Focused Ultrasound-Enhanced Liquid Biopsy: A Promising Diagnostic Tool for Brain Tumor Patients
Abstract
Simple Summary
Abstract
1. Introduction
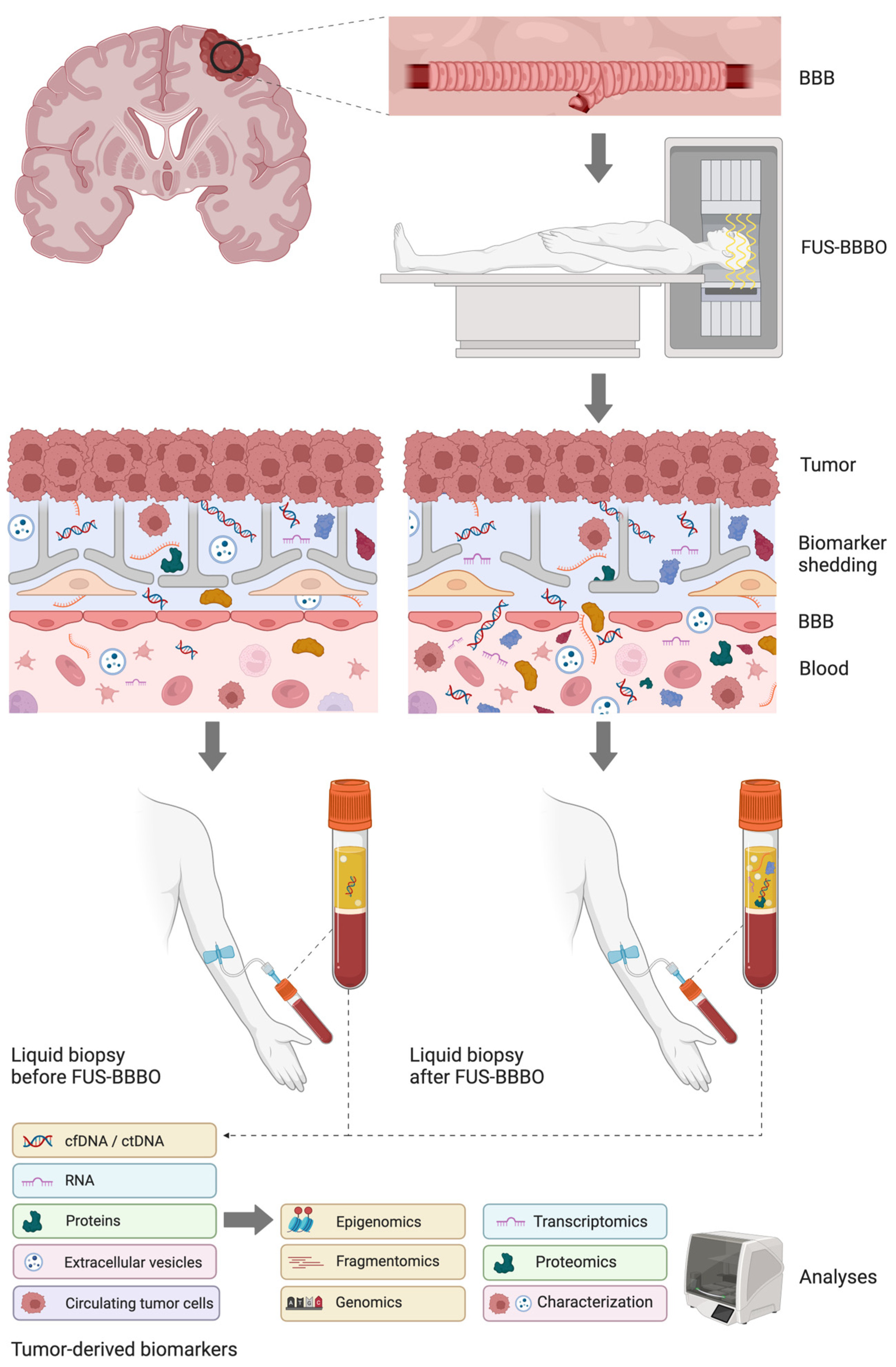
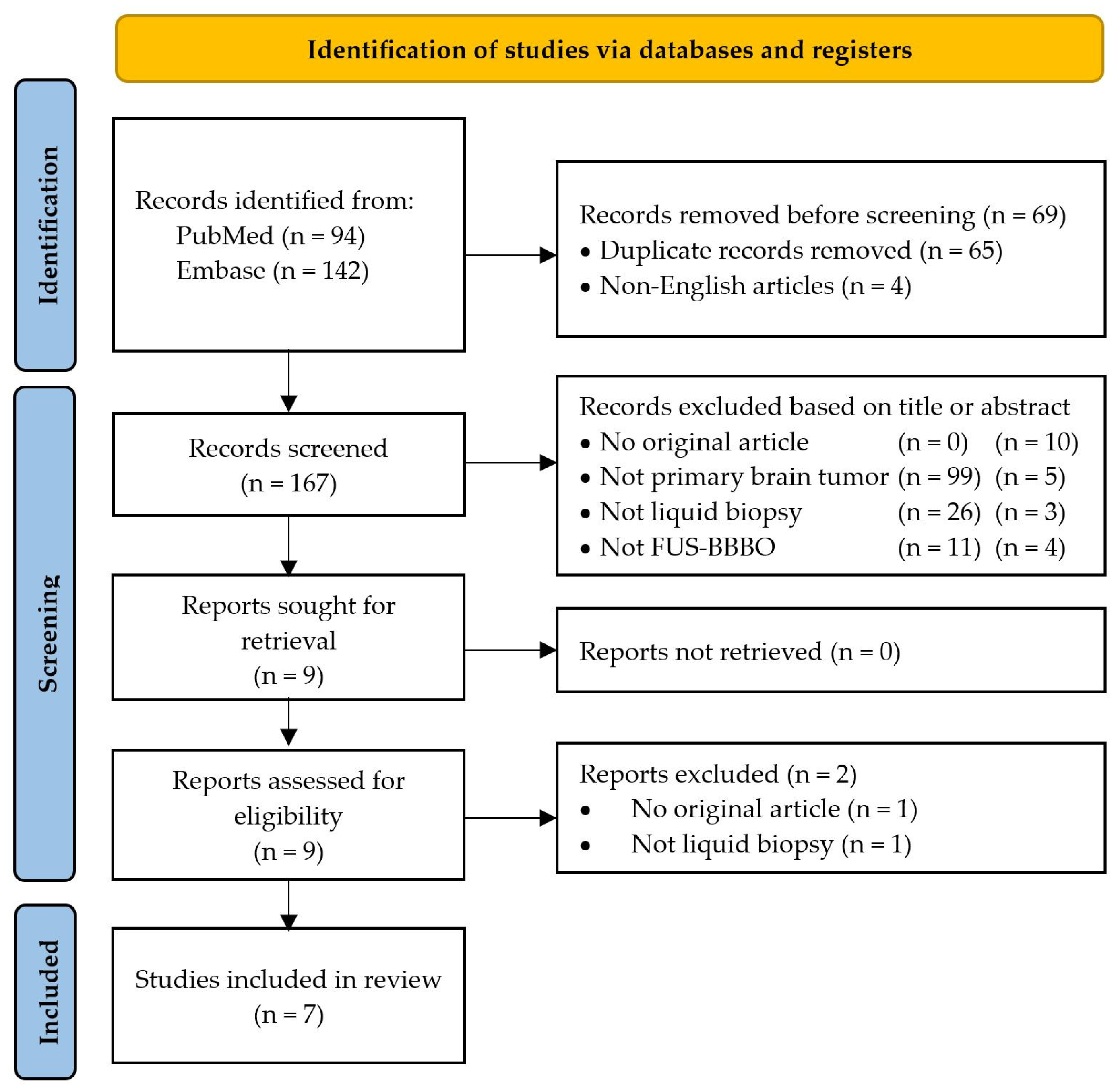
2. Materials and Methods
3. Results
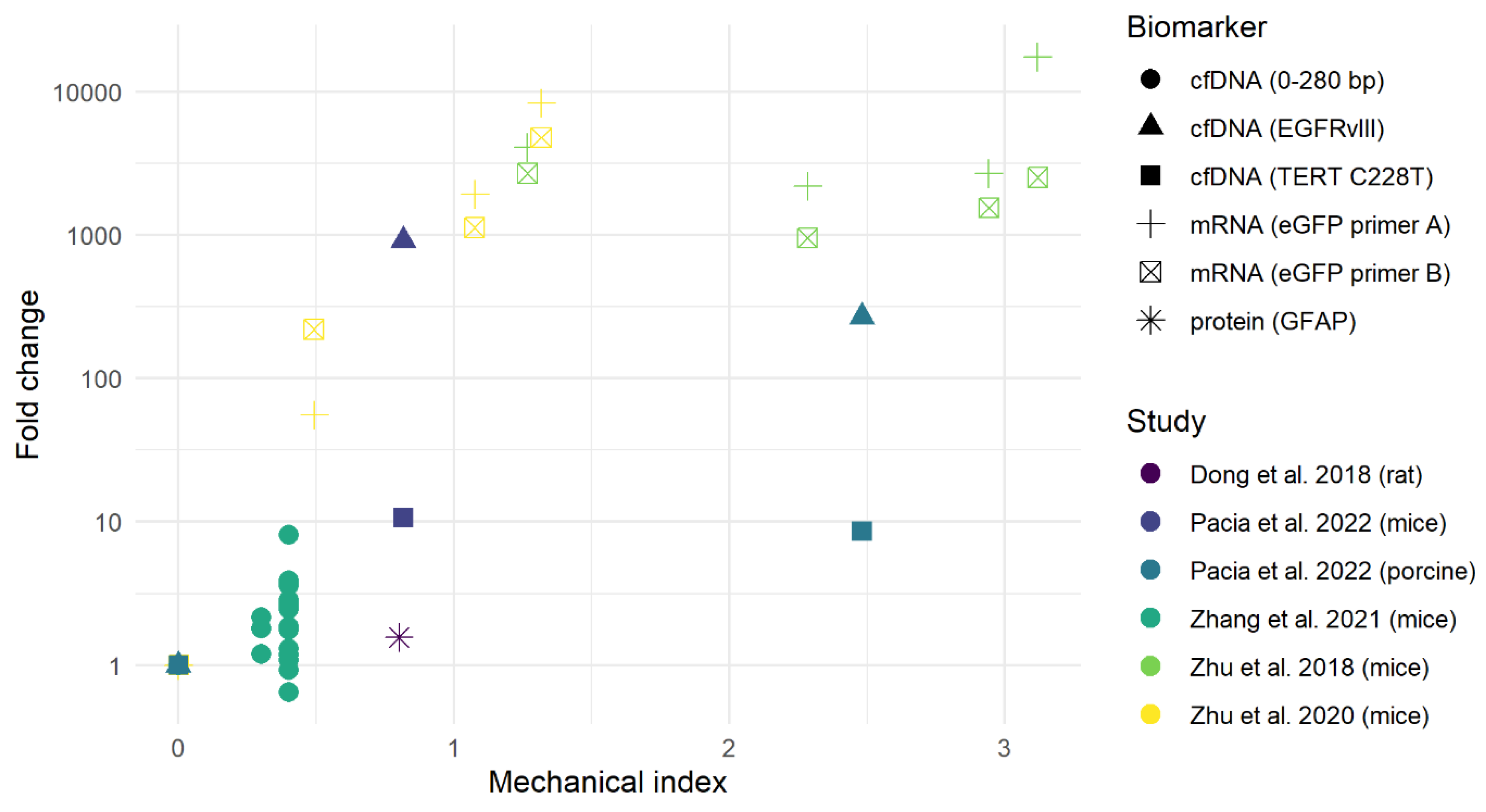
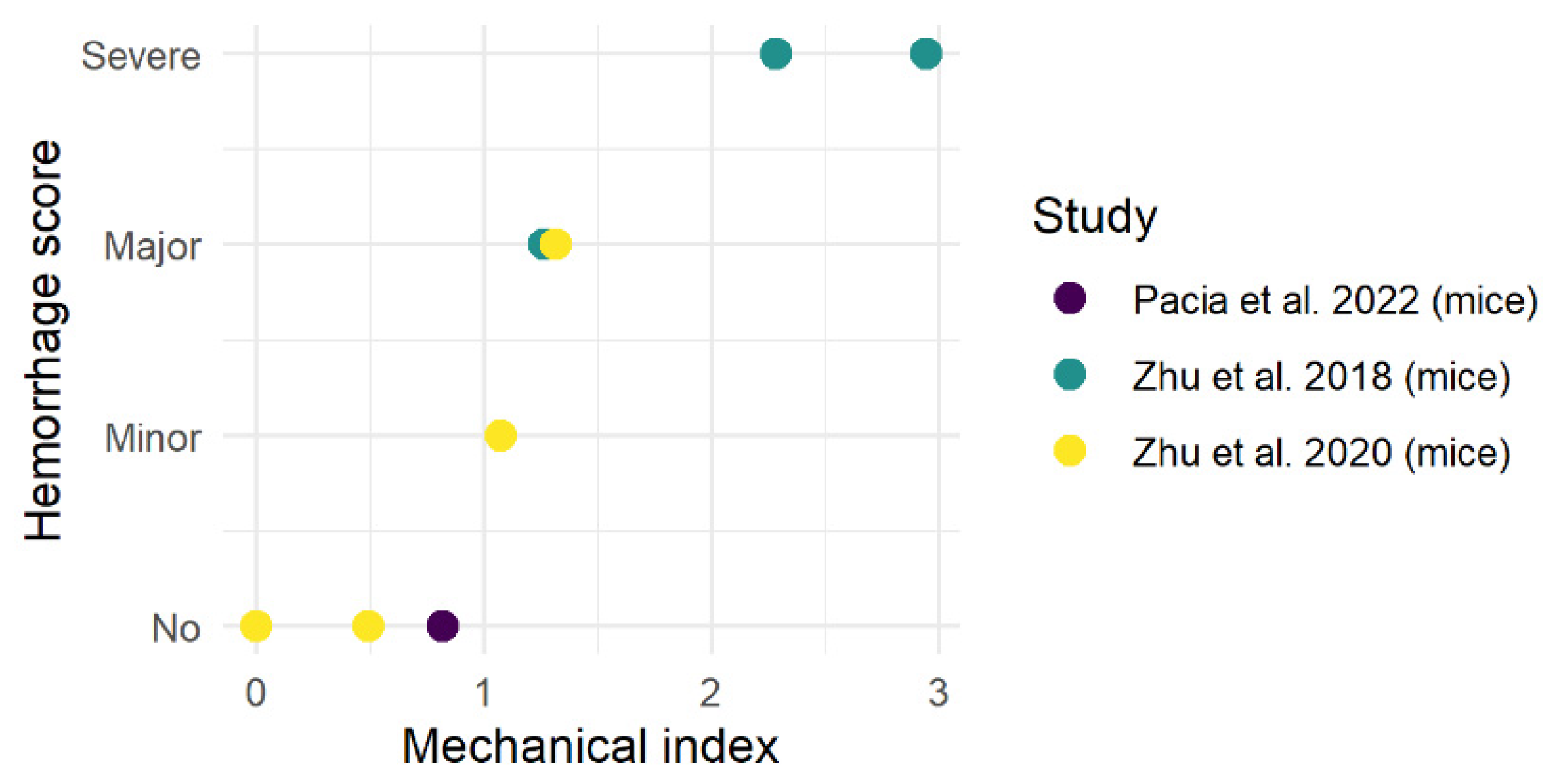
3.1. Preclinical Studies
Complications
3.2. Clinical Studies
Complications
4. Discussion
4.1. Ongoing and Future Clinical Trials
4.2. Limitations
4.3. Risk of Bias
4.4. Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Jusue-Torres, I.; Hulbert, A.; Barton, K.; Melian, E.; Anderson, D.E.; Quinones-Hinojosa, A.; Prabhu Vikram, C. Survival Benefit of Concomitant Chemoradiation in Adult Supratentorial Primary Glioblastoma. A Propensity Score Weighted Population-Based Analysis. J. Neurosurg. Sci. 2022, 66, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Li, B.K.; Al-Karmi, S.; Huang, A.; Bouffet, E. Pediatric Embryonal Brain Tumors in the Molecular Era. Expert Rev. Mol. Diagn. 2020, 20, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood Brain Tumors: Current Management, Biological Insights, and Future Directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, K.A.; Nemer, O.P.; Kessler, A.T.; Bhatt, A.A. Post-Operative Complications of Craniotomy and Craniectomy. Emerg. Radiol. 2019, 26, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Willeit, P.; Simon, T.; Ruge, M.I. Diagnostic Value and Safety of Stereotactic Biopsy for Brainstem Tumors: A Systematic Review and Meta-Analysis of 1480 Cases. Neurosurgery 2013, 72, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, K.; Chatzipli, A.; Taphoorn, M.; Kerkhof, M.; Weyerbrock, A.; Sanson, M.; Hoeben, A.; Lukacova, S.; Lombardi, G.; Leenstra, S.; et al. Molecular Evolution of IDH Wild-Type Glioblastomas Treated With Standard of Care Affects Survival and Design of Precision Medicine Trials: A Report From the EORTC 1542 Study. J. Clin. Oncol. 2019, 38, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Warton, K.; Mahon, K.L.; Samimi, G. Methylated Circulating Tumor DNA in Blood: Power in Cancer Prognosis and Response. Endocr. Relat. Cancer 2016, 23, R157–R171. [Google Scholar] [CrossRef] [PubMed]
- Berzero, G.; Pieri, V.; Mortini, P.; Filippi, M.; Finocchiaro, G. The Coming of Age of Liquid Biopsy in Neuro-Oncology. Brain 2023, 146, 4015–4024. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Seoane, J.; Salzet, M.; Soffietti, R.; Weller, M. Liquid Biopsies for Diagnosing and Monitoring Primary Tumors of the Central Nervous System. Cancer Lett. 2020, 480, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early-and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed]
- Fontanilles, M.; Duran-Peña, A.; Idbaih, A. Liquid Biopsy in Primary Brain Tumors: Looking for Stardust! Curr. Neurol. Neurosci. Rep. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.H.; Schneemann, M. Liquid Biopsy and Primary Brain Tumors. Cancers 2021, 13, 5429. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging-Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 441763. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, E. Effects of Shear Stress on Endothelial Cells: Possible Relevance for Ultrasound Applications. Prog. Biophys. Mol. Biol. 2007, 93, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding Ultrasound Induced Sonoporation: Definitions and Underlying Mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-Mediated Drug Delivery to the Brain: Principles, Progress and Prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Neurosurg. 2020, 19, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular Mechanisms of the Blood-Brain Barrier Opening Induced by Ultrasound in Presence of Microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, H.Y.; Han, M.; Choi, J.R.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized Down-Regulation of P-Glycoprotein by Focused Ultrasound and Microbubbles Induced Blood-Brain Barrier Disruption in Rat Brain. Sci. Rep. 2016, 6, 31201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cheng, G.; Ye, D.; Nazeri, A.; Yue, Y.; Liu, W.; Wang, X.; Dunn, G.P.; Petti, A.A.; Leuthardt, E.C.; et al. Focused Ultrasound-Enabled Brain Tumor Liquid Biopsy. Sci. Rep. 2018, 8, 6553. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted Disruption of the Blood-Brain Barrier with Focused Ultrasound: Association with Cavitation Activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.; ter Haar, G. Recommendations for Reporting Therapeutic Ultrasound Treatment Parameters. Ultrasound Med. Biol. 2022, 48, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- NHLBI. Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 18 June 2023).
- Zhang, D.Y.; Gould, A.; Happ, H.C.; Youngblood, M.W.; Dmello, C.; Kang, S.J.; Canney, M.; Stupp, R.; Carvill, G.L.; Sonabend, A.M. Ultrasound-Mediated Blood–Brain Barrier Opening Increases Cell-Free DNA in a Time-Dependent Manner. Neurooncol. Adv. 2021, 3, vdab165. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; He, L.; Chen, L.; Deng, Q. Opening the Blood-Brain Barrier and Improving the Efficacy of Temozolomide Treatments of Glioblastoma Using Pulsed, Focused Ultrasound with a Microbubble Contrast Agent. Biomed. Res. Int. 2018, 2018, 6501508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Nazeri, A.; Pacia, C.P.; Yue, Y.; Chen, H. Focused Ultrasound for Safe and Effective Release of Brain Tumor Biomarkers into the Peripheral Circulation. PLoS ONE 2020, 15, e0234182. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Pople, C.B.; Suppiah, S.; Llinas, M.; Huang, Y.; Sahgal, A.; Perry, J.; Keith, J.; Davidson, B.; Hamani, C.; et al. MR-Guided Focused Ultrasound Liquid Biopsy Enriches Circulating Biomarkers in Patients with Brain Tumors. Neuro Oncol. 2021, 23, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Pacia, C.P.; Yuan, J.; Yue, Y.; Xu, L.; Nazeri, A.; Desai, R.; Gach, H.M.; Wang, X.; Talcott, M.R.; Chaudhuri, A.A.; et al. Sonobiopsy for Minimally Invasive, Spatiotemporally-Controlled, and Sensitive Detection of Glioblastoma-Derived Circulating Tumor DNA. Theranostics 2022, 27, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, L.; Chien, C.-Y.; Yang, Y.; Yue, Y.; Fadera, S.; Stark, A.H.; Schwetye, K.E.; Nazeri, A.; Desai, R.; et al. First-in-Human Prospective Trial of Sonobiopsy in High-Grade Glioma Patients Using Neuronavigation-Guided Focused Ultrasound. NPJ Precis. Oncol. 2023, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chu, P.C.; Chai, W.Y.; Kang, S.T.; Tsai, C.H.; Fan, C.H.; Yeh, C.K.; Liu, H.L. Characterization of Different Microbubbles in Assisting Focused Ultrasound-Induced Blood-Brain Barrier Opening. Sci. Rep. 2017, 7, 46689. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Poon, C.; Hynynen, K. Evaluating the Safety Profile of Focused Ultrasound and Microbubble-Mediated Treatments to Increase Blood-Brain Barrier Permeability. Expert Opin. Drug Deliv. 2019, 16, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, M.; De Groot, J.; Lee, J.; Mogilner, A.; Schwartz, T.H.; Burns, T.; Shah, B.; McDermott, M.; Bettegowda, C.; Khosla, A.; et al. Pivotal Study to Evaluate Safety and Efficacy of Exablate Model 4000 Using Microbubble Resonators to Temporarily Mediate Blood-Brain Barrier Disruption for Liquid Biopsy in Glioblastoma (LIBERATE). Neuro Oncol. 2022, 24, vii82. [Google Scholar] [CrossRef]
- Miller, A.; Ramamurthy, B.; Pike, L.; Keralapura, M.; Hofius, J.; Marshall, J.; Jones, M.; Lee, C.-Y.; Stember, J.; Brennan, C.; et al. Focused Ultrasound Mediated Blood-Brain Barrier Penetrance to Enable Cell-Free DNA (CfDNA) as a Liquid Biopsy in Recurrent Primary Brain Tumors. Neuro Oncol. 2022, 24, vii87. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Batts, A.J.; Mathew, A.S.; Andrew Thim, E.; Price, R.J. Focused Ultrasound Hyperthermia Augments Release of Glioma-Derived Extracellular Vesicles with Differential Immunomodulatory Capacity. Theranostics 2020, 10, 7436–7447. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Lovejoy, A.F.; Klass, D.M.; Kurtz, D.M.; Chabon, J.J.; Scherer, F.; Stehr, H.; Liu, C.L.; Bratman, S.V.; Say, C.; et al. Integrated Digital Error Suppression for Improved Detection of Circulating Tumor DNA. Nat. Biotechnol. 2016, 34, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Robert Kane, J.; Magnusson, L.P.; Baran, A.; David James, C.; Horbinski, C.; et al. Ultrasound-Mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-Bound versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
| Circulating Factor | Advantage | Disadvantage |
|---|---|---|
| Circulating tumor cell (CTC) |
|
|
| Protein |
|
|
| Extracellular vesicles (EV) |
|
|
| ctDNA |
|
|
| RNA |
|
|
| Study | Patient | Tumor Type | FUS Procedures | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Sex | Type | TERT | MGMT Promotor | # | Sonication Volume (cm3) | Estimated MI | |
| [35] | G01 | 73 | M | Glioblastoma, IDH-wildtype | C228T | n.r. | 1 | 0.13 | 0.58 |
| G02 | 58 | M | Glioblastoma, IDH-wildtype | C250T | n.r. | 1 | 0.13 | 0.38 | |
| G03 | 66 | F | Glioblastoma, IDH-wildtype | C228T | n.r. | 1 | 0.13 | 0.8 | |
| G04 | 66 | M | Glioblastoma, IDH-wildtype | C250T | n.r. | 1 | 0.13 | 0.66 | |
| G05 | 36 | M | High-grade glioma NOS, IDH-wildtype | Wild type | n.r. | 1 | 0.13 | 0.5 | |
| [33] | P1 | 49 | F | Glioblastoma, IDH-wildtype | n.r. | NA | 4 | 3.75 | n.r. |
| P2 | 52 | M | Glioblastoma, IDH-wildtype | n.r. | Methylated | 6 | 4.28 | n.r. | |
| P3 | 56 | F | Glioblastoma, IDH-wildtype | n.r. | Unmethylated | 4 | 2.48 | n.r. | |
| P4 | 35 | M | Glioblastoma, IDH-wildtype | n.r. | NA | 3 | 4.97 | n.r. | |
| P5 | 56 | F | Glioblastoma, IDH-wildtype | n.r. | Unmethylated | 4 | 6.88 | n.r. | |
| P6 | 42 | F | Astrocytoma, IDH-R132-H-mutant, grade 4 | n.r. | NA | 6 | 8.13 | n.r. | |
| P7 | 40 | F | Glioblastoma, IDH-wildtype | n.r. | Unmethylated | 4 | 9.08 | n.r. | |
| P8 | 36 | F | Glioblastoma, IDH-wildtype | n.r. | Unmethylated | 5 | 21.16 | n.r. | |
| P9 | 68 | M | Glioblastoma, IDH-wildtype | n.r. | Methylated | 2 | 5.55 | n.r. | |
| Biomarker | Specific Target | n | Fold Change | Patients with Fold Increase (%) | Data From |
|---|---|---|---|---|---|
| ctDNA | 5 | 1.5 ± 0.4 | 60 | [35] | |
| Mutant copies TERT C228T/C250T | 2 | 4.3 and 5.6 | 50 | [35] | |
| Mutant copies IDH1-R132H | 1 | 2 to 3 | 100 | [33] | |
| cfDNA: fragment size selection | 9 | 2.6 ± 1.2 | 100 | [33] | |
| 0–280 bp | 9 | 3.5 | 100 | [33] | |
| 120–280 bp | 5 | 1.3 ± 0.2 | 80 | [35] | |
| Protein | S100b | 9 | 1.4 ± 0.2 | 89 | [33] |
| Extracellular vesicles | NCAM and L1CAM | 9 | 3.2 ± 1.9 | 100 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, A.; Ixkes, A.E.; Venugopal, H.; Ries, M.G.; Lak, N.S.M.; de Vos, F.Y.F.L.; van Vuurden, D.G.; Snijders, T.J. Focused Ultrasound-Enhanced Liquid Biopsy: A Promising Diagnostic Tool for Brain Tumor Patients. Cancers 2024, 16, 1576. https://doi.org/10.3390/cancers16081576
Bakker A, Ixkes AE, Venugopal H, Ries MG, Lak NSM, de Vos FYFL, van Vuurden DG, Snijders TJ. Focused Ultrasound-Enhanced Liquid Biopsy: A Promising Diagnostic Tool for Brain Tumor Patients. Cancers. 2024; 16(8):1576. https://doi.org/10.3390/cancers16081576
Chicago/Turabian StyleBakker, Akke, Anna E. Ixkes, Hema Venugopal, Mario G. Ries, Nathalie S. M. Lak, Filip Y. F. L. de Vos, Dannis G. van Vuurden, and Tom J. Snijders. 2024. "Focused Ultrasound-Enhanced Liquid Biopsy: A Promising Diagnostic Tool for Brain Tumor Patients" Cancers 16, no. 8: 1576. https://doi.org/10.3390/cancers16081576
APA StyleBakker, A., Ixkes, A. E., Venugopal, H., Ries, M. G., Lak, N. S. M., de Vos, F. Y. F. L., van Vuurden, D. G., & Snijders, T. J. (2024). Focused Ultrasound-Enhanced Liquid Biopsy: A Promising Diagnostic Tool for Brain Tumor Patients. Cancers, 16(8), 1576. https://doi.org/10.3390/cancers16081576






