Optimally Delivered R-da-EPOCH Versus R-CHOP-21 in Primary Mediastinal Large B-Cell Lymphoma: A Real-Life Comparison in a Single Academic Center
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
3.1. Patient Characteristics and Delivery of Chemotherapy
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Martelli, M.; Ferreri, A.; Rocco, A.D.; Ansuinelli, M.; Johnson, P.W.M. Primary Mediastinal Large B-Cell Lymphoma. Crit. Rev. Oncol. Hematol. 2017, 113, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Leroy, K.; Yu, X.; Gaulard, P.; Gascoyne, R.D.; Chan, W.C.; Zhao, T.; Haioun, C.; Greiner, T.C.; et al. Molecular Diagnosis of Primary Mediastinal B Cell Lymphoma Identifies a Clinically Favorable Subgroup of Diffuse Large B Cell Lymphoma Related to Hodgkin Lymphoma. J. Exp. Med. 2003, 198, 851–862. [Google Scholar] [CrossRef]
- Green, M.R.; Monti, S.; Rodig, S.J.; Juszczynski, P.; Currie, T.; O’Donnell, E.; Chapuy, B.; Takeyama, K.; Neuberg, D.; Golub, T.R.; et al. Integrative Analysis Reveals Selective 9p24.1 Amplification, Increased PD-1 Ligand Expression, and Further Induction via JAK2 in Nodular Sclerosing Hodgkin Lymphoma and Primary Mediastinal Large B-Cell Lymphoma. Blood 2010, 116, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leuk 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A Report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, S.; Papageorgiou, S.G.; Michail, M.; Angelopoulou, M.K.; Kalpadakis, C.; Leonidopoulou, T.; Katodritou, E.; Kotsopoulou, M.; Kotsianidis, I.; Hatzimichael, E.; et al. Subdiaphragmatic Extranodal Localizations at Diagnosis of Primary Mediastinal Large B- Cell Lymphoma: An Impressive, Rare Presentation with No Independent Effect on Prognosis. Leuk. Res. 2021, 107, 106595. [Google Scholar] [CrossRef] [PubMed]
- Liaskas, A.; Dimopoulou, M.N.; Piperidou, A.; Angelopoulou, M.K.; Vassilakopoulos, T.P. Current Issues and Future Perspectives of Targeted Therapies in Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Med. 2025, 14, 1191. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Pangalis, G.A.; Katsigiannis, A.; Papageorgiou, S.G.; Constantinou, N.; Terpos, E.; Zorbala, A.; Vrakidou, E.; Repoussis, P.; Poziopoulos, C.; et al. Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone with or without Radiotherapy in Primary Mediastinal Large B-Cell Lymphoma: The Emerging Standard of Care. Oncologist 2012, 17, 239–249. [Google Scholar] [CrossRef]
- Savage, K.J.; Al-Rajhi, N.; Voss, N.; Paltiel, C.; Klasa, R.; Gascoyne, R.D.; Connors, J.M. Favorable Outcome of Primary Mediastinal Large B-Cell Lymphoma in a Single Institution: The British Columbia Experience. Ann. Oncol. 2006, 17, 123–130. [Google Scholar] [CrossRef]
- Rieger, M.; Österborg, A.; Pettengell, R.; White, D.; Gill, D.; Walewski, J.; Kuhnt, E.; Loeffler, M.; Pfreundschuh, M.; Ho, A.D. Primary Mediastinal B-Cell Lymphoma Treated with CHOP-like Chemotherapy with or without Rituximab: Results of the Mabthera International Trial Group Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 664–670. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Hellmann, M.D.; Feng, Y.; Sohani, A.R.; Toomey, C.E.; Barnes, J.A.; Takvorian, R.W.; Neuberg, D.; Hochberg, E.P.; Abramson, J.S. Treatment of Primary Mediastinal B-Cell Lymphoma with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone Is Associated with a High Rate of Primary Refractory Disease. Leuk. Lymphoma 2014, 55, 538–543. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Stefoni, V.; Finolezzi, E.; Brusamolino, E.; Cabras, M.G.; Chiappella, A.; Salvi, F.; Rossi, A.; Broccoli, A.; Martelli, M. Rituximab Combined with MACOP-B or VACOP-B and Radiation Therapy in Primary Mediastinal Large B-Cell Lymphoma: A Retrospective Study. Clin. Lymphoma Myeloma 2009, 9, 381–385. [Google Scholar] [CrossRef]
- Avigdor, A.; Sirotkin, T.; Kedmi, M.; Ribakovsy, E.; Berkowicz, M.; Davidovitz, Y.; Kneller, A.; Merkel, D.; Volchek, Y.; Davidson, T.; et al. The Impact of R-VACOP-B and Interim FDG-PET/CT on Outcome in Primary Mediastinal Large B Cell Lymphoma. Ann. Hematol. 2014, 93, 1297–1304. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Papageorgiou, S.G.; Angelopoulou, M.K.; Chatziioannou, S.; Prassopoulos, V.; Karakatsanis, S.; Arapaki, M.; Mellios, Z.; Sachanas, S.; Kalpadakis, C.; et al. Positron Emission Tomography after Response to Rituximab-CHOP in Primary Mediastinal Large B-Cell Lymphoma: Impact on Outcomes and Radiotherapy Strategies. Ann. Hematol. 2021, 100, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.P.; Pangalis, G.A.; Chatziioannou, S.; Papageorgiou, S.; Angelopoulou, M.K.; Galani, Z.; Kourti, G.; Prassopoulos, V.; Leonidopoulou, T.; Terpos, E.; et al. PET/CT in Primary Mediastinal Large B-Cell Lymphoma Responding to Rituximab-CHOP: An Analysis of 106 Patients Regarding Prognostic Significance and Implications for Subsequent Radiotherapy. Leukemia 2016, 30, 238–242. [Google Scholar] [CrossRef]
- Xu, L.M.; Fang, H.; Wang, W.H.; Jin, J.; Wang, S.L.; Liu, Y.P.; Song, Y.W.; Ren, H.; Zhou, L.Q.; Li, Y.X. Prognostic Significance of Rituximab and Radiotherapy for Patients with Primary Mediastinal Large B-Cell Lymphoma Receiving Doxorubicin-Containing Chemotherapy. Leuk. Lymphoma 2013, 54, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Binkley, M.S.; Hiniker, S.M.; Wu, S.; Natkunam, Y.; Mittra, E.S.; Advani, R.H.; Hoppe, R.T. A Single-Institution Retrospective Analysis of Outcomes for Stage I-II Primary Mediastinal Large B-Cell Lymphoma Treated with Immunochemotherapy with or without Radiotherapy. Leuk. Lymphoma 2016, 57, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.; Ceriani, L.; Zucca, E.; Zinzani, P.L.; Ferreri, A.J.M.; Vitolo, U.; Stelitano, C.; Brusamolino, E.; Cabras, M.G.; Rigacci, L.; et al. [18F]Fluorodeoxyglucose Positron Emission Tomography Predicts Survival after Chemoimmunotherapy for Primary Mediastinal Large B-Cell Lymphoma: Results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J. Clin. Oncol. 2014, 32, 1769–1775. [Google Scholar] [CrossRef]
- Gleeson, M.; Hawkes, E.A.; Cunningham, D.; Chadwick, N.; Counsell, N.; Lawrie, A.; Jack, A.; Smith, P.; Mouncey, P.; Pocock, C.; et al. Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone (R-CHOP) in the Management of Primary Mediastinal B-Cell Lymphoma: A Subgroup Analysis of the UK NCRI R-CHOP 14 Vers. Br. J. Haematol. 2016, 175, 668–672. [Google Scholar] [CrossRef]
- Camus, V.; Rossi, C.; Sesques, P.; Lequesne, J.; Tonnelet, D.; Haioun, C.; Durot, E.; Willaume, A.; Gauthier, M.; Moles-Moreau, M.P.; et al. Outcomes after First-Line Immunochemotherapy for Primary Mediastinal B-Cell Lymphoma: A LYSA Study. Blood Adv. 2021, 5, 3862–3872. [Google Scholar] [CrossRef]
- Wästerlid, T.; Hasselblom, S.; Joelsson, J.; Weibull, C.E.; Rassidakis, G.; Sander, B.; Smedby, K.E. Real-World Data on Treatment and Outcomes of Patients with Primary Mediastinal Large B-Cell Lymphoma: A Swedish Lymphoma Register Study. Blood Cancer J. 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, S.J.; Bouzani, M.; Symeonidis, A.; Angelopoulou, M.K.; Papageorgiou, S.G.; Michail, M.; Gainaru, G.; Kourti, G.; Sachanas, S.; Kalpadakis, C.; et al. Real-Life Experience With Rituximab-CHOP Every 21 or 14 Days in Primary Mediastinal Large B-Cell Lymphoma. In Vivo 2022, 36, 1302–1315. [Google Scholar] [CrossRef]
- Avigdor, A.; Sirotkin, T.; Shemtov, N.; Berkowicz, M.; Davidovitz, Y.; Kneller, A.; Hardan, I.; Shimoni, A.; Apter, S.; Ben-Bassat, I.; et al. Combination of Rituximab with Initial Chemotherapy Improves Outcome of Primary Mediastinal B-Cell Lymphoma: A Retrospective Analysis of a Single Institution Cohort. Blood 2007, 110, 1283. [Google Scholar] [CrossRef]
- Martelli, M.; Ceriani, L.; Ciccone, G.; Ricardi, U.; Kriachok, I.; Botto, B.; Balzarotti, M.; Tucci, A.; Usai, S.V.; Zilioli, V.R.; et al. Omission of Radiotherapy in Primary Mediastinal B-Cell Lymphoma: IELSG37 Trial Results. J. Clin. Oncol. 2024, 42, 4071–4083. [Google Scholar] [CrossRef]
- Thurner, L.; Ziepert, M.; Berdel, C.; Schmidt, C.; Borchmann, P.; Kaddu-Mulindwa, D.; Viardot, A.; Witzens-Harig, M.; Dierlamm, J.; Haenel, M.; et al. Radiation and Dose-Densification of R-CHOP in Aggressive B-Cell Lymphoma With Intermediate Prognosis: The UNFOLDER Study. HemaSphere 2023, 7, E904. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.H.; Schöder, H.; Teruya-Feldstein, J.; Sima, C.; Iasonos, A.; Portlock, C.S.; Straus, D.; Noy, A.; Palomba, M.L.; O’Connor, O.A.; et al. Risk-Adapted Dose-Dense Immunochemotherapy Determined by Interim FDG-PET in Advanced-Stage Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2010, 28, 1896–1903. [Google Scholar] [CrossRef]
- Hamlin, P.A.; Portlock, C.S.; Straus, D.J.; Noy, A.; Singer, A.; Horwitz, S.M.; Oconnor, O.A.; Yahalom, J.; Zelenetz, A.D.; Moskowitz, C.H. Primary Mediastinal Large B-Cell Lymphoma: Optimal Therapy and Prognostic Factor Analysis in 141 Consecutive Patients Treated at Memorial Sloan Kettering from 1980 to 1999. Br. J. Haematol. 2005, 130, 691–699. [Google Scholar] [CrossRef]
- Martelli, M.; Zucca, E.; Botto, B.; Kryachok, I.; Ceriani, L.; Balzarotti, M.; Tucci, A.; Cabras, M.G.; Zilioli, V.R.; Rusconi, C.; et al. Impact of Different Induction Regimens on the Outcome of Primary Mediastinal B Cell Lymphoma In The Prospective Ielsg 37 Trial. Hematol. Oncol. 2021, 39, 52. [Google Scholar] [CrossRef]
- De Sanctis, V.; Alfò, M.; Di Rocco, A.; Ansuinelli, M.; Russo, E.; Osti, M.F.; Valeriani, M.; Minniti, G.; Grapulin, L.; Musio, D.; et al. Second Cancer Incidence in Primary Mediastinal B-Cell Lymphoma Treated with Methotrexate with Leucovorin Rescue, Doxorubicin, Cyclophosphamide, Vincristine, Prednisone, and Bleomycin Regimen with or without Rituximab and Mediastinal Radiotherapy: Results from a Monoinstitutional Cohort Analysis of Long-Term Survivors. Hematol. Oncol. 2017, 35, 554–560. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.P.; van Eggermond, A.M.; Janus, C.P.M.; Krol, A.D.G.; van der Maazen, R.W.M.; Roesink, J.; Raemaekers, J.M.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Shbib Dabaja, B.; Boyce-Fappiano, D.; Dong, W.; Damron, E.; Fang, P.; Gunther, J.; Rodriguez, M.A.; Strati, P.; Steiner, R.; Nair, R.; et al. Second Malignancies in Patients with Hodgkin’s Lymphoma: Half a Century of Experience. Clin. Transl. Radiat. Oncol. 2022, 35, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Eich, H.T.; Diehl, V.; Görgen, H.; Pabst, T.; Markova, J.; Debus, J.; Ho, A.; Dörken, B.; Rank, A.; Grosu, A.L.; et al. Intensified Chemotherapy and Dose-Reduced Involved-Field Radiotherapy in Patients with Early Unfavorable Hodgkin’s Lymphoma: Final Analysis of the German Hodgkin Study Group HD11 Trial. J. Clin. Oncol. 2010, 28, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation Induced Secondary Malignancies: A Review Article. Radiat. Oncol. J. 2018, 36, 85. [Google Scholar] [CrossRef] [PubMed]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef]
- De Vries, S.; Haaksma, M.L.; Jóźwiak, K.; Schaapveld, M.; Hodgson, D.C.; Lugtenburg, P.J.; Krol, A.D.G.; Petersen, E.J.; Van Spronsen, D.J.; Ahmed, S.; et al. Development and Validation of Risk Prediction Models for Coronary Heart Disease and Heart Failure After Treatment for Hodgkin Lymphoma. J. Clin. Oncol. 2023, 41, 86–95. [Google Scholar] [CrossRef]
- Roberti, S.; Van Leeuwen, F.E.; Ronckers, C.M.; Krul, I.M.; De Vathaire, F.; Veres, C.; Diallo, I.; Janus, C.P.M.; Aleman, B.M.P.; Russell, N.S.; et al. Radiotherapy-Related Dose and Irradiated Volume Effects on Breast Cancer Risk Among Hodgkin Lymphoma Survivors. J. Natl. Cancer Inst. 2022, 114, 1270–1278. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Ntentas, G.; Darby, S.C.; Schaapveld, M.; Hauptmann, M.; Lugtenburg, P.J.; Janus, C.P.M.; Daniels, L.; Van Leeuwen, F.E.; Cutter, D.J.; et al. Risk of Heart Failure in Survivors of Hodgkin Lymphoma: Effects of Cardiac Exposure to Radiation and Anthracyclines. Blood 2017, 129, 2257–2265. [Google Scholar] [CrossRef]
- Dunleavy, K.; Pittaluga, S.; Maeda, L.S.; Advani, R.; Chen, C.C.; Hessler, J.; Steinberg, S.M.; Grant, C.; Wright, G.; Varma, G.; et al. Dose-Adjusted EPOCH-Rituximab Therapy in Primary Mediastinal B-Cell Lymphoma. N. Engl. J. Med. 2013, 368, 1408–1416. [Google Scholar] [CrossRef]
- Melani, C.; Advani, R.; Roschewski, M.; Walters, K.M.; Chen, C.C.; Baratto, L.; Ahlman, M.A.; Miljkovic, M.D.; Steinberg, S.M.; Lam, J.; et al. End-of-Treatment and Serial PET Imaging in Primary Mediastinal B-Cell Lymphoma Following Dose-Adjusted EPOCH-R: A Paradigm Shift in Clinical Decision Making. Haematologica 2018, 103, 1337–1344. [Google Scholar] [CrossRef]
- Shah, N.N.; Szabo, A.; Huntington, S.F.; Epperla, N.; Reddy, N.; Ganguly, S.; Vose, J.; Obiozor, C.; Faruqi, F.; Kovach, A.E.; et al. R-CHOP versus Dose-Adjusted R-EPOCH in Frontline Management of Primary Mediastinal B-Cell Lymphoma: A Multi-Centre Analysis. Br. J. Haematol. 2018, 180, 534–544. [Google Scholar] [CrossRef]
- Morgenstern, Y.; Aumann, S.; Goldschmidt, N.; Gatt, M.E.; Nachmias, B.; Horowitz, N.A. Dose-Adjusted EPOCH-R Is Not Superior to Sequential R-CHOP/R-ICE as a Frontline Treatment for Newly Diagnosed Primary Mediastinal B-Cell Lymphoma: Results of a Bi-Center Retrospective Study. Cancer Med. 2021, 10, 8866–8875. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Ferhanoglu, B.; Horowitz, N.; Mellios, Z.; Kaynar, L.; Zektser, M.; Symeonidis, A.; Piperidou, A.; Giotas, A.; Agathocleous, A.; et al. Real-Life Experience with Rituximab-Dose-Adjusted EPOCH (R-Da-EPOCH) in Primary Mediastinal Large B-Cell Lymphoma (PMLBCL): A Multinational Analysis of 274 Patients. Hematol. Oncol. 2023, 41, 410–411. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Dabaja, B.; Ahmed, M.A.; Chuang, H.H.; Costelloe, C.; Wogan, C.F.; Reed, V.; Romaguera, J.E.; Neelapu, S.; Oki, Y.; et al. Single-Institution Experience in the Treatment of Primary Mediastinal B Cell Lymphoma Treated with Immunochemotherapy in the Setting of Response Assessment by 18fluorodeoxyglucose Positron Emission Tomography. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 113–121. [Google Scholar] [CrossRef]
- Giulino-Roth, L.; O’Donohue, T.; Chen, Z.; Bartlett, N.L.; LaCasce, A.; Martin-Doyle, W.; Barth, M.J.; Davies, K.; Blum, K.A.; Christian, B.; et al. Outcomes of Adults and Children with Primary Mediastinal B-Cell Lymphoma Treated with Dose-Adjusted EPOCH-R. Br. J. Haematol. 2017, 179, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.; Mellios, Z.; Verigou, E.; Papageorgiou, S.; Chatzidimitriou, C.; Kalpadaki, C.; Katodritou, E.; Giatra, H.; Xanthopoulos, V.; Gainaru, G.; et al. Comparison of Immunochemotherapy with Rituximab-Dose-Adjusted EPOCH (R-DA-EPOCH) or Rituximab-CHOP (R-CHOP) in Primary Mediastinal Large-B-Cell Lymphoma (PMLBCL). In Proceedings of the 26th EHA Congress, Madrid, Spain, 13–16 June 2021; p. EP551. [Google Scholar]
- Chan, E.H.L.; Koh, L.P.; Lee, J.; De Mel, S.; Jeyasekharan, A.; Liu, X.; Tang, T.; Lim, S.T.; Tao, M.; Quek, R.; et al. Real World Experience of R-CHOP with or without Consolidative Radiotherapy vs. DA-EPOCH-R in the First-line Treatment of Primary Mediastinal B-cell Lymphoma. Cancer Med. 2019, 8, 4626. [Google Scholar] [CrossRef] [PubMed]
- Malenda, A.; Kołkowska-Leśniak, A.; Puła, B.; Długosz-Danecka, M.; Chełstowska, M.; Końska, A.; Giza, A.; Lech-Marańda, E.; Jurczak, W.; Warzocha, K. Outcomes of Treatment with Dose-Adjusted EPOCH-R or R-CHOP in Primary Mediastinal Large B-Cell Lymphoma. Eur. J. Haematol. 2020, 104, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Stepanishyna, Y.; Skrypets, T.; Pastushenko, Y.; Tytorenko, I.; Kriachok, I. Analysis of Prognostic Factors in Patients with Primary Mediastinal Large B-Cell Lymphoma Treated by Da-EPOCH-R and R-CHOP. In Proceedings of the 26th EHA Congress, Madrid, Spain, 13–16 June 2021; p. EP557. [Google Scholar]
- Vassilakopoulos, T.P.; Piperidou, A.; Mellios, Z.; Verigou, E.; Katodritou, E.; Kalpadakis, C.; Papageorgiou, S.G.; Chatzidimitriou, C.; Prassopoulos, V.; Siakantaris, M.P.; et al. PET for Response Assessment to R-Da-EPOCH in Primary Mediastinal Large B-Cell Lymphoma: Who Is Worthy to Be Irradiated? HemaSphere 2023, 7, e965. [Google Scholar] [CrossRef]
- Shipp, M.A.; Harrington, D.P.; Anderson, J.R.; Armitage, J.O.; Bonadonna, G.; Brittinger, G.; Cabanillas, F.; Canellos, G.P.; Coiffier, B.; Connors, J.M.; et al. A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Michail, M.; Papageorgiou, S.; Kourti, G.; Angelopoulou, M.K.; Panitsas, F.; Sachanas, S.; Kalpadakis, C.; Katodritou, E.; Leonidopoulou, T.; et al. Identification of Very Low-Risk Subgroups of Patients with Primary Mediastinal Large B-Cell Lymphoma Treated with R-CHOP. Oncologist 2021, 26, 597–609. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of Survival Data and Two New Rank Order Statistics Arising in Its Consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar] [PubMed]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Rehman, M.E.U.; Ali, R.; Basit, J.; Akbar, U.A.; Saeed, S.; Fatima, M.; Farrukh, L.; Masood, A.; Iftikhar, A.; Husnain, M.; et al. R-CHOP Versus R-EPOCH in Primary Mediastinal Large B-Cell Lymphoma: A Systematic Review and Meta-Analysis. Blood 2022, 140 (Suppl. S1), 9506–9507. [Google Scholar] [CrossRef]
- Lisenko, K.; Dingeldein, G.; Cremer, M.; Kriegsmann, M.; Ho, A.D.; Rieger, M.; Witzens-Harig, M. Addition of Rituximab to CHOP-like Chemotherapy in First Line Treatment of Primary Mediastinal B-Cell Lymphoma. BMC Cancer 2017, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Kim, S.J.; Yun, J.; Yi, J.H.; Kim, J.H.; Won, Y.W.; Kim, K.; Ko, Y.H.; Kim, W.S. Improved Treatment Outcome of Primary Mediastinal Large B-Cell Lymphoma after Introduction of Rituximab in Korean Patients. Int. J. Hematol. 2010, 91, 456–463. [Google Scholar] [CrossRef]
- Tai, W.M.; Chung, J.; Tang, P.L.; Koo, Y.X.; Hou, X.; Tay, K.W.; Quek, R.; Tao, M.; Lim, S.T. Central Nervous System (CNS) Relapse in Diffuse Large B Cell Lymphoma (DLBCL): Pre- and Post-Rituximab. Ann. Hematol. 2011, 90, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Yenson, P.R.; Shenkier, T.; Klasa, R.; Villa, D.; Goktepe, O.; Steidl, C.; Slack, G.W.; Gascoyne, R.D.; Connors, J.M.; et al. The Outcome of Primary Mediastinal Large B-Cell Lymphoma (PMBCL) in the R-CHOP Treatment Era. Blood 2012, 120, 303. [Google Scholar] [CrossRef]
- Aoki, T.; Izutsu, K.; Suzuki, R.; Nakaseko, C.; Arima, H.; Shimada, K.; Tomita, A.; Sasaki, M.; Takizawa, J.; Mitani, K.; et al. Prognostic Significance of Pleural or Pericardial Effusion and the Implication of Optimal Treatment in Primary Mediastinal Large B-Cell Lymphoma: A Multicenter Retrospective Study in Japan. Haematologica 2014, 99, 1817–1825. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Piperidou, A.; Hadjiharissi, E.; Panteliadou, A.K.; Panitsas, F.; Vassilopoulos, I.; Variamis, E.; Boutsis, D.; Michail, M.; Papageorgiou, S.; et al. Development of Classic Hodgkin Lymphoma after Successful Treatment of Primary Mediastinal Large B-Cell Lymphoma: Results from a Well-Defined Database. Leuk. Res. 2021, 100, 106479. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N.; et al. Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study. J. Clin. Oncol. 2019, 37, 3081–3089. [Google Scholar] [CrossRef]
- Merryman, R.W.; Armand, P.; Wright, K.T.; Rodig, S.J. Checkpoint Blockade in Hodgkin and Non-Hodgkin Lymphoma. Blood Adv. 2017, 1, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Shaulov, A.; Gross Even-Zohar, N.; Aumann, S.; Haran, A.; Linetsky, E. Nivolumab for CNS Relapsed Refractory Primary Mediastinal B-Cell Lymphoma: Case Report and Review of the Literature. Leuk. Lymphoma 2024, 65, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Crombie, J.L.; Nastoupil, L.J.; Redd, R.; Tang, K.; Shouse, G.; Herrera, A.F.; Chow, V.A.; Shadman, M.; Puglianini, O.C.; Saucier, A.; et al. Real-World Outcomes of Axicabtagene Ciloleucel in Adult Patients with Primary Mediastinal B-Cell Lymphoma. Blood Adv. 2021, 5, 3563–3567. [Google Scholar] [CrossRef]
- Galtier, J.; Mesguich, C.; Sesques, P.; Dupont, V.; Bachy, E.; Di Blasi, R.; Thieblemont, C.; Gastinne, T.; Cartron, G.; Brisou, G.; et al. Outcomes of Patients with Relapsed or Refractory Primary Mediastinal B-Cell Lymphoma Treated with Anti-CD19 CAR-T Cells: CARTHYM, a Study from the French National DESCAR-T Registry. HemaSphere 2025, 9, e70091. [Google Scholar] [CrossRef]
- Fakhri, B.; Ai, W. Current and Emerging Treatment Options in Primary Mediastinal B-Cell Lymphoma. Ther. Adv. Hematol. 2021, 12, 20406207211048959. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N.; et al. Nivolumab Combined with Brentuximab Vedotin for R/R Primary Mediastinal Large B-Cell Lymphoma: A 3-Year Follow-Up. Blood Adv. 2023, 7, 5272–5280. [Google Scholar] [CrossRef]
- Renaud, L.; Wencel, J.; Pagès, A.; Jijakli, A.A.; Moatti, H.; Quero, L.; Camus, V.; Brice, P. Nivolumab Combined with Brentuximab Vedotin with or without Mediastinal Radiotherapy for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma. Haematologica 2024, 109, 3019–3023. [Google Scholar] [CrossRef]
- Armand, P.; Rodig, S.J.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, D.; et al. Pembrolizumab in Patients with Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma (PMBCL): Data from the Keynote-013 and Keynote-170 Studies. Blood 2018, 132 (Suppl. S1), 228. [Google Scholar] [CrossRef]
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 3291–3299. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Thieblemont, C.; Melnichenko, V.; Bouabdallah, K.; Walewski, J.; Majlis, A.; Fogliatto, L.; Garcia-Sancho, A.M.; Christian, B.; Gulbas, Z.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma: Final Analysis of KEYNOTE-170. Blood 2023, 142, 141–145. [Google Scholar] [CrossRef] [PubMed]
- De-la-Fuente, C.; Nuñez, F.; Cortés-Romera, M.; Franch-Sarto, M.; Ribera, J.M.; Sancho, J.M. Pembrolizumab for Refractory Primary Mediastinal B-Cell Lymphoma with Central Nervous System Involvement. Hematol. Oncol. 2021, 39, 419–422. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristics | R-da-EPOCH % n = 35 | R-CHOP % n = 35 | p-Value | ||
|---|---|---|---|---|---|
| # | % | # | % | ||
| Age (Median, Range) | 35 (16–51) | 28 (16–59) | 0.01 | ||
| Age ≥ 38 years old | 15/35 | 43 | 3/35 | 9 | 0.001 |
| Females | 26/35 | 74 | 22/35 | 63 | 0.30 |
| Β-symptoms | 7/35 | 20 | 7/33 | 21 | 0.90 |
| PS ≥ 2 | 7/35 | 20 | 8/34 | 24 | 0.72 |
| Stage ΙΙΙ/IV | 10/35 | 29 | 6/33 | 18 | 0.31 |
| Any extranodal site | 13/35 | 37 | 17/35 | 49 | 0.33 |
| Extranodal sites ≥ 2 | 4/35 | 11 | 9/35 | 26 | 0.12 |
| Extrathoracic disease | 8/35 | 23 | 4/35 | 11 | 0.21 |
| Kidney/Adrenal involvement | 3/35 | 9 | 2/35 | 6 | 0.64 |
| Serous effusions | 13/35 | 37 | 21/33 | 64 | 0.03 |
| Bulky disease ≥ 10 cm | 25/35 | 71 | 21/32 | 66 | 0.61 |
| Serum LDH elevated | 29/35 | 83 | 30/34 | 88 | 0.53 |
| Serum LDH ≥ 2x | 15/35 | 43 | 11/34 | 32 | 0.37 |
| IPI ≥ 2 | 13/35 | 37 | 16/35 | 46 | 0.47 |
| aaIPI ≥ 2 | 13/35 | 37 | 12/35 | 34 | 0.80 |
| Model A: E/IV and LDH ≥ 2xUNL * | 0.89 | ||||
| No adverse factors | 15/35 | 43 | 15/34 | 44 | |
| 1 adverse factor | 12/35 | 34 | 10/34 | 29 | |
| 2 adverse factors | 8/35 | 23 | 9/34 | 27 | |
| Model B: E/IV and bulk | 0.91 | ||||
| No adverse factors | 8/35 | 23 | 6/32 | 19 | |
| 1 adverse factor | 16/35 | 46 | 15/32 | 47 | |
| 2 adverse factors | 11/35 | 31 | 11/32 | 34 | |
| Maximum R-da-EPOCH Level | All Patients (n = 35) | Strictly Delivered EPOCH Subgroup (n = 33) | ||
|---|---|---|---|---|
| # | % | # | % | |
| 1 | 2 | 5.7 | 2 | 6.1 |
| 2 | 2 | 5.7 | 2 | 6.1 |
| 3 | 14 | 40.0 | 14 | 42.4 |
| 4 | 9 | 25.7 | 8 | 24.2 |
| 5 | 6 | 17.1 | 5 | 15.2 |
| 6 | 2 | 5.7 | 2 | 6.1 |
| Maximum R-da-EPOCH level | All patients (n = 35) | NCI Study [22] (n = 51) | ||
| # | % | # | % | |
| 1 | 2 | 5.7 | 6 | |
| ≥3 | 31 | 88.5 | ||
| ≥4 | 17 | 48.5 | “>50%” | |
| Freedom from Progression | Event-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Multivariate Model including Treatment Regimen, Age and Presence of any Serositis | |||||||||
| R-da-EPOCH vs. R-CHOP-21 | 0.24 | 0.07–0.88 | 0.031 | 0.41 | 0.14–1.20 | 0.103 | 0.16 | 0.02–1.32 | 0.089 |
| Age ≥ 38 years old | NS | NS | NS | ||||||
| Any serositis | NS | NS | NS | ||||||
| Multivariate Model including Treatment Regimen and Prognostic Model A (E/IV and/or LDH ≥ 2x) | |||||||||
| R-da-EPOCH vs. R-CHOP-21 | 0.21 | 0.06–0.76 | 0.017 | 0.37 | 0.12–1.08 | 0.068 | 0.17 | 0.02–1.36 | 0.095 |
| E/IV and/or LDH ≥ 2xUNL * | 0.072 | 0.069 | NS | ||||||
| 1 factor vs. 0 factors | 4.96 | 1.26–19.57 | 0.022 | 4.75 | 1.20–18.73 | 0.026 | |||
| 2 factors vs. 0 factors | 2.66 | 0.60–11.89 | 0.20 | 4.01 | 1.002–16.03 | 0.050 | |||
| Multivariate Model including Treatment Regimen and Prognostic Model A (E/IV and/or bulk) | |||||||||
| R-da-EPOCH vs. R-CHOP-21 | 0.26 | 0.07–0.97 | 0.044 | 0.45 | 0.15–1.33 | 0.147 (NS) | 0.18 | 0.02–1.54 | 0.12 (NS) |
| E/IV and/or bulk | NS | NS | NS | ||||||
| 1 factor vs. 0 factors | |||||||||
| 2 factors vs. 0 factors | |||||||||
| Patients (#) | Progression Free Survival (PFS) | Overall Survival (OS) | |||
|---|---|---|---|---|---|
| Author [REF] | Type of Study | R-da-EPOCH Group | Comparator Group | R-da-EPOCH vs. Comparator | R-da-EPOCH vs. Comparator |
| Shah et al. [41] | Propensity-weighted retrospective analysis | 76 | 56 * | HR: 0.62; 95% CI: 0.24–1.47 p = 0.28 | HR: 0.63; 95% CI 0.19–2.15 p = 0.46 |
| Chan et al. [47] | Retrospective study | 46 | 78 ** | 88.5% vs. (90% vs. 56%) ** at 5 years p = 0.002 | NR |
| Malenda et al. [48] | Retrospective study | 28 | 25 * | 87% vs. 74% at 1 year p = 0.21 | 100% vs. 92% at 1 year p = 0.81 |
| Morgenstern et al. [42] | Retrospective study | 31 | 25 *** | 26% vs. 16% relapse at 2 years p = 0.37 | NR |
| Stepanishyna et al. [49] | Prospective rando-mized clinical trial | 44 | 40 * | 90.9% vs. 64.1% at 5 years p = 0.002 | 97.7% vs. 73.8% at 5 years p = 0.002 |
| Rehman et al. [56] | Systematic review | 256 | 213 ¶ | RR: 0.76; 95% CI: 0.57–1.02 p = 0.06 | RR:0.84; 95% CI 0.72–0.97 p = 0.02 ¶¶ |
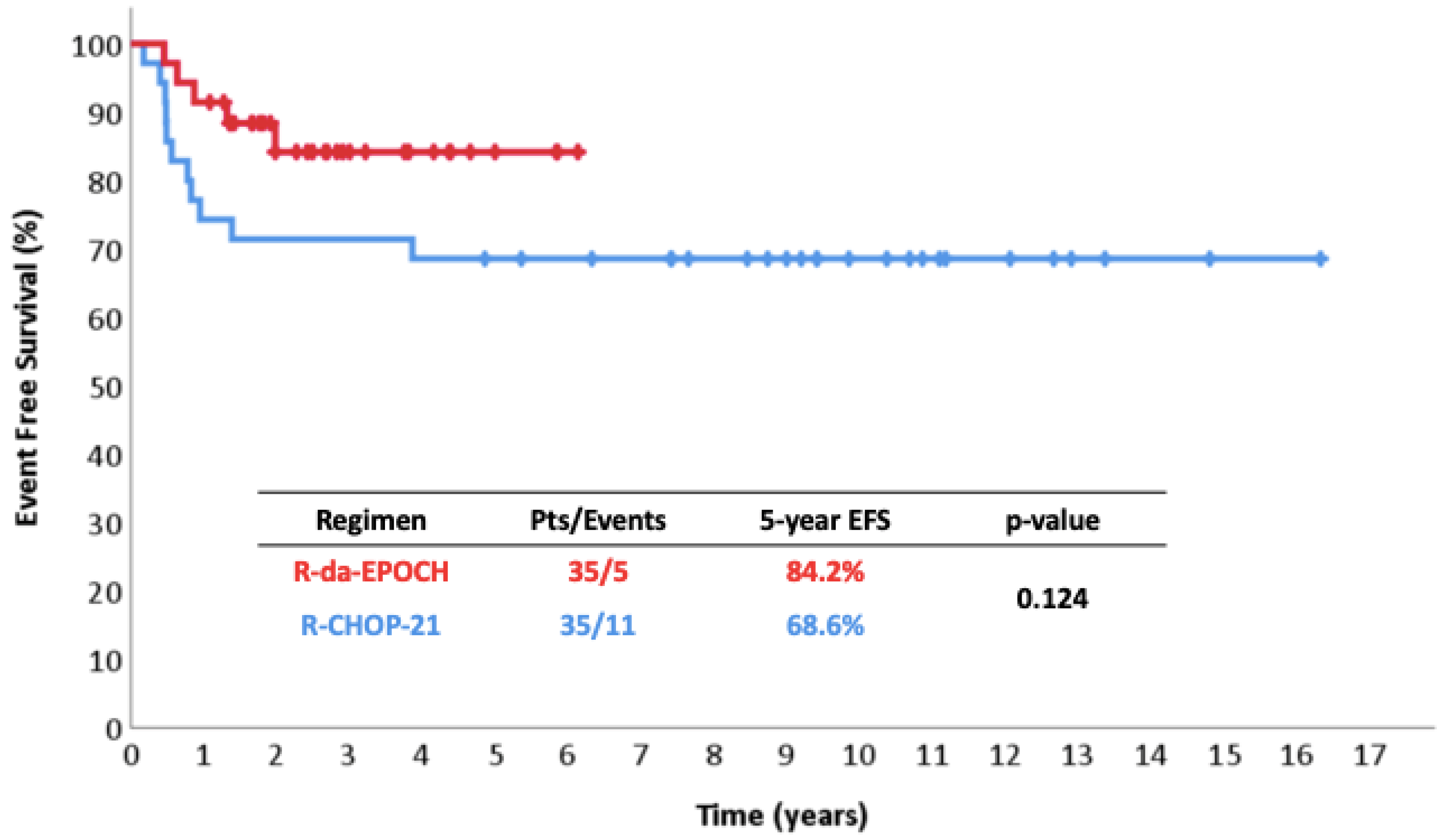
| Piperidou et al. present study | Retrospective study | 35 | 35 * | 91% vs. 69% at 5 years p = 0.027 | 97% vs. 80% at 5 years p = 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperidou, A.; Angelopoulou, M.K.; Chatzidimitriou, C.; Asimakopoulos, J.V.; Arapaki, M.; Panitsas, F.; Tsourouflis, G.; Belia, M.; Konstantinou, I.; Kopsaftopoulou, A.; et al. Optimally Delivered R-da-EPOCH Versus R-CHOP-21 in Primary Mediastinal Large B-Cell Lymphoma: A Real-Life Comparison in a Single Academic Center. Cancers 2025, 17, 1699. https://doi.org/10.3390/cancers17101699
Piperidou A, Angelopoulou MK, Chatzidimitriou C, Asimakopoulos JV, Arapaki M, Panitsas F, Tsourouflis G, Belia M, Konstantinou I, Kopsaftopoulou A, et al. Optimally Delivered R-da-EPOCH Versus R-CHOP-21 in Primary Mediastinal Large B-Cell Lymphoma: A Real-Life Comparison in a Single Academic Center. Cancers. 2025; 17(10):1699. https://doi.org/10.3390/cancers17101699
Chicago/Turabian StylePiperidou, Alexia, Maria K. Angelopoulou, Chrysovalantou Chatzidimitriou, John V. Asimakopoulos, Maria Arapaki, Fotios Panitsas, Gerassimos Tsourouflis, Marina Belia, Iliana Konstantinou, Anastasia Kopsaftopoulou, and et al. 2025. "Optimally Delivered R-da-EPOCH Versus R-CHOP-21 in Primary Mediastinal Large B-Cell Lymphoma: A Real-Life Comparison in a Single Academic Center" Cancers 17, no. 10: 1699. https://doi.org/10.3390/cancers17101699
APA StylePiperidou, A., Angelopoulou, M. K., Chatzidimitriou, C., Asimakopoulos, J. V., Arapaki, M., Panitsas, F., Tsourouflis, G., Belia, M., Konstantinou, I., Kopsaftopoulou, A., Liaskas, A., Machairas, A., Lefaki, M.-A., Dimitrakoudi, M., Sachanas, S., Pangalis, G. A., Konstantopoulos, K., Plata, E., Siakantaris, M., & Vassilakopoulos, T. P. (2025). Optimally Delivered R-da-EPOCH Versus R-CHOP-21 in Primary Mediastinal Large B-Cell Lymphoma: A Real-Life Comparison in a Single Academic Center. Cancers, 17(10), 1699. https://doi.org/10.3390/cancers17101699







