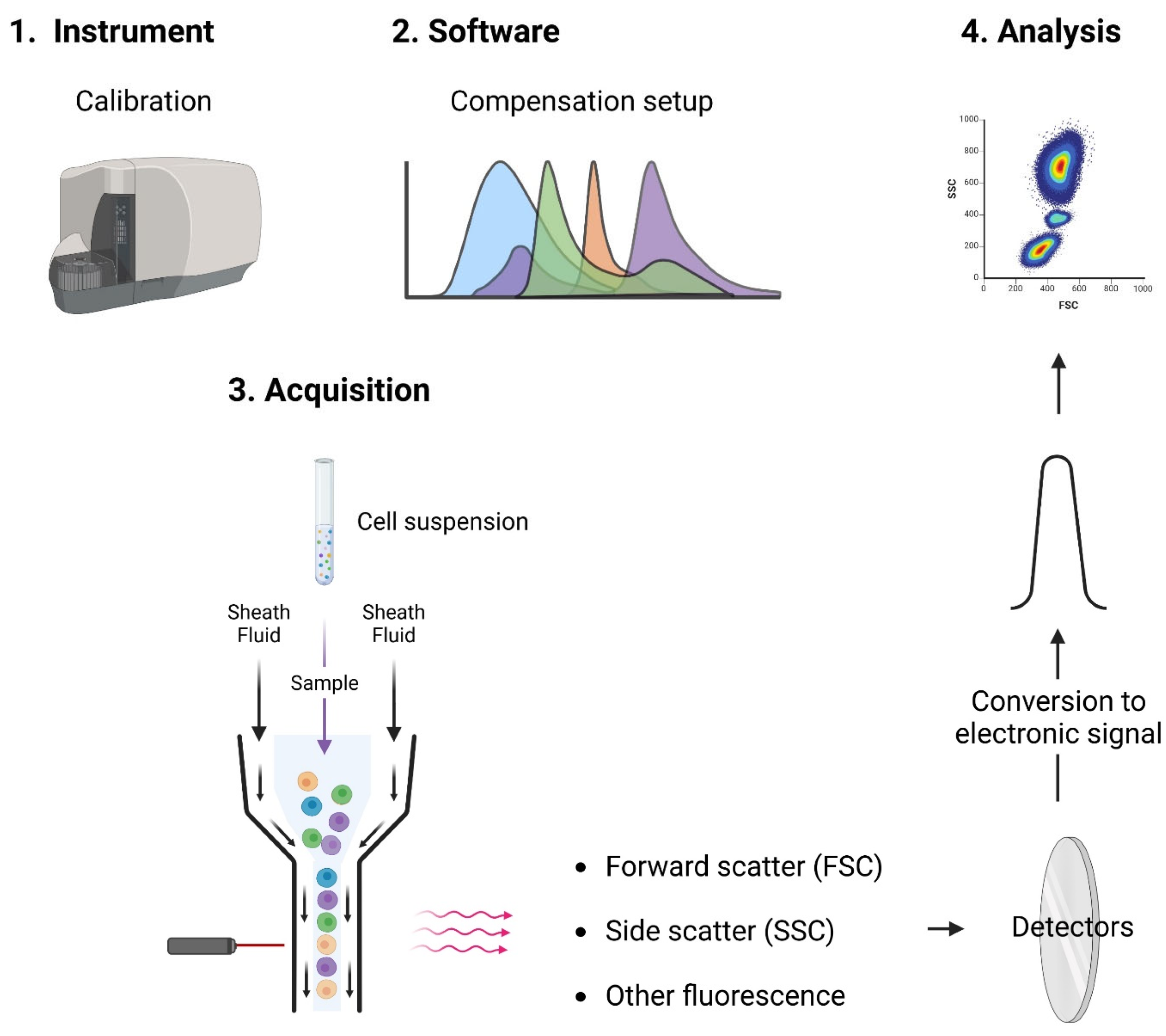
1. Principles of Flow Cytometry
- •
- Cytometer preparation: Experiments require calibration and optimization of cytometer setup (such as compensation) to simplify subsequent analysis.
- •
- Sample preparation: Dedicated protocols are used (e.g., red blood cell lysis) and cells must be in a single-cell suspension, free from clumps and debris.
- •
- Sample staining: Cells are stained with fluorescently labeled antibodies or dyes that bind to specific cellular components, such as surface markers, intracellular proteins, or DNA.
- •
- Gating: The process of selecting specific cell populations based on their scatter and fluorescence characteristics. Gating is crucial for identifying and analyzing subpopulations of interest.
- •
- Histograms and Dot Plots: Data are displayed in various formats, such as histograms (single-parameter plots) and dot plots (dual-parameter plots), to visualize the distribution and relationships of different cell populations.
- •
- Quantitative analysis: The software allows for the semi-quantitative (units are mean fluorescence intensities) assessment of marker expression, cell counts, and other parameters.
- •
- Speed and efficiency (high throughput): Analyze thousands of cells per second, providing rapid results that are essential for timely diagnosis and treatment decisions such as for acute leukemias where prompt treatment initiation is critical.
- •
- Multiparametric analysis at the single-cell level: Simultaneously measure multiple parameters on individual cells for a comprehensive analysis of populations, enhanced accuracy of diagnosis (distinguish different types of malignancies).
- •
- Quantitative assessment: Provide semi-quantitative data on the expression levels of markers, which can be useful for monitoring disease progression and response to therapy (minimal residual disease (MRD)). Quantitative mean fluorescence intensities (MFIs) can be obtained by calibration against defined binding sites.
- •
- Versatility: This can be applied to a wide range of sample types, including peripheral blood, bone marrow, and lymph node biopsies.
2. Importance of Flow Cytometry for Hematological Malignancy Diagnosis
2.1. Low Complexity Analysis
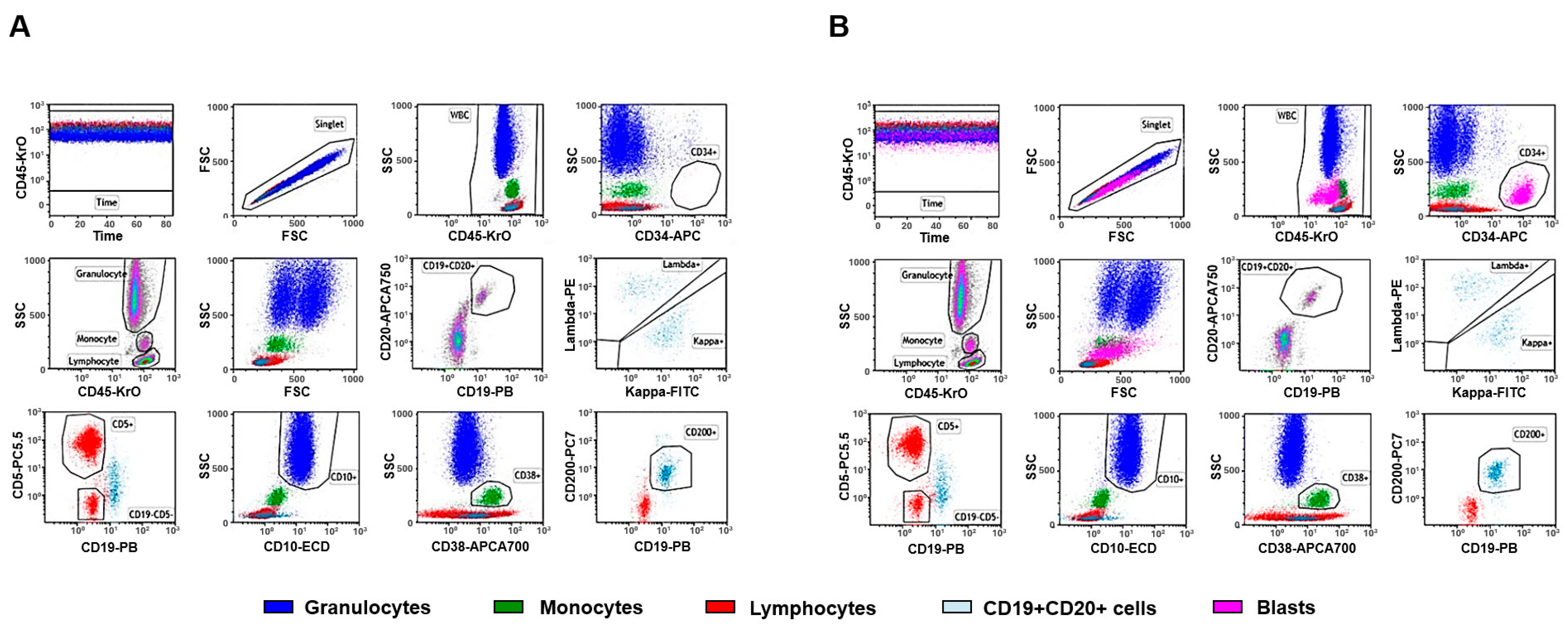
2.2. Higher Complexity Analysis
2.3. The Limitations and Complementarity of Flow Cytometry
3. Pitfalls in Flow Cytometry Analysis
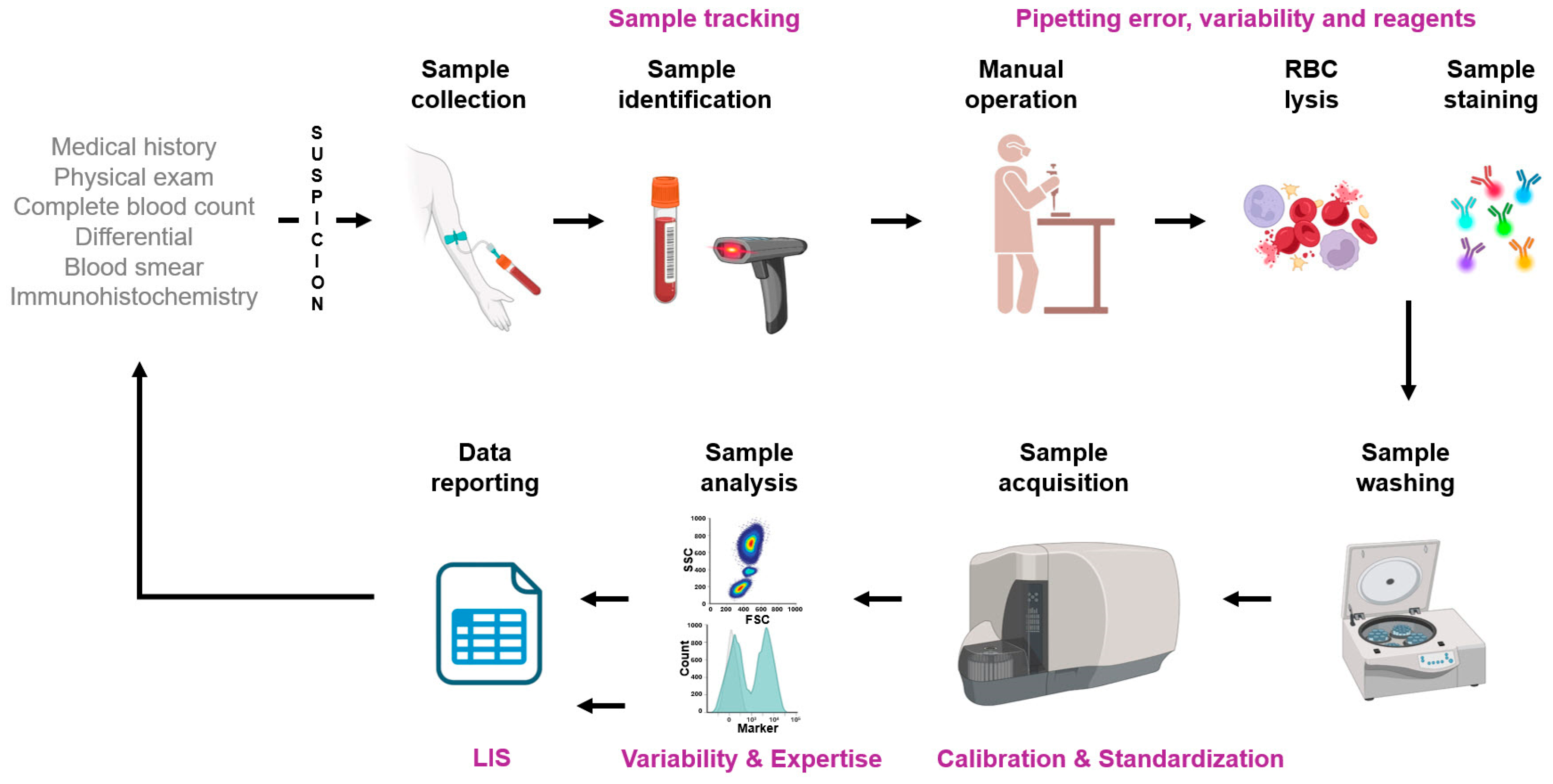
3.1. Overview
- •
- Expertise: The interpretation of flow cytometry data requires specialized training and expertise. Skilled cytometrists are essential for accurate diagnosis, as biology and aberrant expression are not always predictable [37,38]. Practitioners must have a deep understanding of the whole process from panel design, sample preparation, instrument calibration, and quality control to complex data interpretation. This involves understanding the normal/expected expression of specific antigens during the development/maturation of hematopoietic cells compared to aberrant/unexpected expression in the case of leukemia/lymphoma populations [39,40,41,42].
- •
- Standardization: Inadequate laboratory performance can have far-reaching consequences for practical medicine, the healthcare system, and ultimately for the patient. Poor-quality results may lead to incorrect interpretations by clinicians, potentially worsening the patient’s condition [43]. Hence, standardization of methods and quality control measures are important to ensure consistency and reliability [44]. Implementing standardization is not necessarily an easy process [43,45] but could be achieved via (i) establishing a reference system that includes reference methods and materials, (ii) calibrating measurement procedures using the established reference system, (iii) verifying the comparability of measurements used in patient care, typically by measuring a set of authentic patient samples to ensure uniformity of results across different methods, and (iv) simplifying workflows, reducing operator intervention to remove potential bias and to set the stage for automation.
- •
- Cost and Accessibility: Efforts to reduce costs and improve access are important for broader implementation [46,47]. Of note, implementing flow cytometry screening for patient monitoring such as in the case of MRD could reduce the overall healthcare cost [48]. Hence, a critical analysis of flow cytometry-based assay costs should be performed with a holistic view of costs for the initial diagnostic, treatment, and follow-up phases. The number of markers used for diagnosis can be limited by costs for the patients or the reimbursement policy, which is highly variable depending on the country or region [49].
3.2. Validation of Flow Cytometry Assays
4. A Deeper Look into Flow Cytometry Standardization
4.1. The Need for Standardization
4.2. Standardization and Harmonization
- •
- Reagent and Instrument Standardization: Use standardized reagents, protocols, and calibration of flow cytometers to ensure consistent and reproducible results.
- •
- Quality Control: Implement robust quality control measures to monitor instrument performance and reagent quality regularly.
- •
- Comprehensive Marker Panels: Design antibody panels that cover a broad range of lineage-specific and differentiation markers to accurately identify and classify hematological malignancies.
- •
- Disease-Specific Panels: Customize panels based on suspected malignancy, such as acute leukemia, chronic leukemia, or lymphoma.
- •
- Sample Preparation: Ensure proper handling and preparation of samples to maintain cell integrity and viability and prepare single-cell suspensions to avoid clumping and ensure accurate analysis.
- •
- Data Acquisition: Acquire high-quality data using appropriate laser settings and compensation controls, ensuring proper instrument alignment, and employing rigorous gating strategies to accurately identify and analyze subpopulations of interest.
- •
- Data Analysis and Interpretation: Data should be analyzed by experienced personnel who are proficient in flow cytometry techniques and the interpretation of hematological malignancies and utilize advanced software tools for comprehensive analysis, including the identification of MRD.
- •
- Clear Reporting: Provide clear and detailed reports that include information on the markers used, gating strategies, and interpretation of results.
- •
- Clinical Correlation: Correlate flow cytometry findings with clinical features and other laboratory results for accurate diagnosis and management.
- •
- Continuous Education and Training: Ensure continuous education and training for laboratory personnel to stay updated with the latest advancements and best practices in flow cytometry.
4.3. Implementation of Standardization
5. Update on Solutions Improving Resolution and Reducing Variability
5.1. Ready-to-Use Reagents
5.2. Minimal Residual Disease: The Reproducibility Perspective
5.3. Minimal Residual Disease: The Sensitivity Perspective
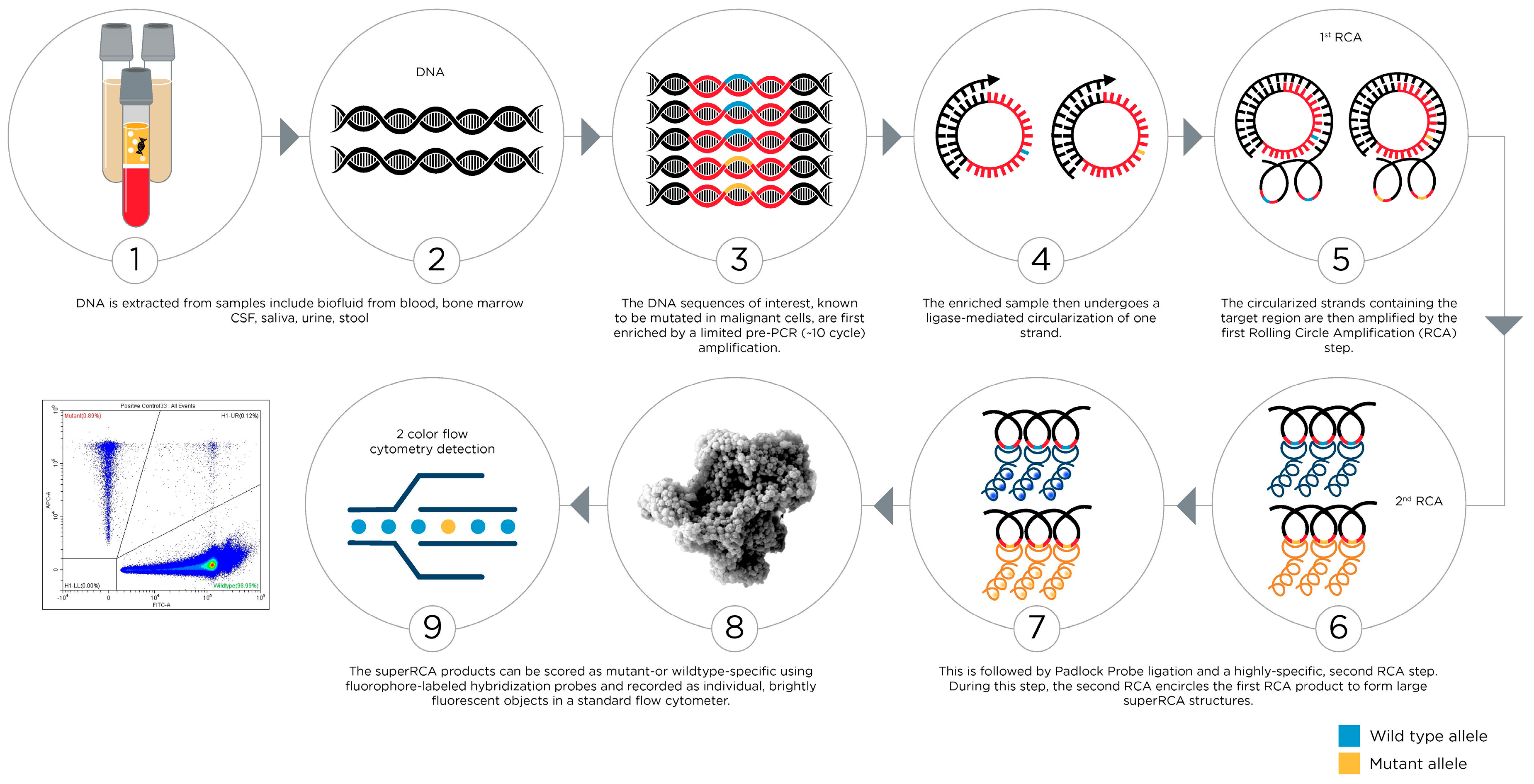
6. The Future of Flow Cytometry for Hematological Malignancies
6.1. Spectral Flow Cytometry
6.2. Approaches Requiring Further Developments
6.3. The Emergence of Assistive Analytical Tools
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, M.; Wittwer, C. Flow cytometry: Principles and clinical applications in hematology. Clin. Chem. 2000, 46 Pt 2, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Laerum, O.D.; Farsund, T. Clinical application of flow cytometry: A review. Cytometry 1981, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cenariu, M.; Grewal, R.; Bumbea, H.; Sauma, D.; Tomuleasa, C. Editorial: Flow cytometry—A powerful tool for diagnosis and therapy monitoring in hematology and immunology. Front. Med. 2023, 10, 1282060. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Hergott, C.B. Advances in Acute Myeloid Leukemia Classification, Prognostication and Monitoring by Flow Cytometry. Clin. Lab. Med. 2023, 43, 377–398. [Google Scholar] [CrossRef]
- Manohar, S.M.; Shah, P.; Nair, A. Flow cytometry: Principles, applications and recent advances. Bioanalysis 2021, 13, 181–198. [Google Scholar] [CrossRef]
- Song, Y.; Lee, Y. Brief guide to flow cytometry. Mol. Cells 2024, 47, 100129. [Google Scholar] [CrossRef]
- Radcliff, G.; Jaroszeski, M.J. Basics of flow cytometry. Methods Mol. Biol. 1998, 91, 1–24. [Google Scholar] [CrossRef]
- Telford, W.G. Laser Sources for Traditional and Spectral Flow Cytometry. Methods Mol. Biol. 2024, 2779, 33–68. [Google Scholar] [CrossRef]
- Lagoo, A.S. How to Design and Validate a Clinical Flow Cytometry Assay. Clin. Lab. Med. 2023, 43, 333–349. [Google Scholar] [CrossRef]
- Scheuermann, R.H.; Bui, J.; Wang, H.Y.; Qian, Y. Automated Analysis of Clinical Flow Cytometry Data: A Chronic Lymphocytic Leukemia Illustration. Clin. Lab. Med. 2017, 37, 931–944. [Google Scholar] [CrossRef]
- Muronova, L.; Soucek, O.; Zihala, D.; Sevcikova, T.; Popkova, T.; Plonkova, H.; Venglar, O.; Pour, L.; Stork, M.; Rihova, L.; et al. Real-World Evidence on Prognostic Value of MRD in Multiple Myeloma Using Flow Cytometry. Eur. J. Haematol. 2025, 114, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J. Flow Cytometry of Hematological Malignancies. Br. J. Haematol. 2022, 199, 158. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Andreeff, M.; Mertelsmann, R.; Haghbin, M.; Tan, C.; Miller, D.R.; Good, R.A.; Gupta, S. Childhood CML in blastic stage: An analysis of cell markers and cell kinetics. Am. J. Hematol. 1980, 9, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L.; Warner, N.L.; Ledbetter, J.A.; Herzenberg, L.A. Quantitative immunofluorescent analysis of surface phenotypes of murine B cell lymphomas and plasmacytomas with monoclonal antibodies. J. Immunol. 1981, 127, 1691–1697. [Google Scholar] [CrossRef]
- Alatawi, D.; Bakhsh, I.; Tashkandi, S.; Alqarni, A.; Shaik, A.P.; Abudawood, M. Diagnostic Accuracy of Flow Cytometric DNA Index in Saudi Children with B Cell Acute Lymphoblastic Leukemia. Children 2023, 10, 1299. [Google Scholar] [CrossRef]
- Soilleux, E.J.; Rodgers, D.T.; Situ, J.J.; Evans, S.C.; Konda, V.N.; Yang, H.C.; Pang, J.; Gilbey Smith, I.; Rajesh, P.; Salimi, M.; et al. Demonstration of T-Cell Monotypia Using Anti-TCRbeta1/2 (TRBC1/2) Immunostaining as a Rapid and Cost-Effective Alternative to PCR-Based Clonality Studies for the Diagnosis of T-Cell Lymphoma. Diagnostics 2024, 14, 2479. [Google Scholar] [CrossRef]
- Eyre, T.A.; Bishton, M.J.; McCulloch, R.; O’Reilly, M.; Sanderson, R.; Menon, G.; Iyengar, S.; Lewis, D.; Lambert, J.; Linton, K.M.; et al. Diagnosis and management of mantle cell lymphoma: A British Society for Haematology Guideline. Br. J. Haematol. 2024, 204, 108–126. [Google Scholar] [CrossRef]
- Killick, S.B.; Wiseman, D.H.; Quek, L.; Cargo, C.; Culligan, D.; Enright, H.; Green, S.; Ingram, W.; Jones, G.L.; Kell, J.; et al. British Society for Haematology guidelines for the diagnosis and evaluation of prognosis of Adult Myelodysplastic Syndromes. Br. J. Haematol. 2021, 194, 282–293. [Google Scholar] [CrossRef]
- Sive, J.; Cuthill, K.; Hunter, H.; Kazmi, M.; Pratt, G.; Smith, D.; British Society of Haematology. Guidelines on the diagnosis, investigation and initial treatment of myeloma: A British Society for Haematology/UK Myeloma Forum Guideline. Br. J. Haematol. 2021, 193, 245–268. [Google Scholar] [CrossRef]
- Ng, A.P.; Adams, R.; Tiong, I.S.; Seymour, L.; Talaulikar, D.; Palfreyman, E.; Enjeti, A.; Tate, C. Reporting bone marrow biopsies for myelodysplastic neoplasms and acute myeloid leukaemia incorporating WHO 5th edition and ICC 2022 classification systems: ALLG/RCPA joint committee consensus recommendations. Pathology 2024, 56, 459–467. [Google Scholar] [CrossRef]
- Debliquis, A.; Ahle, G.; Houillier, C.; Soussain, C.; Hoang-Xuan, K.; Le Garff-Tavernier, M.; CytHem and in partnership with the LOC Network. Analysis of cerebrospinal fluid for the diagnosis of CNS lymphoma: Comparison of the ESCCA/ISCCA protocol and real-world data of the CytHem/LOC French network. Cytom. Part B Clin. Cytom. 2024, 106, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Trka, J.; Fronkova, E. Minimal residual disease detection for acute lymphoblastic leukaemia in peripheral blood-Are we there yet? Br. J. Haematol. 2025, 206, 397–398. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Westers, T.M.; Ireland, R.; Kern, W.; Alhan, C.; Balleisen, J.S.; Bettelheim, P.; Burbury, K.; Cullen, M.; Cutler, J.A.; Della Porta, M.G.; et al. Standardization of flow cytometry in myelodysplastic syndromes: A report from an international consortium and the European LeukemiaNet Working Group. Leukemia 2012, 26, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.; Young, N.S. Bethesda Handbook of Clinical Hematology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2024. [Google Scholar]
- Rawstron, A.C.; Paiva, B.; Stetler-Stevenson, M. Assessment of minimal residual disease in myeloma and the need for a consensus approach. Cytom. Part B Clin. Cytom. 2016, 90, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Gupta, R.; Balzer, B. Diagnosis of metastatic neoplasms: A clinicopathologic and morphologic approach. Arch. Pathol. Lab. Med. 2010, 134, 194–206. [Google Scholar] [CrossRef]
- Chen, X.; Fromm, J.R.; Naresh, K.N. “Blasts” in myeloid neoplasms—How do we define blasts and how do we incorporate them into diagnostic schema moving forward? Leukemia 2022, 36, 327–332. [Google Scholar] [CrossRef]
- Martig, D.S.; Fromm, J.R. A comparison and review of the flow cytometric findings in classic Hodgkin lymphoma, nodular lymphocyte predominant Hodgkin lymphoma, T cell/histiocyte rich large B cell lymphoma, and primary mediastinal large B cell lymphoma. Cytom. Part B Clin. Cytom. 2022, 102, 14–25. [Google Scholar] [CrossRef]
- Tembhare, P.; Yuan, C.M.; Xi, L.; Morris, J.C.; Liewehr, D.; Venzon, D.; Janik, J.E.; Raffeld, M.; Stetler-Stevenson, M. Flow cytometric immunophenotypic assessment of T-cell clonality by Vβ repertoire analysis: Detection of T-cell clonality at diagnosis and monitoring of minimal residual disease following therapy. Am. J. Clin. Pathol. 2011, 135, 890–900. [Google Scholar] [CrossRef]
- Chen, J.; Gale, R.P.; Hu, Y.; Yan, W.; Wang, T.; Zhang, W. Measurable residual disease (MRD)-testing in haematological and solid cancers. Leukemia 2024, 38, 1202–1212. [Google Scholar] [CrossRef]
- Seferna, K.; Svaton, M.; Rennerova, A.; Skotnicova, A.; Reznickova, L.; Valova, T.; Sedlacek, P.; Riha, P.; Formankova, R.; Keslova, P.; et al. NGS-MRD negativity in post-HSCT ALL spares unnecessary therapeutic interventions triggered by borderline qPCR results without an increase in relapse risk. HemaSphere 2025, 9, e70124. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Hedley, B.D.; Keeney, M. Common flow cytometry pitfalls in diagnostic hematopathology. Cytom. Part B Clin. Cytom. 2019, 96, 449–463. [Google Scholar] [CrossRef]
- Song, J.Y.; Pan, Z. Aberrant expression in lymphoma, a diagnostic pitfall. Hum. Pathol. 2025, 156, 105706. [Google Scholar] [CrossRef] [PubMed]
- Frye Naharro, E.; Peterson, D.; Yohe, S.L.; Linden, M.A. Application and pitfalls of immunophenotyping in challenging plasma cell neoplasms: A case series. Hum. Pathol. 2024, 150, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Placek, A.; Lockhart, B.; Miller, K.P.; Wertheim, G.B.; Maude, S.L.; Wood, B.L.; Kovach, A.E. Maturational dyssynchrony in benign B-cell precursors following lymphocyte depleting chemotherapy: A potential pitfall for B-lymphoblastic leukemia minimal/measurable residual disease (MRD) flow cytometry analysis. Cytom. Part B Clin. Cytom. 2024, 106, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Soma, L.; Wu, D.; Chen, X.; Edlefsen, K.; Fromm, J.R.; Wood, B. Apparent CD19 expression by natural killer cells: A potential confounder for minimal residual disease detection by flow cytometry in B lymphoblastic leukemia. Cytom. Part B Clin. Cytom. 2015, 88, 145–147. [Google Scholar] [CrossRef]
- Wood, B.L.; Levin, G.R. Interactions between mouse IgG2 antibodies are common and mediated by plasma C1q. Cytom. Part B Clin. Cytom. 2006, 70, 321–328. [Google Scholar] [CrossRef]
- Golzari-Sorkheh, M.; Yoganathan, K.; Chen, E.L.Y.; Singh, J.; Zúñiga-Pflücker, J.C. T Cell Development: From T-Lineage Specification to Intrathymic Maturation. Adv. Exp. Med. Biol. 2025, 1471, 81–137. [Google Scholar] [CrossRef]
- Xing, R.; Wang, M.; Wang, L.; Pan, M.; Wang, Y.; Zhou, H. Clinical updates of B-cell maturation antigen-targeted therapy in multiple myeloma (MM) and relapsed/refractory MM (Review). Int. J. Mol. Med. 2025, 55, 27. [Google Scholar] [CrossRef]
- Costa, B.A.; Ortiz, R.J.; Lesokhin, A.M.; Richter, J. Soluble B-cell maturation antigen in multiple myeloma. Am. J. Hematol. 2024, 99, 727–738. [Google Scholar] [CrossRef]
- Weiskopf, K.; Schnorr, P.J.; Pang, W.W.; Chao, M.P.; Chhabra, A.; Seita, J.; Feng, M.; Weissman, I.L. Myeloid Cell Origins, Differentiation, and Clinical Implications. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.W.; Myers, G.L.; Miller, W.G. Current practices and challenges in the standardization and harmonization of clinical laboratory tests. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 907S–912S. [Google Scholar] [CrossRef]
- Panteghini, M.; Forest, J.C. Standardization in laboratory medicine: New challenges. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 355, 1–12. [Google Scholar] [CrossRef]
- Wears, R.L. Standardisation and Its Discontents. Cogn. Technol. Work 2015, 17, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rajab, A.; Axler, O.; Leung, J.; Wozniak, M.; Porwit, A. Ten-color 15-antibody flow cytometry panel for immunophenotyping of lymphocyte population. Int. J. Lab. Hematol. 2017, 39 (Suppl. 1), 76–85. [Google Scholar] [CrossRef][Green Version]
- Chong, H.M.; Zhang, Z.W.; Li, J.M.; Ren, X.D.; Gong, C.M.; Zhu, Z.X.; Xiang, N.; Ni, Z.H.; Huang, Q. Cost-effective in-house-made whole blood materials for internal quality control in clinical flow cytometry analysis. Anal. Bioanal. Chem. 2025, 417, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Tettero, J.M.; Al-Badri, W.K.W.; Ngai, L.L.; Bachas, C.; Breems, D.A.; van Elssen, C.H.M.J.; Fischer, T.; Gjertsen, B.T.; van Gorkom, G.N.Y.; Gradowska, P.; et al. Concordance in measurable residual disease result after first and second induction cycle in acute myeloid leukemia: An outcome- and cost-analysis. Front. Oncol. 2022, 12, 999822. [Google Scholar] [CrossRef]
- Ross, A.; Rudd, D.; Wight, J. Low flow: Selecting a limited flow cytometry panel where resources are constrained. Blood Rev. 2025, 101284, advance online publication. [Google Scholar] [CrossRef]
- Valle, A.; Maugeri, N.; Manfredi, A.A.; Battaglia, M. Standardization in flow cytometry: Correct sample handling as a priority. Nat. Rev. Immunol. 2012, 12, 864. [Google Scholar] [CrossRef]
- Plank, K.; Dorn, C.; Krause, S.W. The effect of erythrocyte lysing reagents on enumeration of leukocyte subpopulations compared with a no-lyse-no-wash protocol. Int. J. Lab. Hematol. 2021, 43, 939–947. [Google Scholar] [CrossRef]
- Kasinrerk, W. A flow cytometric method for enumeration of lymphocyte sub-populations in sample containing lysis-resistant red blood cells. Immunol. Lett. 2003, 86, 259–264. [Google Scholar] [CrossRef]
- Johansson, U.; Macey, M. Tandem dyes: Stability in cocktails and compensation considerations. Cytom. Part B Clin. Cytom. 2014, 86, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Livák, F. Principles of Advanced Flow Cytometry: A Practical Guide. Methods Mol. Biol. 2023, 2580, 89–114. [Google Scholar] [CrossRef]
- Kala, P.S.; Zubair, M. Flow Cytometry Blood Cell Identification. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Meyerson, H. Flow Cytometry in Hematology. In Concise Guide to Hematology; Lazarus, H., Schmaier, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Craig, F.E.; Foon, K.A. Flow cytometric immunophenotyping for hematologic neoplasms. Blood 2008, 111, 3941–3967. [Google Scholar] [CrossRef]
- Gorczyca, W. Flow Cytometry in Neoplastic Hematology. Morphologic-Immunophenotypic-Genetic Correlation, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Wang, L.; Hoffman, R.A. Standardization, Calibration, and Control in Flow Cytometry. Curr. Protoc. Cytom. 2017, 79, 1.3.1–1.3.27. [Google Scholar] [CrossRef] [PubMed]
- Gonneau, C.; Wang, L.; Mitra-Kaushik, S.; Trampont, P.C.; Litwin, V. Progress towards global standardization for quantitative flow cytometry. Bioanalysis 2021, 13, 1591–1595. [Google Scholar] [CrossRef]
- Kalina, T. Reproducibility of Flow Cytometry Through Standardization: Opportunities and Challenges. Cytom. Part A J. Int. Soc. Anal. Cytol. 2020, 97, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, S.; Mehta, D.; Vitaliti, A.; Allinson, J.; Amur, S.; Eck, S.; Green, C.; Hedrick, M.; Hopper, S.; Ji, A.; et al. 2019 White Paper on Recent Issues in Bioanalysis: FDA Immunogenicity Guidance, Gene Therapy, Critical Reagents, Biomarkers and Flow Cytometry Validation (Part 3—Recommendations on 2019 FDA Immunogenicity Guidance, Gene Therapy Bioanalytical Challenges, Strategies for Critical Reagent Management, Biomarker Assay Validation, Flow Cytometry Validation & CLSI H62). Bioanalysis 2019, 11, 2207–2244. [Google Scholar] [CrossRef]
- Stelzer, G.T.; Marti, G.; Hurley, A.; McCoy, P.; Jr Lovett, E.J.; Schwartz, A. U.S.-Canadian Consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: Standardization and validation of laboratory procedures. Cytometry 1997, 30, 214–230. [Google Scholar] [CrossRef]
- ICSH/ICCS Workgroup. Validation of cell-based fluorescence assays: Practice guidelines from the International Council for Standardization of Haematology and International Clinical Cytometry Society. Cytom. Part B Clin. Cytom. 2013, 84, 281. [Google Scholar] [CrossRef]
- Available online: https://www.cytometry.org/web/quality.php (accessed on 17 April 2025).
- Le Lann, L.; Jouve, P.E.; Alarcón-Riquelme, M.; Jamin, C.; Pers, J.O.; PRECISESADS Flow Cytometry Study Group, PRECISESADS Clinical Consortium. Standardization procedure for flow cytometry data harmonization in prospective multicenter studies. Sci. Rep. 2020, 10, 11567. [Google Scholar] [CrossRef]
- Porwit, A.; Béné, M.C.; Duetz, C.; Matarraz, S.; Oelschlaegel, U.; Westers, T.M.; Wagner-Ballon, O.; Kordasti, S.; Valent, P.; Preijers, F.; et al. Multiparameter flow cytometry in the evaluation of myelodysplasia: Analytical issues: Recommendations from the European LeukemiaNet/International Myelodysplastic Syndrome Flow Cytometry Working Group. Cytometry. Part B Clin. Cytom. 2023, 104, 27–50. [Google Scholar] [CrossRef]
- Keeney, M.; Barnett, D.; Gratama, J.W. Impact of standardization on clinical cell analysis by flow cytometry. J. Biol. Regul. Homeost. Agents 2004, 18, 305–312. [Google Scholar] [PubMed]
- Monaghan, S.A.; Eck, S.; Bunting, S.; Dong, X.X.; Durso, R.J.; Gonneau, C.; Hays, A.; Illingworth, A.; League, S.C.; Linskens, E.; et al. Flow cytometry assay modifications: Recommendations for method validation based on CLSI H62 guidelines. Cytom. Part B Clin. Cytom. 2024. advance online publication. [Google Scholar] [CrossRef]
- Westera, L.; van Viegen, T.; Jeyarajah, J.; Azad, A.; Bilsborough, J.; van den Brink, G.R.; Cremer, J.; Danese, S.; D’Haens, G.; Eckmann, L.; et al. Centrally Determined Standardization of Flow Cytometry Methods Reduces Interlaboratory Variation in a Prospective Multicenter Study. Clin. Transl. Gastroenterol. 2017, 8, e126. [Google Scholar] [CrossRef]
- Glier, H.; Heijnen, I.; Hauwel, M.; Dirks, J.; Quarroz, S.; Lehmann, T.; Rovo, A.; Arn, K.; Matthes, T.; Hogan, C.; et al. Standardization of 8-color flow cytometry across different flow cytometer instruments: A feasibility study in clinical laboratories in Switzerland. J. Immunol. Methods 2019, 475, 112348. [Google Scholar] [CrossRef] [PubMed]
- Debliquis, A.; Baseggio, L.; Bouyer, S.; Guy, J.; Garnache-Ottou, F.; Genevieve, F.; Mayeur-Rousse, C.; Letestu, R.; Chapuis, N.; Harrivel, V.; et al. Multicentric MFI30 study: Standardization of flow cytometry analysis of CD30 expression in non-Hodgkin lymphoma. Cytom. Part B Clin. Cytom. 2021, 100, 488–496. [Google Scholar] [CrossRef]
- Soh, K.T.; Wallace, P.K. Evaluation of measurable residual disease in multiple myeloma by multiparametric flow cytometry: Current paradigm, guidelines, and future applications. Int. J. Lab. Hematol. 2021, 43 (Suppl. 1), 43–53. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Ossenkoppele, G.J.; Kelder, A.; Cloos, J. Measurable residual disease in acute myeloid leukemia using flow cytometry: Approaches for harmonization/standardization. Expert Rev. Hematol. 2018, 11, 921–935. [Google Scholar] [CrossRef]
- Ikoma-Colturato, M.R.V.; Bertolucci, C.M.; Conti-Spilari, J.E.; Oliveira, E.; Simioni, A.J.; Figueredo-Pontes, L.L.; Furtado, F.M.; Alegretti, A.P.; Azambuja, A.P.; Gevert, F.; et al. Multicentric standardization of minimal/measurable residual disease in B-cell precursor acute lymphoblastic leukaemia using next-generation flow cytometry in a low/middle-level income country. Br. J. Haematol. 2023, 200, 381–384. [Google Scholar] [CrossRef]
- Sommer, U.; Eck, S.; Marszalek, L.; Stewart, J.J.; Bradford, J.; McCloskey, T.W.; Green, C.; Vitaliti, A.; Oldaker, T.; Litwin, V. High-sensitivity flow cytometric assays: Considerations for design control and analytical validation for identification of Rare events. Cytom. Part B Clin. Cytom. 2021, 100, 42–51. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Kreuzer, K.A.; Soosapilla, A.; Spacek, M.; Stehlikova, O.; Gambell, P.; McIver-Brown, N.; Villamor, N.; Psarra, K.; Arroz, M.; et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytom. Part B Clin. Cytom. 2018, 94, 121–128. [Google Scholar] [CrossRef]
- Engelmann, R.; Flores-Montero, J.; Schilperoord-Vermeulen, J.; Ritgen, M.; Hengeveld, P.J.; Kohlscheen, S.; Grigore, G.; Rodriguez, R.F.; Lecrevisse, Q.; Philippé, J.; et al. Novel Flow Cytometric Antibody Panel and Dedicated Analysis Algorithm for Automated Fully Standardized Minimal Residual Disease Detection in Chronic Lymphocytic Leukemia. Am. J. Hematol. 2025, 100, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Terra, R.; Éthier, V.; Busque, L.; Morin-Quintal, A.; D’Angelo, G.; Hébert, J.; Wang, X.; Lépine, G.; LeBlanc, R.; Bergeron, J. Improved identification of clinically relevant Acute Leukemia subtypes using standardized EuroFlow panels versus non-standardized approach. Cytom. Part B Clin. Cytom. 2025, 108, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.W.C.; van der Velden, V.H.J. The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 4881. [Google Scholar] [CrossRef]
- Matarraz, S.; Leoz, P.; Yeguas-Bermejo, A.; van der Velden, V.; Bras, A.E.; Sánchez Gallego, J.I.; Lecrevisse, Q.; Ayala-Bueno, R.; Teodosio, C.; Criado, I.; et al. Baseline immunophenotypic profile of bone marrow leukemia cells in acute myeloid leukemia with nucleophosmin-1 gene mutation: A EuroFlow study. Blood Cancer J. 2023, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://euroflow.org (accessed on 28 April 2025).
- Raskovalova, T.; Scheffen, L.; Jacob, M.C.; Chevalier, S.; Tondeur, S.; Bulabois, B.; Meunier, M.; Szymanski, G.; Lefebvre, C.; Planta, C.; et al. Flow cytometry lyophilised-reagent tube for quantifying peripheral blood neutrophil myeloperoxidase expression in myelodysplastic syndromes (MPO-MDS-Develop): Protocol for a diagnostic accuracy study. BMJ Open 2022, 12, e065850. [Google Scholar] [CrossRef]
- Hedley, B.D.; Cheng, G.; Luider, J.; Kern, W.; Lozanski, G.; Chin-Yee, I.; Lowes, L.E.; Keeney, M.; Careaga, D.; Magari, R.; et al. Initial flow cytometric evaluation of the Clearllab lymphoid screen. Cytom. Part B Clin. Cytom. 2018, 94, 707–713. [Google Scholar] [CrossRef]
- Hedley, B.D.; Cheng, G.; Keeney, M.; Kern, W.; Padurean, A.; Luider, J.; Chin-Yee, I.; Lowes, L.E.; Rohrbach, J.; Ortega, R.; et al. A multicenter study evaluation of the ClearLLab 10C panels. Cytom. Part B Clin. Cytom. 2021, 100, 225–234. [Google Scholar] [CrossRef]
- Smallwood, C.; Galama, L.; Apoll, L.; Heinrich, K.; Buchanan, S.; Demers, J. Examining the economic impact of laboratory developed testing in flow cytometry immunophenotyping for hematologic malignancies: An analysis of health resource utilization. Value Health 2015, 18, A361. [Google Scholar] [CrossRef]
- George, G.V.; Kajstura, M.; Evans, A.G.; Syposs, C.R. Gamma-delta T-cell acute lymphoblastic lymphoma/leukemia: A report of a rare entity. J. Hematop. 2024, 17, 103–107. [Google Scholar] [CrossRef]
- Glencross, D.K.; Swart, L.; Pretorius, M.; Lawrie, D. Evaluation of fixed-panel, multicolour ClearLLab 10C at an academic flow cytometry laboratory in Johannesburg, South Africa. Afr. J. Lab. Med. 2022, 11, 1458. [Google Scholar] [CrossRef]
- Espasa, A.; Torrents, S.; Morales-Indiano, C.; Rico, L.G.; Bardina, J.; Ancochea, A.; Bistué-Rovira, À.; Linio, R.; Raya, M.; Vergara, S.; et al. Diagnostic performance of the ClearLLab 10C B cell tube. Cytom. Part B Clin. Cytom. 2021, 100, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Streitz, M.; Miloud, T.; Kapinsky, M.; Reed, M.R.; Magari, R.; Geissler, E.K.; Hutchinson, J.A.; Vogt, K.; Schlickeiser, S.; Kverneland, A.H.; et al. Standardization of whole blood immune phenotype monitoring for clinical trials: Panels and methods from the ONE study. Transplant. Res. 2013, 25, 17. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P., Jr.; the FOCIS Human Immunophenotyping Consortium; Amos, M.; Elliott, J.; Gaigalas, A.; Wang, L.; Aranda, R.; Banchereau, J.; Boshoff, C.; et al. A model for harmonizing flow cytometry in clinical trials. Nat. Immunol. 2010, 11, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, M.; LaBarge, T.; Evans, A.G. ClearLLab 10C reagents panel can be applied to analyze paucicellular samples by flow cytometry. Cytom. Part B Clin. Cytom. 2025, 108, 128–136. [Google Scholar] [CrossRef]
- Frater, J.L.; Shirai, C.L.; Brestoff, J.R. Technological features of blast identification in the cerebrospinal fluid: A systematic review of flow cytometry and laboratory haematology methods. Int. J. Lab. Hematol. 2022, 44 (Suppl. 1), 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, L.; Bernot, D.; Nivaggioni, V.; Arnoux, I.; Loosveld, M. Detection of Minimal Residual Disease in B Cell Acute Lymphoblastic Leukemia Using an Eight-Color Tube with Dried Antibody Reagents. Cytom. Part B Clin. Cytom. 2019, 96, 158–163. [Google Scholar] [CrossRef]
- Ho, C.; Syed, M.; Roshal, M.; Petrova-Drus, K.; Moung, C.; Yao, J.; Quesada, A.E.; Benhamida, J.; Vanderbilt, C.; Liu, Y.; et al. Routine Evaluation of Minimal Residual Disease in Myeloma Using Next-Generation Sequencing Clonality Testing: Feasibility, Challenges, and Direct Comparison with High-Sensitivity Flow Cytometry. J. Mol. Diagn. JMD 2021, 23, 181–199. [Google Scholar] [CrossRef]
- Royston, D.J.; Gao, Q.; Nguyen, N.; Maslak, P.; Dogan, A.; Roshal, M. Single-Tube 10-Fluorochrome Analysis for Efficient Flow Cytometric Evaluation of Minimal Residual Disease in Plasma Cell Myeloma. Am. J. Clin. Pathol. 2016, 146, 41–49. [Google Scholar] [CrossRef]
- Nilsson, M.; Malmgren, H.; Samiotaki, M.; Kwiatkowski, M.; Chowdhary, B.P.; Landegren, U. Padlock probes: Circularizing oligonucleotides for localized DNA detection. Science 1994, 265, 2085–2088. [Google Scholar] [CrossRef]
- Larsson, C.; Koch, J.; Nygren, A.; Janssen, G.; Raap, A.K.; Landegren, U.; Nilsson, M. In situ genotyping individual DNA molecules by target-primed rolling-circle amplification of padlock probes. Nat. Methods 2004, 1, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Eriksson, A.; Weström, S.; Pandzic, T.; Lehmann, S.; Cavelier, L.; Landegren, U. Ultra-sensitive monitoring of leukemia patients using superRCA mutation detection assays. Nat. Commun. 2022, 13, 4033. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.P.; Ostafe, R.; Iyengar, S.N.; Rajwa, B.; Fischer, R. Flow Cytometry: The Next Revolution. Cells 2023, 12, 1875. [Google Scholar] [CrossRef]
- Brestoff, J.R. Full spectrum flow cytometry in the clinical laboratory. Int. J. Lab. Hematol. 2023, 45 (Suppl. 2), 44–49. [Google Scholar] [CrossRef]
- Duffield, A.S.; Aoki, J.; Levis, M.; Cowan, K.; Gocke, C.D.; Burns, K.H.; Borowitz, M.J.; Vuica-Ross, M. Clinical and pathologic features of secondary acute promyelocytic leukemia. Am. J. Clin. Pathol. 2012, 137, 395–402. [Google Scholar] [CrossRef]
- Hayden, P.J.; O’Connell, N.M.; O’Brien, D.A.; O’Rourke, P.; Lawlor, E.; Browne, P.V. The value of autofluorescence as a diagnostic feature of acute promyelocytic leukemia. Haematologica 2006, 91, 417–418. [Google Scholar]
- Zhang, T.; Gao, M.; Chen, X.; Gao, C.; Feng, S.; Chen, D.; Wang, J.; Zhao, X.; Chen, J. Demands and technical developments of clinical flow cytometry with emphasis in quantitative, spectral, and imaging capabilities. Nanotechnol. Precis. Eng. 2022, 5, 045002. [Google Scholar] [CrossRef]
- Watson, D.A.; Brown, L.O.; Gaskill, D.F.; Naivar, M.; Graves, S.W.; Doorn, S.K.; Nolan, J.P. A flow cytometer for the measurement of Raman spectra. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 119–128. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, P.; Chen, Y. Surface Plasmon Resonance Biosensors: A Review of Molecular Imaging with High Spatial Resolution. Biosensors 2024, 14, 84. [Google Scholar] [CrossRef]
- Nolan, J.P.; Duggan, E.; Liu, E.; Condello, D.; Dave, I.; Stoner, S.A. Single cell analysis using surface enhanced Raman scattering (SERS) tags. Methods 2012, 57, 272–279. [Google Scholar] [CrossRef]
- Guo, H.; Zhi, S.; Zhao, Z.; Gao, T.; Ma, H.; Luo, S.H.; Zhang, W.; Guo, P.; Ren, B.; Tian, Z.Q.; et al. Rapid SERS Analysis: From Laboratory to Real Sample. ACS Appl. Mater. Interfaces 2025, 17, 33318–33339. [Google Scholar] [CrossRef]
- Spies, N.C.; Rangel, A.; English, P.; Morrison, M.; O’Fallon, B.; Ng, D.P. Machine Learning Methods in Clinical Flow Cytometry. Cancers 2025, 17, 483. [Google Scholar] [CrossRef]
- Seheult, J.N.; Otteson, G.E.; Weybright, M.J.; Timm, M.M.; Han, W.; Jevremovic, D.; Horna, P.; Olteanu, H.; Shi, M. Clinical Validation and Post-Implementation Performance Monitoring of a Neural Network-Assisted Approach for Detecting Chronic Lymphocytic Leukemia Minimal Residual Disease by Flow Cytometry. Cancers 2025, 17, 1688. [Google Scholar] [CrossRef]
- Pedreira, C.E.; Sobral da Costa, E.; Lecrevise, Q.; Grigore, G.; Fluxa, R.; Verde, J.; Hernandez, J.; van Dongen, J.J.M.; Orfao, A. From big flow cytometry datasets to smart diagnostic strategies: The EuroFlow approach. J. Immunol. Methods 2019, 475, 112631. [Google Scholar] [CrossRef] [PubMed]
- Mocking, T.R.; van de Loosdrecht, A.A.; Cloos, J.; Bachas, C. Applications of machine learning for immunophenotypic measurable residual disease assessment has proven its utility in acute myeloid leukemia. HemaSphere 2025, 9, e70138. [Google Scholar] [CrossRef] [PubMed]
- Stagno, F.; Mirabile, G.; Rizzotti, P.; Bottaro, A.; Pagana, A.; Gangemi, S.; Allegra, A. Using Artificial Intelligence to Enhance Myelodysplastic Syndrome Diagnosis, Prognosis, and Treatment. Biomedicines 2025, 13, 835. [Google Scholar] [CrossRef]
- Czechowska, K.; Lannigan, J.; Aghaeepour, N.; Back, J.B.; Begum, J.; Behbehani, G.; Bispo, C.; Bitoun, D.; Fernández, A.B.; Boova, S.T.; et al. Cyt-Geist: Current and Future Challenges in Cytometry: Reports of the CYTO 2019 Conference Workshops. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2019, 95, 1236–1274. [Google Scholar] [CrossRef]
- Gorrese, M.; Bertolini, A.; Fresolone, L.; Campana, A.; Pezzullo, L.; Guariglia, R.; Mettivier, L.; Manzo, P.; Cuffa, B.; D’Alto, F.; et al. Inter-intra instrument comparison and standardization of a 10-color immunophenotyping for B and T cell non-Hodgkin lymphoma diagnosis and monitoring. J. Immunol. Methods 2022, 511, 113374. [Google Scholar] [CrossRef]
- Ng, D.P.; Zuromski, L.M. Augmented Human Intelligence and Automated Diagnosis in Flow Cytometry for Hematologic Malignancies. Am. J. Clin. Pathol. 2021, 155, 597–605. [Google Scholar] [CrossRef]
- Shopsowitz, K.; Lofroth, J.; Chan, G.; Kim, J.; Rana, M.; Brinkman, R.; Weng, A.; Medvedev, N.; Wang, X. MAGIC-DR: An interpretable machine-learning guided approach for acute myeloid leukemia measurable residual disease analysis. Part B Clin. Cytom. 2024, 106, 239–251. [Google Scholar] [CrossRef]
- Al-Attar, A.; Kumar, K.R.; Al-Attar, A.; Untersee, D.; O’Driscoll, M.; Ventura, M.F.S.; Lin, L. Automation in flow cytometry: Guidelines and review of systems. Cytom. Part B Clin. Cytom. 2024, 106, 308–320. [Google Scholar] [CrossRef] [PubMed]
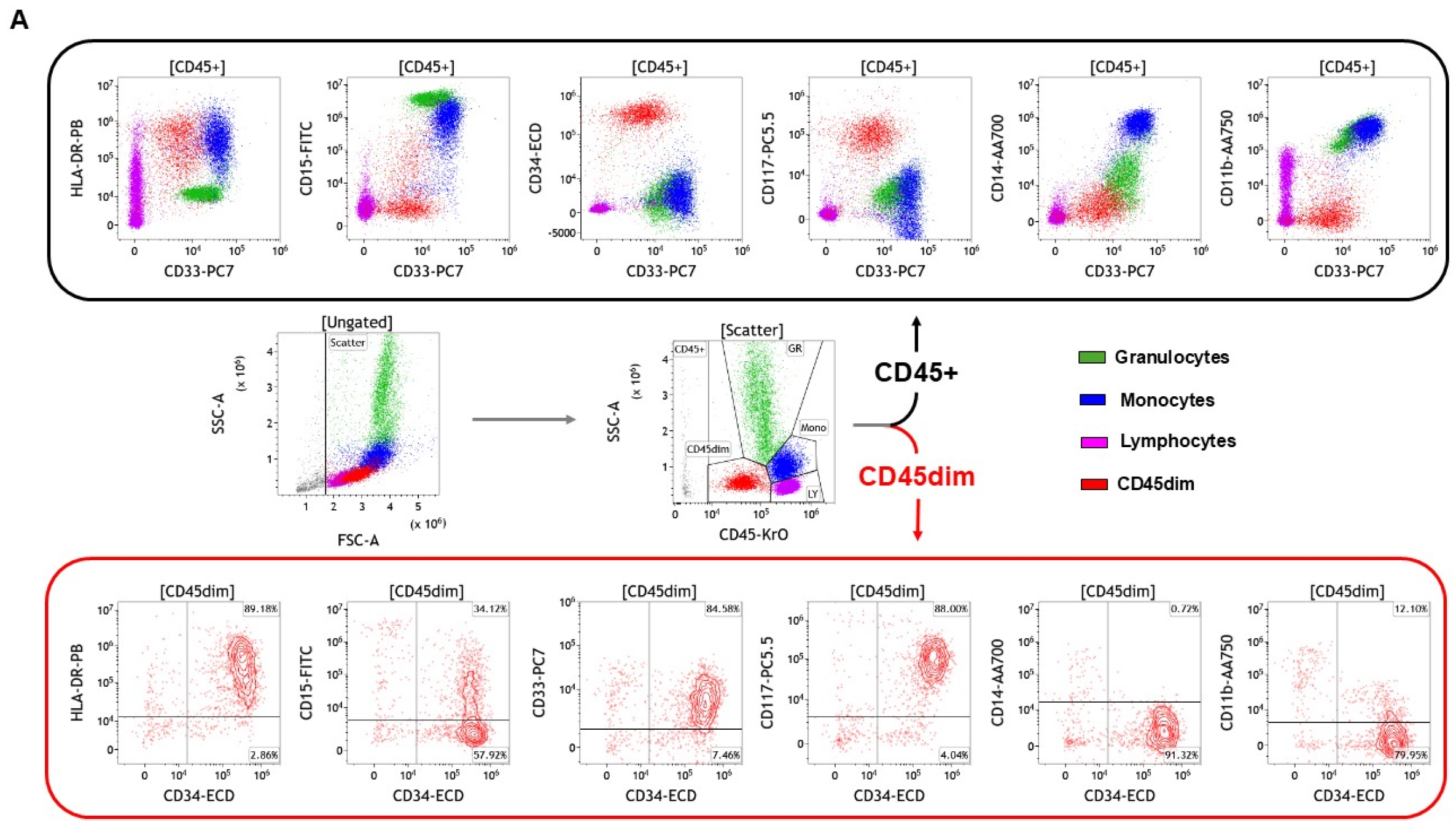
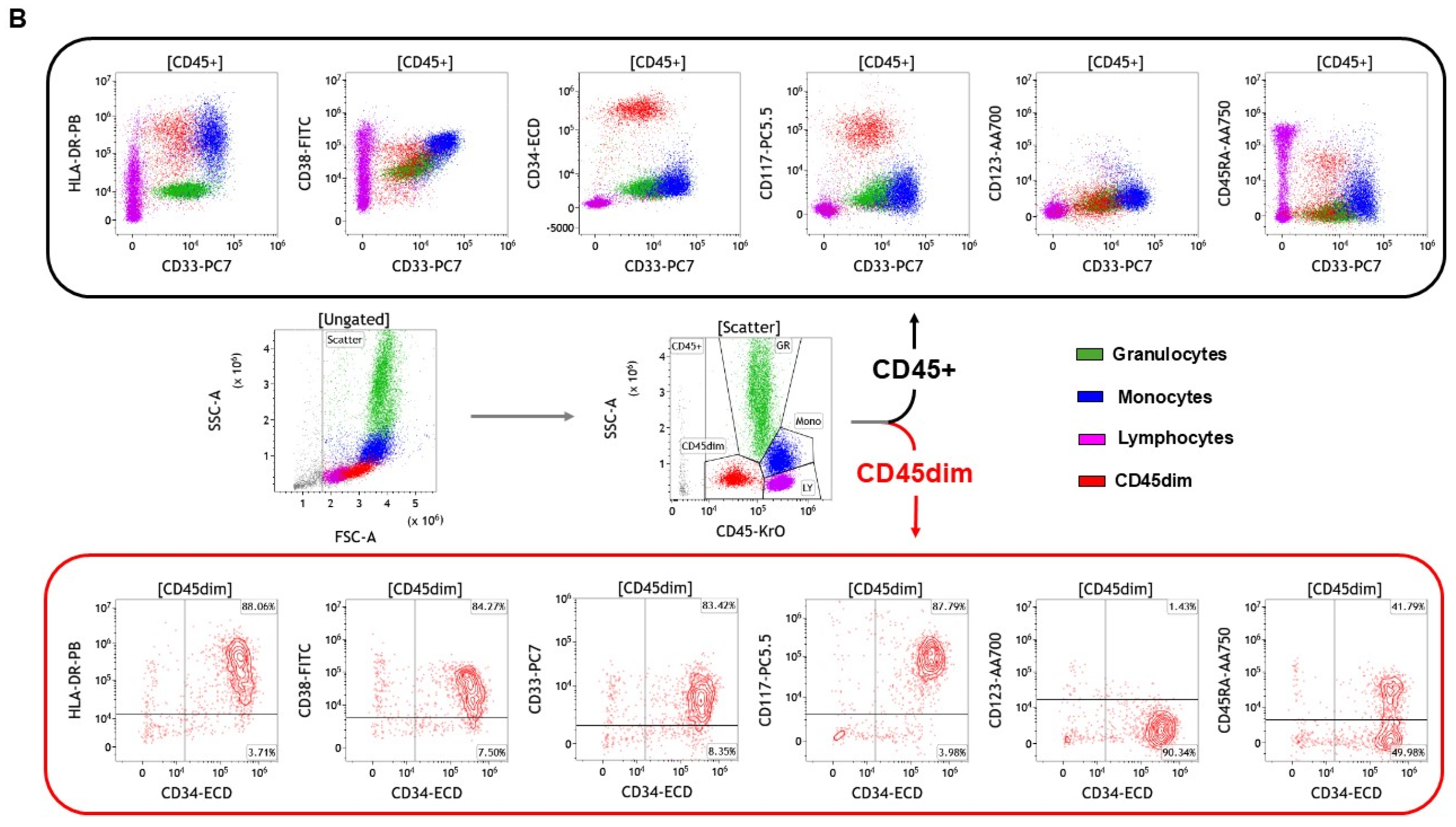
| AML | B-Cell ALL | T-Cell ALL | CLL | CML # | B-Cell Lymphoma | T-Cell Lymphoma |
|---|---|---|---|---|---|---|
| CD13 | CD10 | CD1a | CD5 | CD11b | CD19 | CD2 |
| CD14 | CD19 | CD2 | CD19 | CD13 | CD20 | CD3 |
| CD33 | CD20 | CD3 | CD20 | CD14 | CD22 | CD4 |
| CD34 | CD22 | CD5 | CD23 | CD33 | CD79a | CD5 |
| CD45 | CD34 | CD7 | CD38 | CD34 | CD5 $,& | CD7 |
| CD64 | CD45 | CD45 | CD43 | CD45 | CD10 * | CD8 |
| CD117 | TdT | TdT | CD79b | CD23 & | CD30 § | |
| HLA-DR | CD200 | CD30 § | ||||
| MPO | FMC7 | BCL2 * | ||||
| kappa/lambda | Cyclin D1 $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holl, E.; Kapinsky, M.; Larbi, A. An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization. Cancers 2025, 17, 2045. https://doi.org/10.3390/cancers17122045
Holl E, Kapinsky M, Larbi A. An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization. Cancers. 2025; 17(12):2045. https://doi.org/10.3390/cancers17122045
Chicago/Turabian StyleHoll, Eda, Michael Kapinsky, and Anis Larbi. 2025. "An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization" Cancers 17, no. 12: 2045. https://doi.org/10.3390/cancers17122045
APA StyleHoll, E., Kapinsky, M., & Larbi, A. (2025). An Update on Flow Cytometry Analysis of Hematological Malignancies: Focus on Standardization. Cancers, 17(12), 2045. https://doi.org/10.3390/cancers17122045





