Retrospective Evaluation of Baseline Amino Acid PET for Identifying Future Regions of Tumor Recurrence in High-Grade Glioma Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
2.3. PET-MRI Protocol
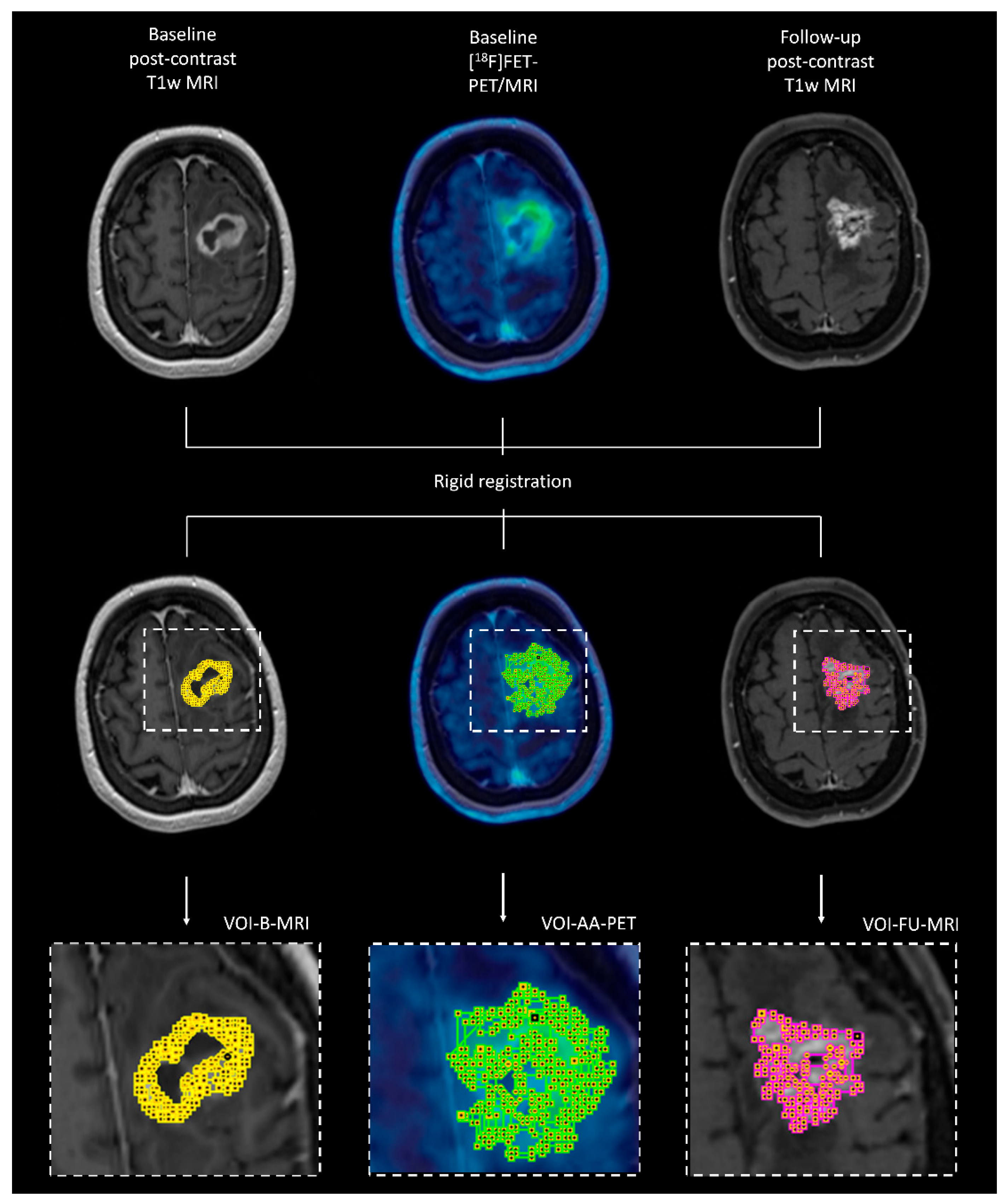
2.4. Co-Registration of Different Imaging Modalities at Different Time Points
2.5. Dice Similarity Coefficient
2.6. PET Statistics
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics
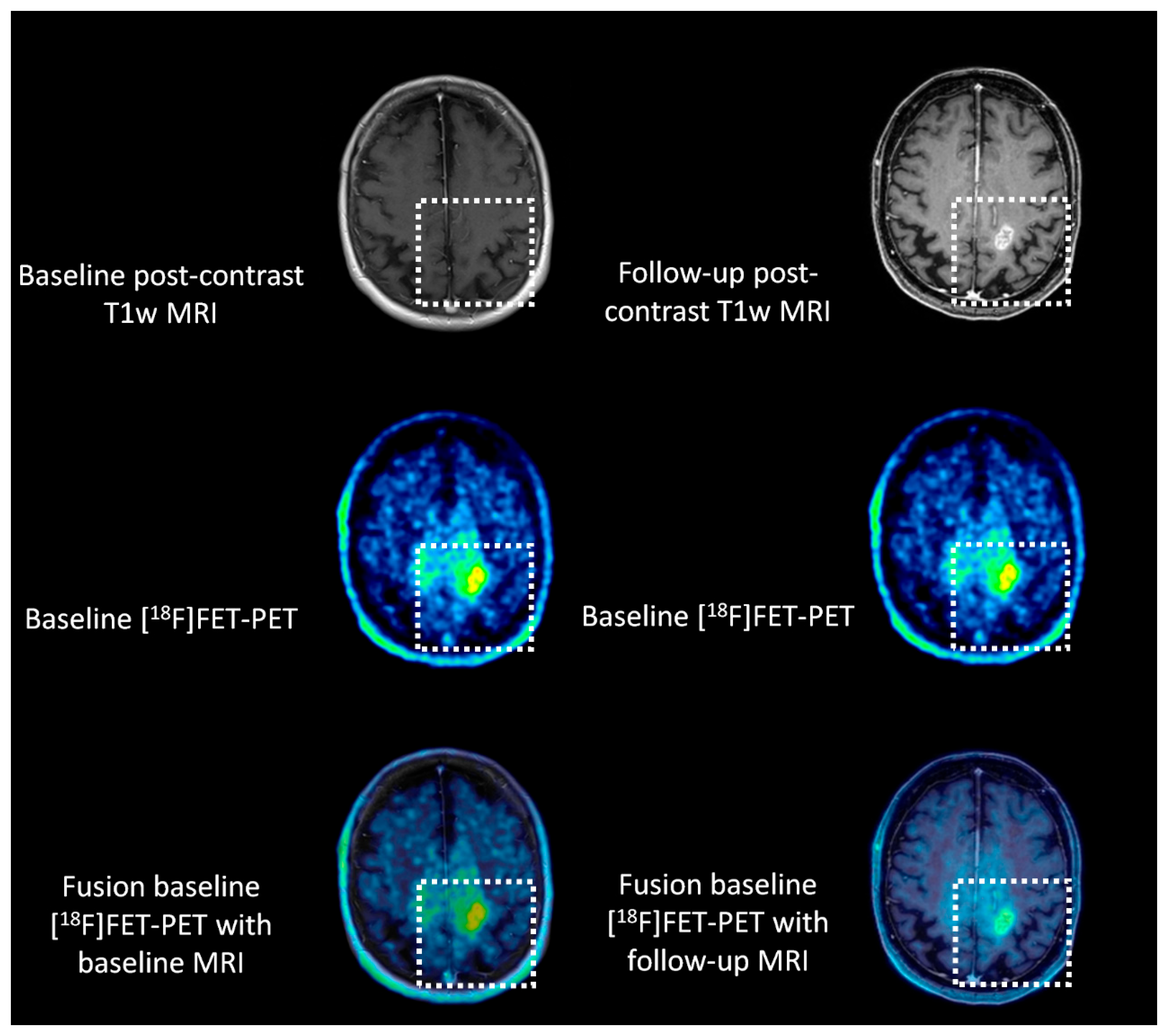
3.2. Volumes of Interest on Baseline Amino Acid PET Yield Significantly Greater Overlap with Regions of Future Tumor Recurrence Compared to Baseline Contrast-Enhanced MRI
3.3. Baseline Static and Dynamic Amino Acid PET Statistics Cannot Be Used for Predicting the Region of Tumor Recurrence
4. Discussion
5. Conclusions
6. Key Points
6.1. Pertinent Findings
6.2. Implications for Patient Care
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Dice similarity coefficient |
| MRI | Magnetic resonance imaging |
| PET | Positron-emission tomography |
| VOI | Volume of interest |
| [11C]MET | [S-methyl-11C]methionine |
| [18F]FET | O-(2-[18F]fluoroethyl)-L-tyrosine |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Scherer, H.J. Structural Development in Gliomas. Am. J. Cancer 1938, 34, 333–351. [Google Scholar] [CrossRef]
- Giese, A.; Kluwe, L.; Laube, B.; Meissner, H.; Berens, M.E.; Westphal, M. Migration of Human Glioma Cells on Myelin. Neurosurgery 1996, 38, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Castellano-Sanchez, A.A.; Hunter, S.B.; Pecot, M.; Cohen, C.; Hammond, E.H.; Devi, S.N.; Kaur, B.; Van Meir, E.G. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004, 64, 920–927. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Henssen, D.; Meijer, F.; Verburg, F.A.; Smits, M. Challenges and opportunities for advanced neuroimaging of glioblastoma. Br. J. Radiol. 2023, 96, 20211232. [Google Scholar] [CrossRef]
- Smits, M. MRI biomarkers in neuro-oncology. Nat. Rev. Neurol. 2021, 17, 486–500. [Google Scholar] [CrossRef]
- Lopez, W.O.; Cordeiro, J.G.; Albicker, U.; Doostkam, S.; Nikkhah, G.; Kirch, R.D.; Trippel, M.; Reithmeier, T. Correlation of (18)F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. Onco Targets Ther. 2015, 8, 3803–3815. [Google Scholar] [CrossRef]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.W.; Zilles, K.; Coenen, H.H.; Langen, K.J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- McGirt, M.J.; Attenello, F.; Gathinji, M.; Than, K.; Chaichana, K.L.; Datoo, G.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with malignant and low-grade brain astrocytoma. J. Neurosurg. 2008, 108, A886. [Google Scholar]
- Chiocca, E.A.; Silbergeld, D.L.; Piepmeier, J.M.; Laws, E.R.; Berger, M.S. Extent of Surgical Resection Is Independently Associated with Survival in Patients with Hemispheric Infiltrating Low-Grade Gliomas Comments. Neurosurgery 2008, 63, 707–708. [Google Scholar]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougere, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro. Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- van der Kolk, A.G.; Henssen, D.; Schroeder, H.W., III; Hall, L.T. PET Agents for Primary Brain Tumor Imaging; Exon Publications: Brisbane, Australia, 2023. [Google Scholar]
- Arbizu, J.; Tejada, S.; Marti-Climent, J.M.; Diez-Valle, R.; Prieto, E.; Quincoces, G.; Vigil, C.; Idoate, M.A.; Zubieta, J.L.; Penuelas, I.; et al. Quantitative volumetric analysis of gliomas with sequential MRI and (1)(1)C-methionine PET assessment: Patterns of integration in therapy planning. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 771–781. [Google Scholar] [CrossRef]
- Suchorska, B.; Jansen, N.L.; Linn, J.; Kretzschmar, H.; Janssen, H.; Eigenbrod, S.; Simon, M.; Popperl, G.; Kreth, F.W.; la Fougere, C.; et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 2015, 84, 710–719. [Google Scholar] [CrossRef]
- Henssen, D.; Rullmann, M.; Schildan, A.; Striepe, S.; Schürer, M.; Scherlach, C.; Jähne, K.; Stassart, R.; Sabri, O.; Seidel, C.; et al. The Diagnostic Accuracy of Dynamic Amino-Acid PET for the Differentiation Between Treatment Related Abnormalities and Tumor Progression in Post-Treatment Neuro-Oncology Patients. 2024. [Google Scholar]
- Rapp, M.; Heinzel, A.; Galldiks, N.; Stoffels, G.; Felsberg, J.; Ewelt, C.; Sabel, M.; Steiger, H.J.; Reifenberger, G.; Beez, T.; et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J. Nucl. Med. 2013, 54, 229–235. [Google Scholar] [CrossRef]
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Jansen, N.L.; Seiz, M.; Schocke, M.; McCoy, M.; Göbel, G.; la Fougère, C.; Virgolini, I.J.; et al. [18F]-fluoro-ethyl-L-tyrosine PET: A valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro. Oncol. 2013, 15, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Cinotti, L.E.; Jouvet, A.; Ramackers, J.M.; Saint Pierre, G.; Thiesse, P.; Jouanneau, E.; Guyotat, J.; Pelissou-Guyotat, I.; Setiey, A.; et al. High and low grade oligodendrogliomas (ODG): Correlation of amino-acid and glucose uptakes using PET and histological classifications. J. Neurooncol. 2004, 68, 263–274. [Google Scholar] [CrossRef]
- Manabe, O.; Hattori, N.; Yamaguchi, S.; Hirata, K.; Kobayashi, K.; Terasaka, S.; Kobayashi, H.; Motegi, H.; Shiga, T.; Magota, K.; et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 896–904. [Google Scholar] [CrossRef]
- Pöpperl, G.; Kreth, F.W.; Mehrkens, J.H.; Herms, J.; Seelos, K.; Koch, W.; Gildehaus, F.J.; Kretzschmar, H.A.; Tonn, J.C.; Tatsch, K. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Huang, K.; Schrimpf, D.; Albert, N.L.; Hielscher, T.; von Deimling, A.; Schüller, U.; Dimitrakopoulou-Strauss, A.; Haberkorn, U. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Thon, N.; Eigenbrod, S.; Hartmann, C.; Egensperger, R.; Herms, J.; Geisler, J.; la Fougere, C.; Lutz, J.; Linn, J.; et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro. Oncol. 2011, 13, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kracht, L.W.; Miletic, H.; Busch, S.; Jacobs, A.H.; Voges, J.; Hoevels, M.; Klein, J.C.; Herholz, K.; Heiss, W.D. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: Local comparison with stereotactic histopathology. Clin. Cancer Res. 2004, 10, 7163–7170. [Google Scholar] [CrossRef]
- Tripathi, M.; Sharma, R.; D’Souza, M.; Jaimini, A.; Panwar, P.; Varshney, R.; Datta, A.; Kumar, N.; Garg, G.; Singh, D.; et al. Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin. Nucl. Med. 2009, 34, 878–883. [Google Scholar] [CrossRef]
- Song, S.; Cheng, Y.; Ma, J.; Wang, L.; Dong, C.; Wei, Y.; Xu, G.; An, Y.; Qi, Z.; Lin, Q.; et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: A biopsy validation study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1458–1467. [Google Scholar] [CrossRef]
- Ort, J.; Hamou, H.A.; Kernbach, J.M.; Hakvoort, K.; Blume, C.; Lohmann, P.; Galldiks, N.; Heiland, D.H.; Mottaghy, F.M.; Clusmann, H.; et al. (18)F-FET-PET-guided gross total resection improves overall survival in patients with WHO grade III/IV glioma: Moving towards a multimodal imaging-guided resection. J. Neurooncol. 2021, 155, 71–80. [Google Scholar] [CrossRef]
| Baseline Amino Acid PET | Lesion Derived from Follow-Up MRI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nr | Age | Sex | Dose Tracer | Tumor Type | PFS (Months) | DSC Baseline Amino Acid PET vs. Follow-Up MRI | DSC Baseline MRI vs. Follow-Up MRI | TTP | Area Under the Time Activity Curve | TBRmax | TBRmean | TTP | Area Under the Time Activity Curve | TBRmax | TBRmean |
| 01 | 59 | F | 763 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 21 | 41.7% | 8.6% | 37.5 | 1980 | 2.7 | 3.8 | 52.5 | 2150 | 2.7 | 4.1 |
| 02 | 72 | M | 707 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 16 | 100.0% | 0% | 52.5 | 909 | 2.0 | 1.5 | 52.5 | 851 | 2.0 | 1.4 |
| 03 | 50 | F | 717 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 13 | 0% | 0% | 345 | 1120 | 1.7 | 1.5 | 157.5 | 1290 | 1.7 | 1.7 |
| 04 | 58 | M | 739 MBq [11C]MET | Oligodendroglioma (IDHmut, 1p/19q co-deleted) grade 3 | 11 | 33.3% | 0% | 90 | 2390 | 2.6 | 2.1 | 90 | 2920 | 2.5 | 2.6 |
| 05 | 46 | M | 448 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 8 | 27.5% | 0% | 157.5 | 1450 | 1.8 | 2.1 | 22.5 | 907 | 3.1 | 1.4 |
| 06 | 40 | M | 204 MBq [18F]FET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 44 | 60.8% | 0% | 52.5 | 909 | 2.0 | 1.5 | 52.5 | 851 | 2.0 | 1.4 |
| 07 | 72 | F | 192 MBq [18F]FET | Glioblastoma (IDHwildtype) grade 4 | 12 | 85.6% | 0% | 37.5 | 1980 | 2.7 | 3.8 | 52.5 | 2150 | 2.7 | 4.1 |
| 08 | 56 | F | 749 MBq [11C]MET | Oligodendroglioma (IDHmut, 1p/19q co-deleted) grade 3 | 8 | 4.6% | 0% | 37.5 | 1920 | 3.0 | 1.5 | 127.5 | 1380 | 1.5 | 1.1 |
| 09 | 79 | F | 711 MBq [11C]MET | Oligodendroglioma (IDHmut, 1p/19q co-deleted) grade 3 | 12 | 38.1% | 0% | 52.5 | 1200 | 1.6 | 1.4 | 2250 | 822 | 1.4 | 1.0 |
| 10 | 36 | M | 750 MBq [11C]MET | Glioblastoma (IDHwildtype) grade 4 | 37 | 59.2% | 37.8% | 37.5 | 1180 | 4.9 | 1.6 | 37.5 | 978 | 2.9 | 1.3 |
| 11 | 37 | F | 738 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 12 | 78.5% | 7.8% | 157.5 | 1470 | 1.4 | 1.7 | 112.5 | 773 | 1.1 | 1.0 |
| 12 | 30 | M | 763 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 11 | 93.3% | 68.5% | 165 | 1970 | 1.1 | 2.0 | 60 | 1270 | 2.1 | 1.4 |
| 13 | 69 | F | 156 MBq [18F]FET | Glioblastoma (IDHwildtype) grade 4 | 10 | 14.0% | 14.5% | 82.5 | 1030 | 1.8 | 0.6 | 97.5 | 930 | 1.2 | 1.4 |
| 14 | 47 | M | 737 MBq [11C]MET | Astrocytoma (IDHmut, 1p/19q intact) grade 3 | 3 | 50.5% | 40.3% | 345 | 1120 | 1.7 | 1.5 | 157.5 | 1290 | 1.7 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henssen, D.; Rullmann, M.; Schildan, A.; Striepe, S.; Schürer, M.; Feraco, P.; Scherlach, C.; Jähne, K.; Stassart, R.; Sabri, O.; et al. Retrospective Evaluation of Baseline Amino Acid PET for Identifying Future Regions of Tumor Recurrence in High-Grade Glioma Patients. Cancers 2025, 17, 1986. https://doi.org/10.3390/cancers17121986
Henssen D, Rullmann M, Schildan A, Striepe S, Schürer M, Feraco P, Scherlach C, Jähne K, Stassart R, Sabri O, et al. Retrospective Evaluation of Baseline Amino Acid PET for Identifying Future Regions of Tumor Recurrence in High-Grade Glioma Patients. Cancers. 2025; 17(12):1986. https://doi.org/10.3390/cancers17121986
Chicago/Turabian StyleHenssen, Dylan, Michael Rullmann, Andreas Schildan, Stephan Striepe, Matti Schürer, Paola Feraco, Cordula Scherlach, Katja Jähne, Ruth Stassart, Osama Sabri, and et al. 2025. "Retrospective Evaluation of Baseline Amino Acid PET for Identifying Future Regions of Tumor Recurrence in High-Grade Glioma Patients" Cancers 17, no. 12: 1986. https://doi.org/10.3390/cancers17121986
APA StyleHenssen, D., Rullmann, M., Schildan, A., Striepe, S., Schürer, M., Feraco, P., Scherlach, C., Jähne, K., Stassart, R., Sabri, O., Seidel, C., & Hesse, S. (2025). Retrospective Evaluation of Baseline Amino Acid PET for Identifying Future Regions of Tumor Recurrence in High-Grade Glioma Patients. Cancers, 17(12), 1986. https://doi.org/10.3390/cancers17121986







