Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study †
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Data Source and Patient Selection
2.2. Setting, Patients and Data Sources
2.3. Statistical Analysis
3. Results
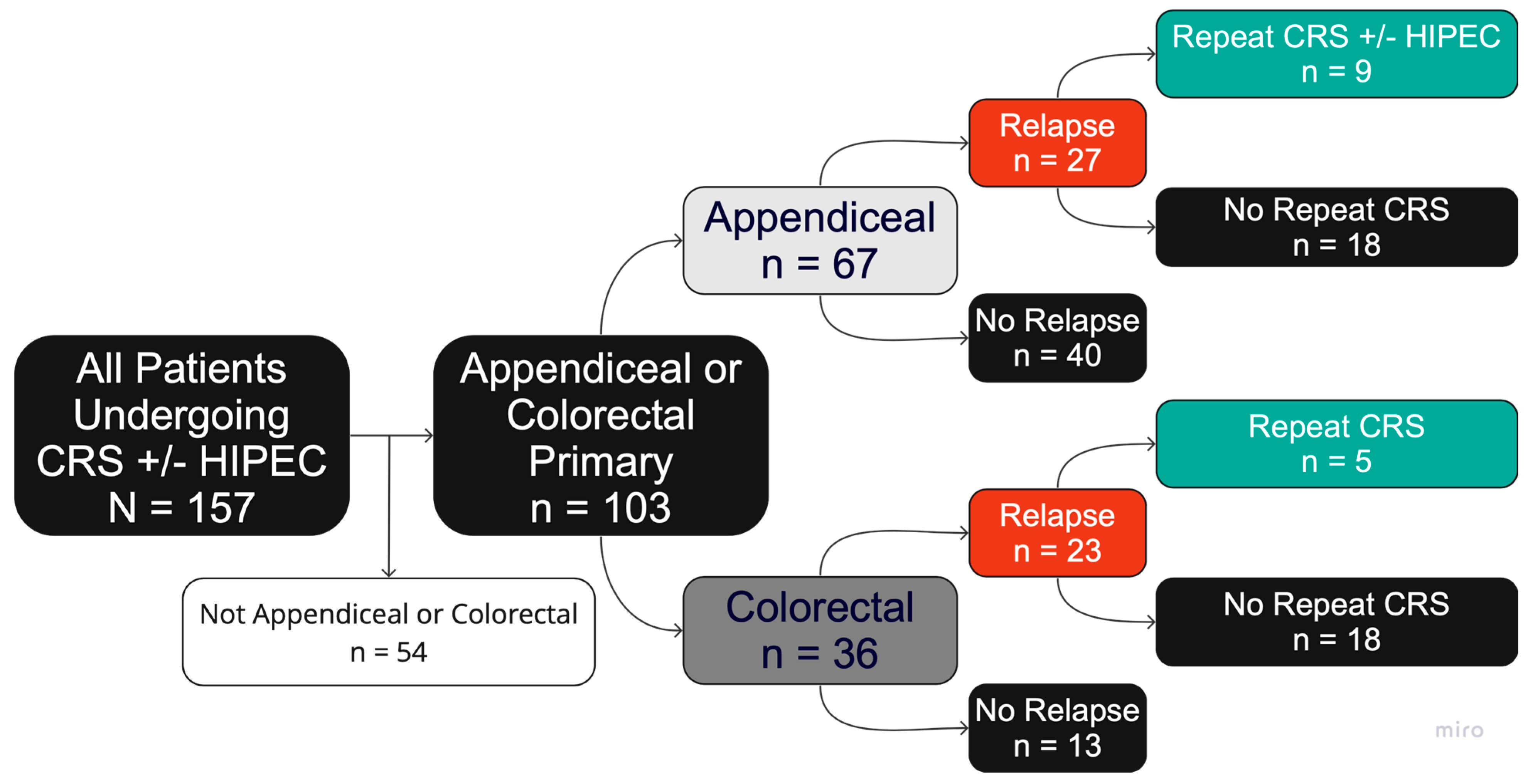
3.1. Patient and Disease Characteristics
3.2. Disease Relapse
3.3. Repeat CRS/HIPEC
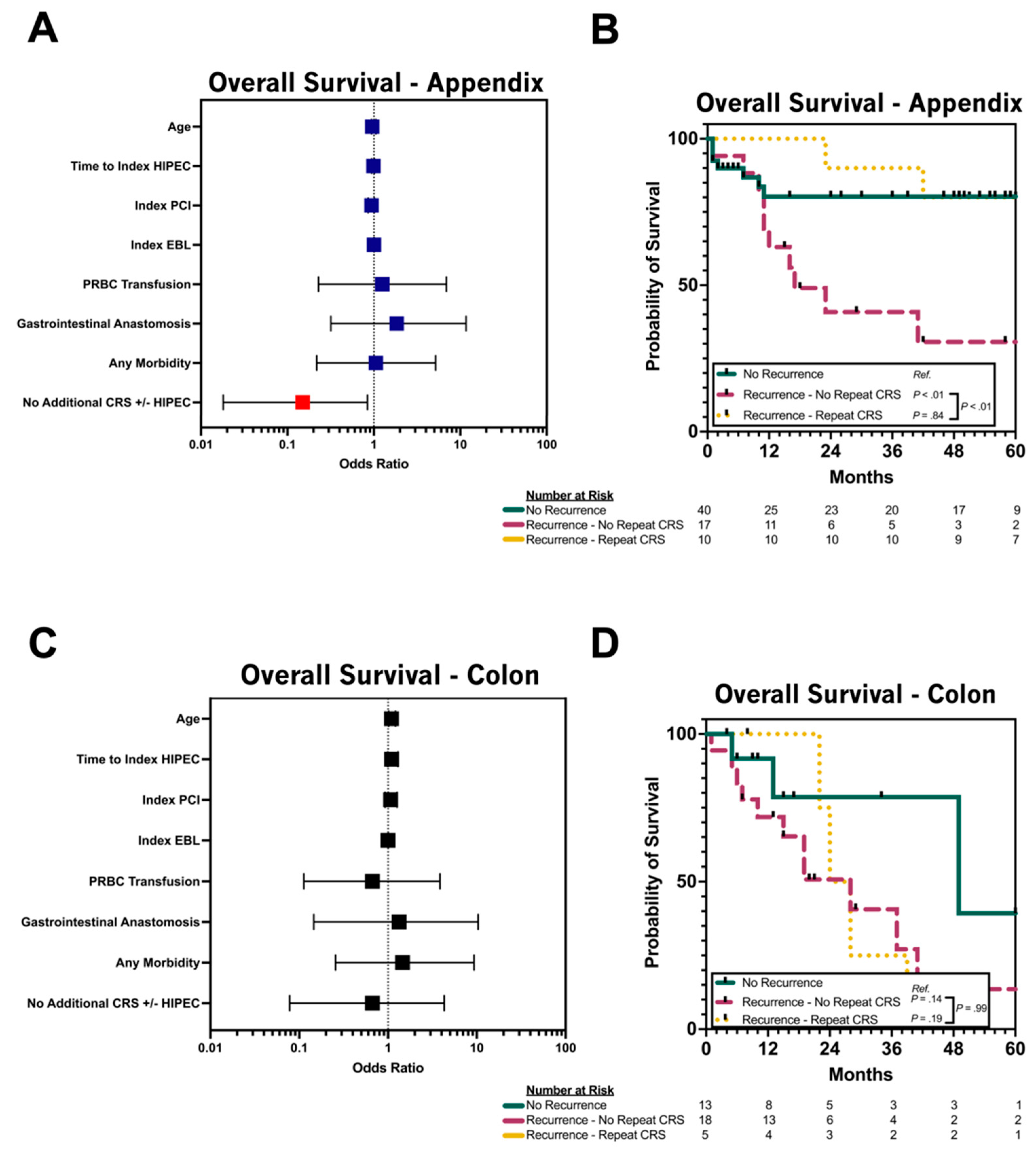
3.4. Repeat CRS/HIPEC for Relapsed pAC
3.5. Repeat CRS/HIPEC for Relapsed pCRC
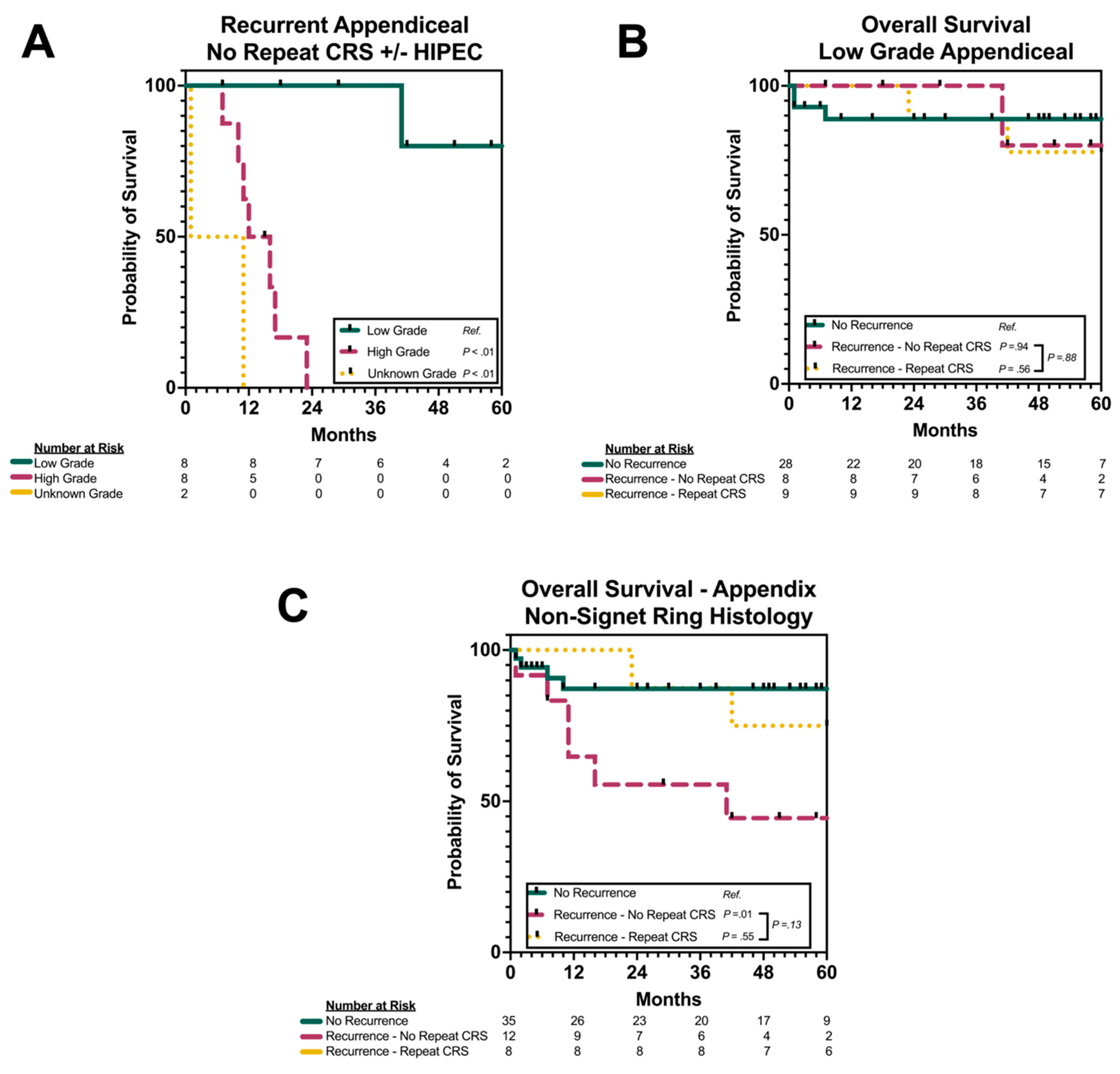
3.6. Subgroup Analysis by Grade and Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, E.A.; Stewart, J.H.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; Boot, H.; Aleman, B.M.P.; van Tinteren, H.; Zoetmulder, F.A.N. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: Location, treatment, and outcome. Ann. Surg. Oncol. 2004, 11, 375–379. [Google Scholar] [CrossRef]
- Bijelic, L.; Yan, T.D.; Sugarbaker, P.H. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J. Surg. Oncol. 2008, 98, 295–299. [Google Scholar] [CrossRef]
- Chua, T.C.; Liauw, W.; Morris, D.L. Early recurrence of pseudomyxoma peritonei following treatment failure of cytoreductive surgery and perioperative intraperitoneal chemotherapy is indicative of a poor survival outcome. Int. J. Colorectal. Dis. 2012, 27, 381–389. [Google Scholar] [CrossRef]
- Chu, D.Z.; Lang, N.P.; Thompson, C.; Osteen, P.K.; Westbrook, K.C. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989, 63, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Choudry, H.A.; Bednar, F.; Shuai, Y.; Jones, H.L.; Pai, R.K.; Pingpank, J.F.; Ahrendt, S.S.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L. Repeat Cytoreductive Surgery-Hyperthermic Intraperitoneal Chemoperfusion is Feasible and Offers Survival Benefit in Select Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2019, 26, 1445–1453. [Google Scholar] [CrossRef]
- Laks, S.; Schtrechman, G.; Adileh, M.; Ben-Yaacov, A.; Purim, O.; Ivanov, V.; Aderka, D.; Shacham-Shmueli, E.; Halpern, N.; Goren, S.; et al. Repeat Cytoreductive Surgery and Intraperitoneal Chemotherapy for Colorectal Cancer Peritoneal Recurrences is Safe and Efficacious. Ann. Surg. Oncol. 2021, 28, 5330–5338. [Google Scholar] [CrossRef]
- Williams, B.H.; Alzahrani, N.A.; Chan, D.L.; Chua, T.; Morris, D. Repeat cytoreductive surgery (CRS) for recurrent colorectal peritoneal metastases: Yes or no? Eur. J. Surg. Oncol. 2014, 40, 943–949. [Google Scholar] [CrossRef]
- Karpes, J.B.; Lansom, J.D.; Alshahrani, M.; Parikh, R.; Shamavonian, R.; A Alzahrani, N.; Liauw, W.; Morris, D.L. Repeat cytoreductive surgery with or without intraperitoneal chemotherapy for recurrent epithelial appendiceal neoplasms. BJS Open 2020, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.D.; Levine, E.A.; Mangieri, C.W.; Gawdi, R.; Moaven, O.; Russell, G.; Lundy, M.E.; Perry, K.C.; Votanopoulos, K.I.; Shen, P. Repeat Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Cancers with Peritoneal Metastasis: A 30-year Institutional Experience. Ann. Surg. Oncol. 2022, 29, 3436–3445. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.A.; O’Dwyer, S.T.; Barriuso, J.; Aziz, O.; Selvasekar, C.; Renehan, A.; Wilson, M. Indications and outcomes for repeat cytoreductive surgery and heated intra-peritoneal chemotherapy in peritoneal surface malignancy. Surg. Oncol. 2021, 38, 101572. [Google Scholar] [CrossRef]
- Powers, B.D.; Felder, S.; Veerapong, J.; Baumgartner, J.M.; Clarke, C.; Mogal, H.; Staley, C.A.; Maithel, S.K.; Patel, S.; Dhar, V.; et al. Repeat Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Is Not Associated with Prohibitive Complications: Results of a Multiinstitutional Retrospective Study. Ann. Surg. Oncol. 2020, 27, 4883–4891. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Levine, E.A.; Chouliaras, K.; Russell, G.; Shen, P.; Votanopoulos, K.I. Interval between cytoreductions as a marker of tumor biology in selecting patients for repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2017, 116, 741–745. [Google Scholar] [CrossRef]
- Feferman, Y.; Solomon, D.; Bhagwandin, S.; Kim, J.; Aycart, S.N.; Feingold, D.; Sarpel, U.; Labow, D.M. Sites of Recurrence After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Carcinomatosis from Colorectal and Appendiceal Adenocarcinoma: A Tertiary Center Experience. Ann. Surg. Oncol. 2019, 26, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I. Repeat CRS/HIPEC: It Comes Down to Tumor Biology and Ability to Achieve a Complete CRS. Ann. Surg. Oncol. 2022, 29, 3366–3368. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann. Intern. Med. 2007, 147, W163–W194. [Google Scholar] [CrossRef]
- Mogal, H.; Chouliaras, K.; Levine, E.A.; Shen, P.; Votanopoulos, K.I. Repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: Review of indications and outcomes. J. Gastrointest. Oncol. 2016, 7, 129–142. [Google Scholar]
- Portilla, A.G.; Sugarbaker, P.H.; Chang, D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: Analysis of prognostic features. World J. Surg. 1999, 23, 23–29. [Google Scholar] [CrossRef]
- Bijelic, L.; Yan, T.D.; Sugarbaker, P.H. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2007, 14, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Polanco, P.M.; Ding, Y.; Knox, J.M.; Ramalingam, L.; Jones, H.; Hogg, M.E.; Zureikat, A.H.; Holtzman, M.P.; Pingpank, J.; Ahrendt, S.; et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann. Surg. Oncol. 2015, 22, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.A.; Pozzar, R.A.; Enzinger, A.C.; Tavormina, A.; Howard, C.; Matulonis, U.A.; Liu, J.F.; Horowitz, N.; Meyer, L.A.; Wright, A.A. Improving the palliative-procedure decision-making process for patients with peritoneal carcinomatosis: A secondary analysis. Gynecol. Oncol. 2024, 188, 125–130. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients Without Relapse (n = 53) | Patients with Relapse (n = 50) | p |
|---|---|---|---|
| Age in Years, mean (SD) | 63.2 (13.5) | 59.1 (13.2) | 0.1229 |
| Race, n (%) | 0.5225 | ||
| White | 38 (71.7%) | 33 (66.0%) | |
| Black | 15 (28.3%) | 16 (32.0%) | |
| Other | 0 (0.0%) | 1 (2.0%) | |
| Female Sex, n (%) | 37 (69.8%) | 34 (68.0%) | >0.9999 |
| Primary Site, n (%) | 0.0224 | ||
| Appendix | 40 (75.5%) | 27 (54.0%) | |
| Colon | 13 (24.5%) | 23 (46.0%) | |
| ASA Score | 0.1622 | ||
| 2 | 15 (28.3%) | 10 (20.0%) | |
| 3 | 37 (69.8%) | 35 (70.0%) | |
| 4 | 1 (1.9%) | 5 (10.0%) | |
| Time to HIPEC in Months, median [IQR] | 4.0 [3.0–8.0] | 6.0 [4–19.0] | 0.0293 |
| Prior Laparotomy, n (%) | 34 (64.2%) | 32 (64.0%) | >0.9999 |
| Previous Chemotherapy, n (%) | 38 (71.7%) | 26 (52.0%) | 0.0447 |
| Peritoneal Cancer Index Score (PCI), median [IQR] | 8.0 [4.0–16.0] | 15.5 [6.8–22.3] | 0.0266 |
| Cytoreduction Score (CCR), n (%) | 0.4453 | ||
| CCR-0 | 43 (79.6%) | 36 (72.0%) | |
| CCR-1 | 9 (16.7%) | 11 (22.0%) | |
| CCR-2 | 1 (1.9%) | 3 (6.0%) | |
| Unknown | 1 (1.9%) | 0 (0.0%) | |
| Operative Time in Minutes, mean (SD) | 407.1 (109.5) | 502.3 (148.3) | 0.0004 |
| Operative Blood Loss in mL, median [IQR] | 250.0 [125.0–500.0] | 500.0 [250.0–850.0] | 0.0006 |
| Red Blood Cell Transfusion, n (%) | 31 (58.5%) | 31 (62.0%) | 0.8407 |
| Fresh Frozen Plasma Transfusion | 3 (5.66%) | 10 (20.0%) | 0.0380 |
| ≥2 Organs Resected, n (%) | 49 (92.5%) | 45 (90.0%) | 0.7367 |
| ≥4 Organs Resected, n (%) | 20 (37.7%) | 32 (53.3%) | 0.1303 |
| ≥1 Anastomosis, n (%) | 43 (81.2%) | 38 (76.0%) | 0.6323 |
| Ostomy Creation, n (%) | 13 (24.5%) | 19 (38.0%) | 0.2009 |
| ICU Length of Stay in Days, median [IQR] | 1.0 [1.0–3.0] | 3.0 [2.0–4.0] | 0.0151 |
| Hospital Length of Stay in Days, [IQR] | 8.0 [6.0–10.0] | 8.5 [7.0–10.3] | 0.2447 |
| Any Complication, n (%) | 28 (52.8%) | 21 (42.0%) | 0.3254 |
| Readmission (30 day), n (%) | 8 (15.7%) | 18 (36.0%) | 0.0238 |
| Mortality (30 day), n (%) | 3 (5.7%) | 0 (0.0%) | 0.2433 |
| Adjuvant Radiation, n (%) | 1 (1.9%) | 2 (4.0%) | 0.6104 |
| Adjuvant Chemotherapy, n (%) | 7 (13.2%) | 36 (72.0%) | <0.0001 |
| Follow-Up Status, n (%) | <0.0001 | ||
| No Evidence of Disease | 42 (79.2%) | 4 (8.0%) | |
| Alive with Disease | 1 (1.9%) | 18 (36.0%) | |
| Died of Disease | 2 (3.8%) | 27 (54.0%) | |
| Died of Other Cause/Unknown | 8 (15.1%) | 1 (2.0%) | |
| Overall Survival, n (%) | 43 (81.1%) | 23 (46.0%) | 0.0002 |
| Follow-Up in Months, median [IQR] | 17.0 [6.0–54.0] | 21.5 [11.0–42.0] | 0.4119 |
| Variable | Repeat CRS/HIPEC (n = 14) | No Repeat CRS/HIPEC (n = 36) | p |
|---|---|---|---|
| Age in Years, mean (SD) | 63.0 [55.8–71.0] | 60.0 [45.0–67.0] | 0.5174 |
| Race, n% | 0.7861 | ||
| White | 9 (64.3%) | 24 (66.7%) | |
| Black | 5 (35.7%) | 11 (30.6%) | |
| Other | 0 (0.0%) | 1 (2.8%) | |
| Primary Site, n (%) | 0.3628 | ||
| Appendix | 9 (64.3%) | 18 (50.0%) | |
| Colon | 5 (35.7%) | 18 (50.0%) | |
| Female Sex, n % | 13 (92.9%) | 21 (58.3%) | 0.0211 |
| ASA Score | 0.7117 | ||
| 2 | 2 (14.3%) | 8 (22.2%) | |
| 3 | 11 (78.6%) | 24 (66.7%) | |
| 4 | 1 (7.1%) | 4 (11.1%) | |
| Time to HIPEC in Months, median [IQR] | 18.0 [4.0–26.0] | 6.0 [3.3–17.8] | 0.4746 |
| Prior Laparotomy, n (%) | 9 (64.3%) | 23 (63.9%) | >0.9999 |
| Previous Chemotherapy, n (%) | 12 (85.7%) | 26 (66.7%) | 0.3002 |
| Peritoneal Cancer Index Score (PCI), mean (SD) | 16 (10.8) | 15.3 (10.4) | 0.8285 |
| Cytoreduction Score (CCR), n (%) | 0.5257 | ||
| CCR-0 | 11 (78.6%) | 25 (69.4%) | |
| CCR-1 | 3 (21.4%) | 8 (22.2%) | |
| CCR-2 | 0 (0.0%) | 3 (8.3%) | |
| Operative Time in Minutes, mean (SD) | 563.1 (157.4) | 479 (140.1) | 0.0823 |
| Operative Blood Loss in mL, mean (SD) | 585.7 (427.6) | 673.6 (494.9) | 0.5618 |
| Red Blood Cell Transfusion, n (%) | 8 (57.1%) | 23 (63.9%) | 0.7500 |
| Fresh Frozen Plasma Transfusion | 2 (14.3%) | 8 (22.2%) | 0.7042 |
| ≥2 Organs Resected, n (%) | 13 (92.9%) | 32 (88.9%) | >0.9999 |
| ≥4 Organs Resected, n (%) | 11 (78.6%) | 21 (58.3%) | 0.1807 |
| ≥1 Anastomosis, n (%) | 9 (64.3%) | 29 (80.6%) | 0.2777 |
| Ostomy Creation, n (%) | 2 (14.3%) | 17 (47.2%) | 0.0504 |
| ICU Length of Stay in Days, median [IQR] | 2.0 [1.0–3.5] | 3.0 [2.0–4.0] | 0.2124 |
| Hospital Length of Stay in Days, [IQR] | 7.0 [5.0–9.3] | 9.0 [7.3–11.8] | 0.0683 |
| Any Complication, n (%) | 4 (28.6%) | 17 (47.2%) | 0.3408 |
| Readmission (30 day), n (%) | 2 (14.3%) | 16 (44.4%) | 0.0563 |
| Mortality (30 day), n (%) | 0 (0.0%) | 0 (0.0%) | >0.9999 |
| Adjuvant Radiation, n (%) | 0 (0.0%) | 2 (5.6%) | >0.9999 |
| Adjuvant Chemotherapy, n (%) | 27 (75.0%) | 9 (64.3%) | 0.49555 |
| Follow-Up Status, n (%) | 0.1564 | ||
| No Evidence of Disease | 3 (21.4%) | 1 (2.8%) | |
| Alive with Disease | 5 (35.7%) | 13 (36.1%) | |
| Died of Disease | 6 (42.9%) | 21 (58.3%) | |
| Died of Other Cause/Unknown | 0 (0.0%) | 1 (2.8%) | |
| Overall Survival, n (%) | 8 (57.1%) | 14 (38.9%) | 0.2430 |
| Follow-Up in Months, median [IQR] | 51 [23.8–92.3] | 16.5 [10.0–29.0] | 0.0003 |
| Appendix (n = 27) | ||||||
|---|---|---|---|---|---|---|
| Variable | Simple Logistic Regression | Multiple Logistic Regression | ||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Age (per year) | 0.995 | 0.934–1.057 | 0.0678 | |||
| Race | ||||||
| White | Ref. | Ref. | Ref. | |||
| Black (vs. White) | 2.571 | 0.490–15.91 | 0.2767 | |||
| Sex | ||||||
| Female | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Male (vs. Female) | 0.119 | 0.014–0.689 | 0.0270 | 0.137 | 0.0136–0.906 | 0.0534 |
| Previous Laparotomy | 1.800 | 0.375–9.123 | 0.4640 | |||
| Time to Index HIPEC (per month) | 0.985 | 0.939–1.016 | 0.4011 | |||
| PCI Score (per point) | 0.938 | 0.858–1.012 | 0.1216 | |||
| Index HIPEC Agent | ||||||
| Mitomycin C Monotherapy | Ref. | Ref. | Ref. | |||
| Platinum Monotherapy (vs. Mitomycin C) | 0.833 | 0.087–7.992 | 0.8669 | |||
| Duration of Index HIPEC | ||||||
| 90 min | Ref. | Ref. | Ref. | |||
| 60 min (vs. 90 min) | 0.446 | 0.084–2.169 | 0.3237 | |||
| Index Operative Time (per minute) | 1.001 | 0.996–1.006 | 0.7199 | |||
| Index EBL (per mL) | 1.001 | 0.999–1.003 | 0.4975 | |||
| Index CCR | ||||||
| CCR-0 | Ref. | Ref. | Ref. | |||
| CCR-1/CCR-2 | 0.400 | 0.073–1.973 | 0.2677 | |||
| Intraoperative PRBC Transfusion | 1.250 | 0.228–6.906 | 0.7933 | |||
| Intraoperative FFP Transfusion | 0.800 | 0.145–4.380 | 0.7933 | |||
| Multivisceral Resection | 1.200 | 0.125–11.54 | 0.8669 | |||
| Gastrointestinal Anastomosis | 1.833 | 0.318–11.64 | 0.4978 | |||
| Intensive Care Unit Length of Stay (per day) | 0.934 | 0.676–1.052 | 0.4336 | |||
| Hospital Length of Stay (per day) | 0.947 | 0.806–1.034 | 0.3497 | |||
| Any Morbidity | 1.050 | 0.217–5.155 | 0.9512 | |||
| No Additional CRS/HIPEC | 0.150 | 0.018–0.839 | 0.0445 | 0.175 | 0.017–1.167 | 0.0906 |
| Colon (n = 23) | |||
| Variable | Simple Logistic Regression | ||
| OR | 95% CI | p | |
| Age (per year) | 1.094 | 1.014–1.213 | 0.0420 |
| Time to Index HIPEC (per month) | 1.099 | 0.996–1.294 | 0.0685 |
| PCI Score (per point) | 1.071 | 0.927–1.265 | 0.3736 |
| Index Operative Time (per minute) | 1.002 | 0.993–1.011 | 0.6583 |
| Index EBL (per mL) | 1.000 | 0.999–1.003 | 0.6110 |
| Intraoperative PRBC Transfusion | 0.667 | 0.113–3.841 | 0.6457 |
| Gastrointestinal Anastomosis | 1.333 | 0.146–10.36 | 0.7822 |
| Intensive Care Unit Length of Stay (per day) | 1.208 | 0.768–2.482 | 0.5094 |
| Hospital Length of Stay (per day) | 1.107 | 0.883–1.515 | 0.4366 |
| Any Morbidity | 1.458 | 0.256–9.312 | 0.6734 |
| No Additional CRS/HIPEC | 0.667 | 0.078–4.291 | 0.6800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, A.M.; Clark, O.M.; Lee, J.J.; Dougherty, K.; Hendrick, L.E.; Raine, J.; Solsky, I.; Dickson, P.V.; Glazer, E.S.; Shibata, D.; et al. Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study. Cancers 2025, 17, 2014. https://doi.org/10.3390/cancers17122014
Fleming AM, Clark OM, Lee JJ, Dougherty K, Hendrick LE, Raine J, Solsky I, Dickson PV, Glazer ES, Shibata D, et al. Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study. Cancers. 2025; 17(12):2014. https://doi.org/10.3390/cancers17122014
Chicago/Turabian StyleFleming, Andrew M., Owen M. Clark, Jaewon J. Lee, Kristen Dougherty, Leah E. Hendrick, Jordan Raine, Ian Solsky, Paxton V. Dickson, Evan S. Glazer, David Shibata, and et al. 2025. "Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study" Cancers 17, no. 12: 2014. https://doi.org/10.3390/cancers17122014
APA StyleFleming, A. M., Clark, O. M., Lee, J. J., Dougherty, K., Hendrick, L. E., Raine, J., Solsky, I., Dickson, P. V., Glazer, E. S., Shibata, D., Gleeson, E., Munene, G., & Deneve, J. L. (2025). Iterative Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Primary Appendiceal and Colorectal Cancers: An Observational Study. Cancers, 17(12), 2014. https://doi.org/10.3390/cancers17122014






