Safety and Efficacy Outcomes of Robotic, Laparoscopic, and Laparotomic Surgery in Severe Obese Endometrial Cancer Patients: A Network Meta-Analysis
Simple Summary
Abstract
1. Introduction
Objective
2. Material and Methods
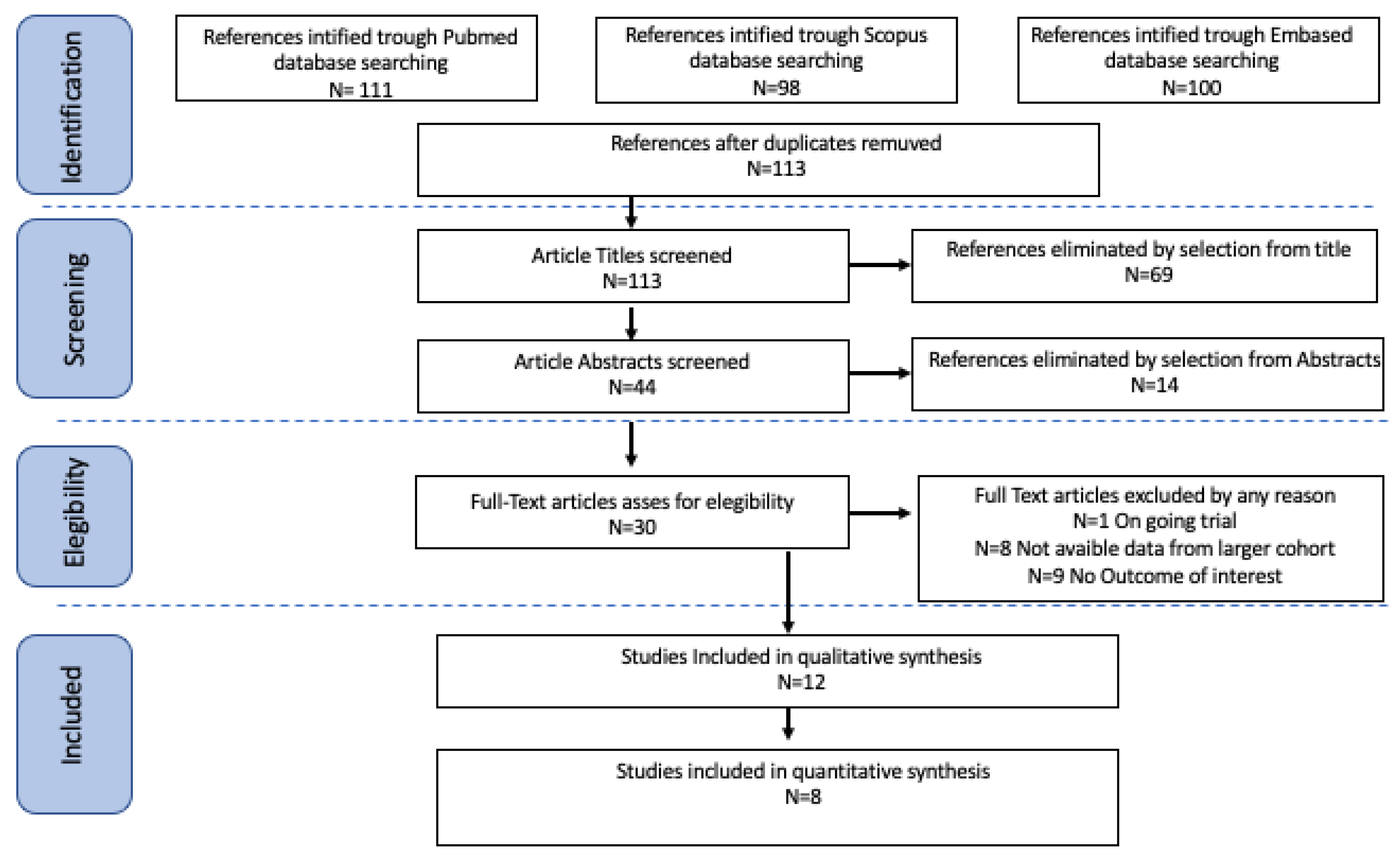
2.1. Search Method
2.2. Study Selection
2.3. Risk of Bias
2.4. Primary and Secondary Endpoints
2.5. Statistical Analysis
2.6. Quality Assessment
2.7. Declaration of Generative AI in Scientific Writing
3. Results
3.1. Studies’ Characteristics
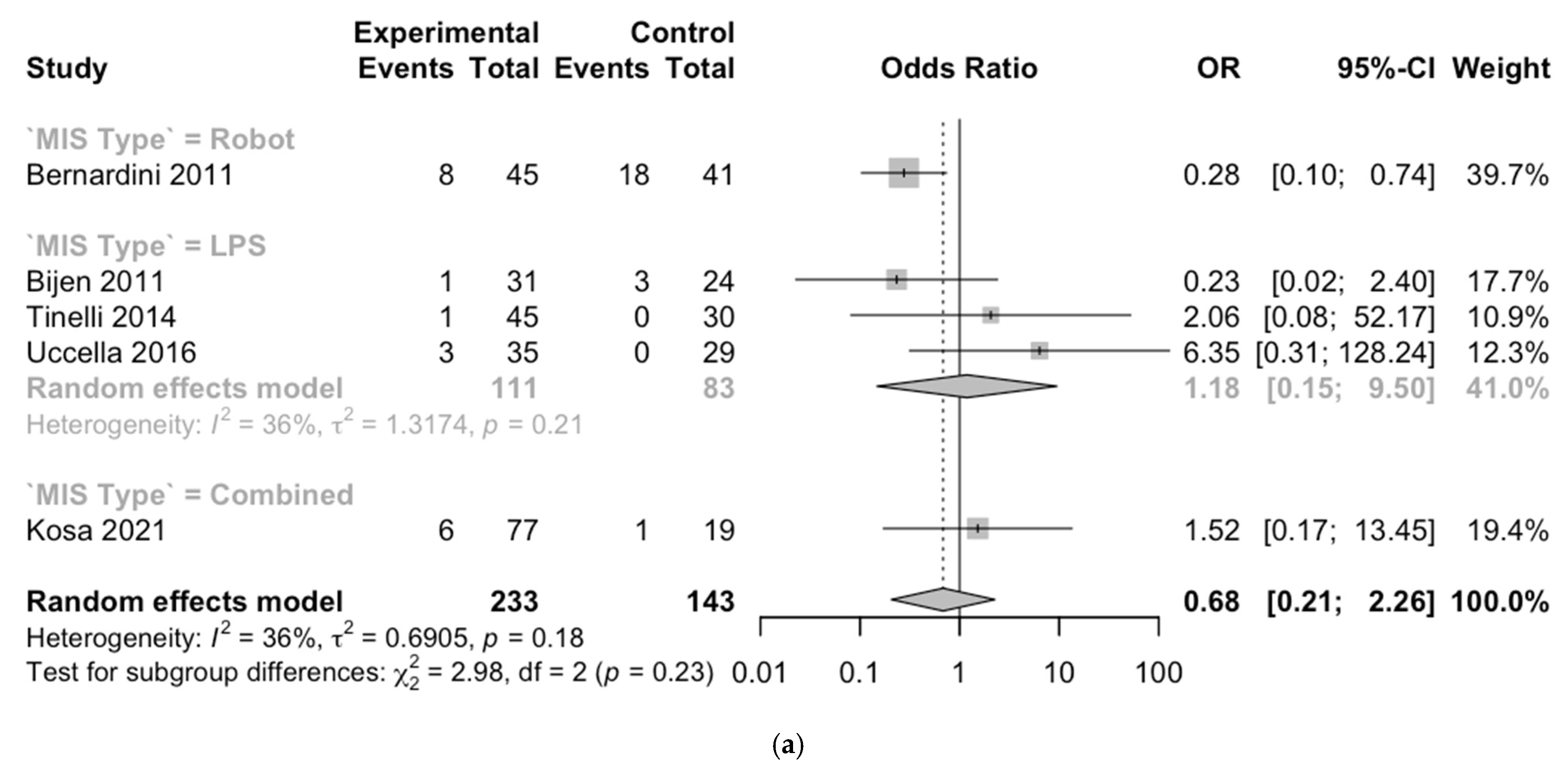
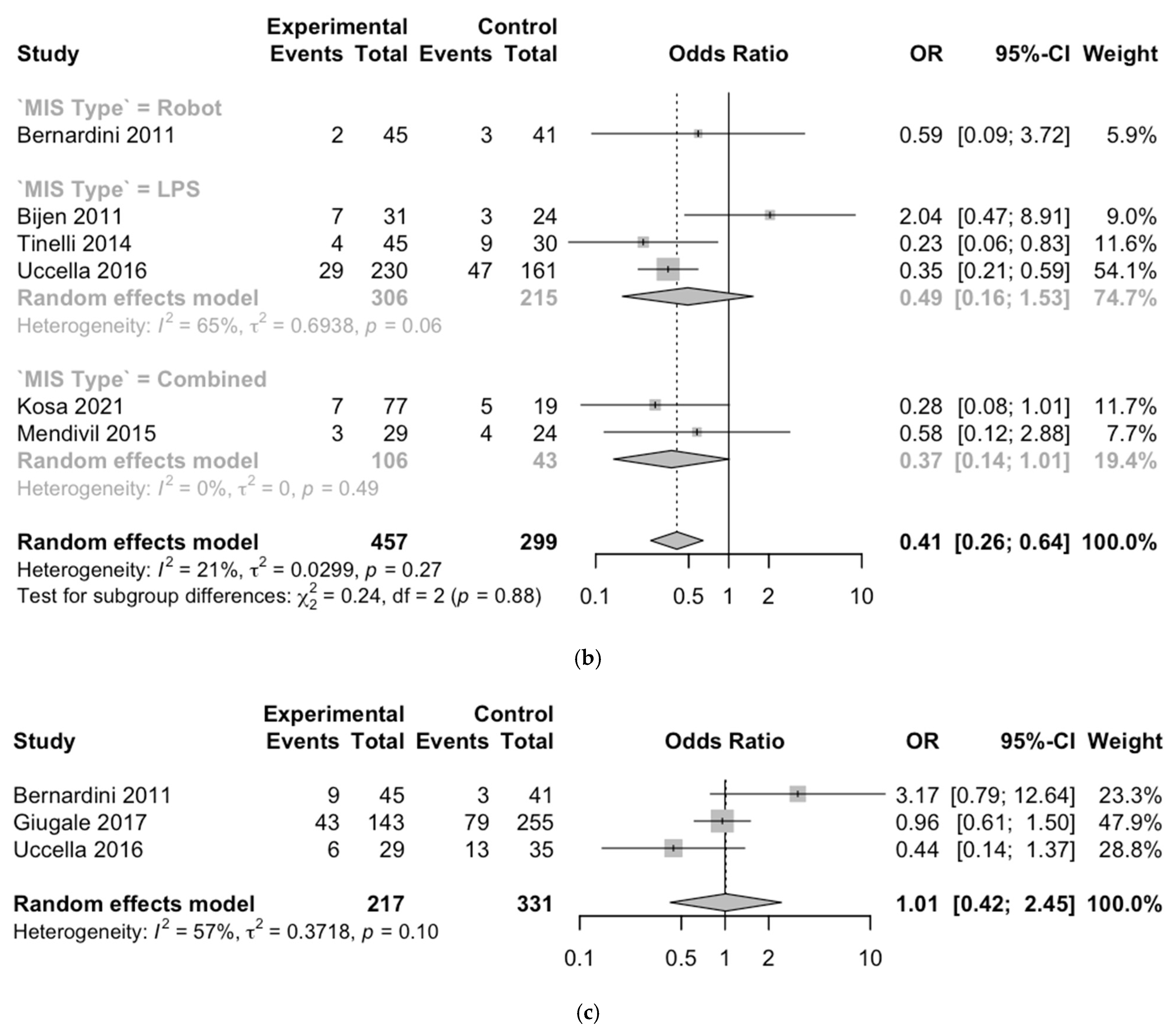
3.2. Outcomes
3.3. Meta-Analysis
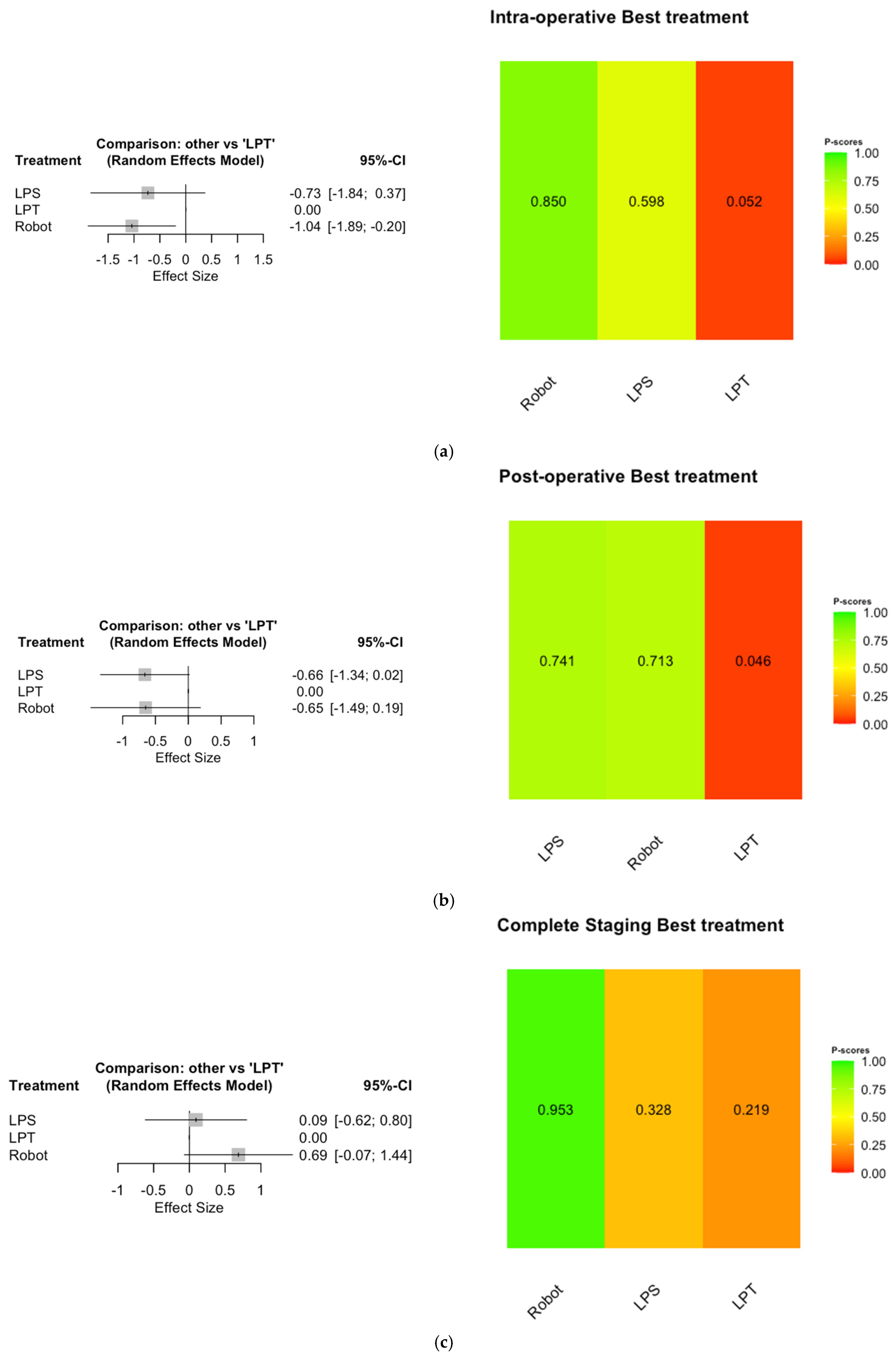
3.4. Network Meta-Analysis
4. Discussion
4.1. Interpretation of Results
4.2. Comparison with Existing Literature
4.3. Clinical Implication
4.4. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Kok, K.S.; Lee, K.H. Obesity and endometrial cancer: An overview. Malays. J. Med. Sci. 2018, 25, 4–10. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- European Society of Gynaecological Oncology (ESGO). ESGO/ESTRO/ESP guidelines for the management of endometrial cancer. Eur. J. Cancer 2020, 138, 197–216. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 21 February 2023).
- Mencaglia, S.; Pippa, L. ESMO Guidelines for the management of endometrial cancer. Ann. Oncol. 2022, 33, 165–176. [Google Scholar] [CrossRef]
- Wright, J.D.; Barrena Medel, N.I.; Sehouli, J.; Fujiwara, K.; Herzog, T.J. Contemporary management of endometrial cancer. Lancet 2012, 379, 1352–1360. [Google Scholar] [CrossRef]
- Schreiner, M.A.; Fennerty, M.B. Endoscopy in the obese patient. Gastroenterol. Clin. N. Am. 2010, 39, 87–97. [Google Scholar] [CrossRef]
- Hägglund, M.; Lönnqvist, P.-A. Anesthesia for obese patients: A review. Acta Anaesthesiol. Scand. 2014, 58, 690–701. [Google Scholar] [CrossRef]
- Pannu, S.; Meena, H. Risk factors for surgical site infections in obese patients: A review. J. Clin. Med. 2020, 9, 478. [Google Scholar] [CrossRef]
- Snyder, E.M.; Driscoll, J.J. The impact of obesity on ventilation: Implications for anesthesia and surgery. Anesth. Analg. 2015, 121, 700–709. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Moher, D. The PRISMA extension statement for reporting systematic reviews incorporating network meta-analyses of healthcare interventions: Explanation and elaboration. BMJ 2015, 350, g5298. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R.; et al. [Table], Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4/ (accessed on 16 August 2021).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Armfield, N.R.; Janda, M.; Obermair, A. Obesity in total laparoscopic hysterectomy for early stage endometrial cancer: Health gain and inpatient resource use. Int. J. Qual. Health Care J. Int. Soc. Qual. Health Care 2019, 31, 283–288. [Google Scholar] [CrossRef]
- Corrado, G.; Chiantera, V.; Fanfani, F.; Cutillo, G.; Lucidi, A.; Mancini, E.; Pedone Anchora, L.; Scambia, G.; Vizza, E. Robotic Hysterectomy in Severely Obese Patients With Endometrial Cancer: A Multicenter Study. J. Minim. Invasive Gynecol. 2016, 23, 94–100. [Google Scholar] [CrossRef]
- Stephan, J.M.; Goodheart, M.J.; McDonald, M.; Hansen, J.; Reyes, H.D.; Button, A.; Bender, D. Robotic surgery in supermorbidly obese patients with endometrial cancer. Am. J. Obstet. Gynecol. 2015, 213, 49.e1–49.e8. [Google Scholar] [CrossRef]
- Corrado, G.; Mereu, L.; Bogliolo, S.; Cela, V.; Gardella, B.; Sperduti, I.; Certelli, C.; Pellegrini, A.; Posar, G.; Zampa, A.; et al. Comparison between single-site and multiport robot-assisted hysterectomy in obese patients with endometrial cancer: An Italian multi-institutional study. Int. J. Med. Robot. + Comput. Assist. Surg. MRCAS 2020, 16, e2066. [Google Scholar] [CrossRef]
- Bernardini, M.Q.; Gien, L.T.; Tipping, H.; Murphy, J.; Rosen, B.P. Surgical outcome of robotic surgery in morbidly obese patient with endometrial cancer compared to laparotomy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2012, 22, 76–81. [Google Scholar] [CrossRef]
- Bijen, C.B.; de Bock, G.H.; Vermeulen, K.M.; Arts, H.J.; ter Brugge, H.G.; van der Sijde, R.; Kraayenbrink, A.A.; Bongers, M.Y.; van der Zee, A.G.; Mourits, M.J. Laparoscopic hysterectomy is preferred over laparotomy in early endometrial cancer patients, however not cost effective in the very obese. Eur. J. Cancer 2011, 47, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Giugale, L.E.; Di Santo, N.; Smolkin, M.E.; Havrilesky, L.J.; Modesitt, S.C. Beyond mere obesity: Effect of increasing obesity classifications on hysterectomy outcomes for uterine cancer/hyperplasia. Gynecol. Oncol. 2012, 127, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Kosa, S.D.; Ferguson, S.E.; Panzarella, T.; Lau, S.; Abitbol, J.; Samouëlian, V.; Giede, C.; Steed, H.; Renkosinski, B.; Gien, L.T.; et al. A prospective comparison of costs between robotics, laparoscopy, and laparotomy in endometrial cancer among women with Class III obesity or higher. J. Surg. Oncol. 2022, 125, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, A.A.; Rettenmaier, M.A.; Abaid, L.N.; Brown, J.V.; 3rd Micha, J.P.; Lopez, K.L.; Goldstein, B.H. A comparison of open surgery, robotic-assisted surgery and conventional laparoscopic surgery in the treatment of morbidly obese endometrial cancer patients. JSLS J. Soc. Laparoendosc. Surg. 2015, 19, e2014.00001. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Vizza, E.; Cela, V.; Mereu, L.; Bogliolo, S.; Legge, F.; Ciccarone, F.; Mancini, E.; Gallotta, V.; Baiocco, E.; et al. Laparoscopic versus robotic hysterectomy in obese and extremely obese patients with endometrial cancer: A multi-institutional analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1935–1941. [Google Scholar] [CrossRef]
- Tinelli, R.; Litta, P.; Meir, Y.; Surico, D.; Leo, L.; Fusco, A.; Angioni, S.; Cicinelli, E. Advantages of laparoscopy versus laparotomy in extremely obese women (BMI > 35) with early-stage endometrial cancer: A multicenter study. Anticancer Res. 2014, 34, 2497–2502. [Google Scholar]
- Uccella, S.; Bonzini, M.; Palomba, S.; Fanfani, F.; Ceccaroni, M.; Seracchioli, R.; Vizza, E.; Ferrero, A.; Roviglione, G.; Casadio, P.; et al. Impact of Obesity on Surgical Treatment for Endometrial Cancer: A Multicenter Study Comparing Laparoscopy vs Open Surgery, with Propensity-Matched Analysis. J. Minim. Invasive Gynecol. 2016, 23, 53–61. [Google Scholar] [CrossRef]
- Derzie, A.J.; Silvestri, F.; Liriano, E.; Benotti, P. Wound closure technique and acute wound complications in gastric surgery for morbid obesity: A prospective randomized trial. J. Am. Coll. Surg. 2000, 191, 238–243. [Google Scholar] [CrossRef]
- Dindo, D.; Muller, M.K.; Weber, M.; Clavien, P.A. Obesity in general elective surgery. Lancet 2003, 361, 2032–2035. [Google Scholar] [CrossRef]
- van der Veen, A.; Brenkman, H.J.F.; Seesing, M.F.J.; Haverkamp, L.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.H.M.B.; Tegels, J.J.W.; Wijnhoven, B.P.L.; Lagarde, S.M.; et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. JCO 2021, 39, 978–989. [Google Scholar] [CrossRef]
- Lacy, A.M.; García-Valdecasas, J.C.; Delgado, S.; Castells, A.; Taurá, P.; Piqué, J.M.; Visa, J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet 2002, 359, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Keating, N.L.; Clemmer, J.T.; Bregar, A.J.; Wright, J.D.; Boruta, D.M.; del Carmen, M.G. Laparoscopic staging for apparent stage I epithelial ovarian cancer. Obstet. Gynecol. 2017, 130, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.A.; Zarmer, L.; West, K.; Chung, F. Obstructive Sleep Apnea and Risk of Postoperative Complications after Non-Cardiac Surgery. J. Clin. Med. 2024, 13, 2538. [Google Scholar] [CrossRef]
- DeMaria, E.J.; Carmody, B.J.; Wolfe, L.G.; Meador, J.G. Obesity increases the risk of wound infection and failure after laparoscopic gastric bypass. Surg. Obes. Relat. Dis. 2000, 2, 76–82. [Google Scholar] [CrossRef]
- Bolenz, C.; Gupta, A.; Hotze, T.; Ho, R.; Cadeddu, J.A.; Roehrborn, C.G.; Lotan, Y. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur. Urol. 2010, 57, 453–458. [Google Scholar] [CrossRef]
- Williams, S.B.; Prado, K.; Hu, J.C. Economics of robotic surgery: Does it make sense and for whom? Urol. Clin. N. Am. 2014, 41, 591–596. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Lai, S.; Li, Y.; Lin, Z.; Hao, L.; Dong, J.; Li, X.; Huang, K. Global trends and prospects in health economics of robotic surgery: A bibliometric analysis. Int. J. Surg. 2023, 109, 3896–3904. [Google Scholar] [CrossRef]
| Non-Comparative Studies | |||||||
| Name | Country | Study Design | Study Year | Approaches | FIGO * Stage | BMI ° Range | N of Participant |
| Armfield 2018 [19] | International | Randomized Clinical Trial | 2005–10 | LPS | I–III | 40–63 | 167 ^ |
| Corrado 2015 [20] | Italy | Prospective Case–control Multicentric | 2010–14 | Robot | I–IV | 40–61 | 70 |
| Stephan 2015 [21] | USA | Retrospective Observational Monocentric | 2005–12 | Robotic | I–II | 50–57 | 56 ^ |
| Vizza 2019 [22] | Italy | Retrospective Case–control Multicentric | 2010–18 | Robotic | I–II | 40–55 | 41 ^ |
| Comparative Studies | |||||||
| Bernardini 2011 [23] | Canada | Prospective Case–control Multicentric | 2008–10 | LPT vs. Robot | I–IV | 40–75 | 86 |
| Bijen 2011 [24] | Netherlands | Randomized Clinical Trial | 2009–11 | LPS vs. LPT | I–IV | 40–55 | 55 ^ |
| Giugale 2012 [25] | USA | Retrospective Cohort Monocentric | 2001–07 | LPS vs. LPT vs. Robotic | I–IV | 40–61 | 398 ^ |
| Kosa 2021 [26] | Canada | Prospective Observational Multicentric | 2012–14 | LPS vs. LPT vs. Robotic | NR | 40–55 | 103 |
| Mendivil 2015 [27] | USA | Retrospective Cohort Monocentric | 2008–11 | LPS vs. LPT vs. Robotic | I–III | 40–67 | 53 |
| Scambia 2018 [28] | Italy | Retrospective Case–control Multicentric | 2001–17 | LPS vs. Robot | I–IV | 40–66 | 134 ^ |
| Tinelli 2014 [29] | Italy | Retrospective Observational Multicentric | 2004–13 | LPS vs. LPT | I–III | 35–64 | 75 |
| Uccella 2016 [30] | Italy | Retrospective Observational Monocentric | 2000–13 | LPS vs. LPT | I–III | 40–62 | 64 ^ |
| Name | Treatment | Intra-Operative Complication (%) | Post-Operative Complication (%) | Severe Complication * (%) | Complete Staging ^ (%) |
|---|---|---|---|---|---|
| Armfield 2018 [19] | LPS | 0.6 | 29.9 | 7.8 | - |
| Bernardini 2011 [23] | LPT | 7.3 | 44.0 | 14.6 | 7.0 |
| Robot | 4.4 | 17.7 | 4.4 | 20.0 | |
| Bijen 2011 [24] | LPS | 3.2 | 22.6 | 25.8 | - |
| LPT | 12.5 | 12.5 | 25.0 | - | |
| Corrado 2015 [20] | Robot | 1.4 | 8.7 | 7.1 | 38.6 |
| Giugale 2012 [25] | LPS | - | - | - | 31.0 |
| LPT | - | - | - | 27.7 | |
| Robot | - | - | - | 34.7 | |
| Kosa 2021 [26] | LPS | 0 | 8.3 | - | - |
| LPT | 0 | 26.3 | - | - | |
| Robot | 7.7 | 9.2 | - | - | |
| Mendivil 2015 [27] | LPS | - | 6.2 | 6.2 | - |
| LPT | - | 16.7 | 8.3 | - | |
| Robot | - | 15.4 | 0 | - | |
| Scambia 2018 [28] | LPS | 3.9 | 2.5 | 2.5 | 14.5 |
| Robot | 3.4 | 8.6 | 8.6 | 31.0 | |
| Stephan 2015 [21] | Robot | - | 19.6 | - | 75.0 |
| Tinelli 2014 [29] | LPS | 2.2 | 8.9 | 0 | - |
| LPT | 0 | 30.0 | 10.0 | - | |
| Uccella 2016 [30] | LPS | 0 | 14.3 | 8.6 | 37.1 |
| LPT | 0 | 20.7 | 6.9 | 20.7 | |
| Vizza 2019 [22] | Robot | 2.4 | 9.8 | 9.8 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; Fordellone, M.; Braca, E.; Di Donna, M.C.; Solazzo, M.C.; Cucinella, G.; Scaffa, C.; De Franciscis, P.; Chiantera, V. Safety and Efficacy Outcomes of Robotic, Laparoscopic, and Laparotomic Surgery in Severe Obese Endometrial Cancer Patients: A Network Meta-Analysis. Cancers 2025, 17, 2018. https://doi.org/10.3390/cancers17122018
Ronsini C, Fordellone M, Braca E, Di Donna MC, Solazzo MC, Cucinella G, Scaffa C, De Franciscis P, Chiantera V. Safety and Efficacy Outcomes of Robotic, Laparoscopic, and Laparotomic Surgery in Severe Obese Endometrial Cancer Patients: A Network Meta-Analysis. Cancers. 2025; 17(12):2018. https://doi.org/10.3390/cancers17122018
Chicago/Turabian StyleRonsini, Carlo, Mario Fordellone, Eleonora Braca, Mariano Catello Di Donna, Maria Cristina Solazzo, Giuseppe Cucinella, Cono Scaffa, Pasquale De Franciscis, and Vito Chiantera. 2025. "Safety and Efficacy Outcomes of Robotic, Laparoscopic, and Laparotomic Surgery in Severe Obese Endometrial Cancer Patients: A Network Meta-Analysis" Cancers 17, no. 12: 2018. https://doi.org/10.3390/cancers17122018
APA StyleRonsini, C., Fordellone, M., Braca, E., Di Donna, M. C., Solazzo, M. C., Cucinella, G., Scaffa, C., De Franciscis, P., & Chiantera, V. (2025). Safety and Efficacy Outcomes of Robotic, Laparoscopic, and Laparotomic Surgery in Severe Obese Endometrial Cancer Patients: A Network Meta-Analysis. Cancers, 17(12), 2018. https://doi.org/10.3390/cancers17122018







