Vitamin D Concentration Among Women with Gynecological Cancers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Patients
3.2. Surgery
3.3. Vitamin D Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piatek, K.; Schepelmann, M.; Kallay, E. The Effect of Vitamin D and Its Analogs in Ovarian Cancer. Nutrients 2022, 14, 3867. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann. Intern. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public. Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef]
- Mueller, P.R.; Kershner, A.J.; Breitrick, B.I.; Keller, K.N.; Radtke, R.L.; Patel, R.J.; Gierach, K.; Arvedson, J.; Moyle-Heyrman, G.E.; Pearson, D.A. Vitamin D and docosahexaenoic acid inhibit proliferation of the ovarian cancer cell line OVCAR4. Nutr. Health 2023. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Cuellar-Partida, G.; Lu, Y.; Fashing, P.A.; Hein, A.; Burghaus, S.; Beckmann, M.W.; Lambrechts, D.; Van Nieuwenhuysen, E.; Vergote, I.; et al. Association of vitamin D levels and risk of ovarian cancer: A Mendelian randomization study. Int. J. Epidemiol. 2016, 45, 1619–1630. [Google Scholar] [CrossRef]
- Ong, J.S.; Dixon-Suen, S.C.; Han, X.; An, J.; Liyanage, U.; Dusingize, J.C.; Schumacher, J.; Gockel, I.; Bohmer, A.; Jankowski, J. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat. Commun. 2021, 12, 246. [Google Scholar] [CrossRef]
- Jiang, F.; Li, P.; Fornace, A.J., Jr.; Nicosia, S.V.; Bai, W. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J. Biol. Chem. 2003, 278, 48030–48040. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Zhao, X.; Zhang, X.; Nicosia, S.V.; Bai, W. p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J. Biol. Chem. 2004, 279, 25260–25267. [Google Scholar] [CrossRef]
- Yin, L.; Ordóñez-Mena, J.M.; Chen, T.; Schöttker, B.; Arndt, V.; Brenner, H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev. Med. 2013, 57, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, S.; Guo, B. Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2. Int. J. Mol. Sci. 2020, 21, 2334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Chen, P.; Li, X.; Li, M.; Guo, H.; Li, J.; Chu, R.; Wang, H. Polymorphisms in the vitamin D Receptor (VDR) and the risk of ovarian cancer: A meta-analysis. PLoS ONE 2013, 8, e66716. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jin, S.; Je, Y. Vitamin D Intake, Blood 25-Hydroxyvitamin D, and Risk of Ovarian Cancer: A Meta-Analysis of Observational Studies. J. Women’s Heal. 2023, 32, 561–573. [Google Scholar] [CrossRef]
- Hosono, S.; Matsuo, K.; Kajiyama, H.; Hirose, K.; Suzuki, T.; Kawase, T.; Kidokoro, K.; Nakanishi, T.; Hamajima, N.; Kikkawa, F. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur. J. Clin. Nutr. 2010, 64, 400–409. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Jamilian, M.; Bahmani, F.; Aghadavod, E.; Karamali, M.; Kashanian, M.; Asemi, Z. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Cancer 2017, 8, 58–67. [Google Scholar] [CrossRef]
- Özgü, E.; Yılmaz, N.; Başer, E.; Güngör, T.; Erkaya, S.; Yakut, H.İ. Could 25-OH vitamin D deficiency be a reason for HPV infection persistence in cervical premalignant lesions? J. Exp. Ther. Oncol. 2016, 11, 177–180. [Google Scholar]
- Wang, G.; Lei, L.; Zhao, X.; Zhang, J.; Zhou, M.; Nan, K. Calcitriol Inhibits Cervical Cancer Cell Proliferation Through Downregulation of HCCR1 Expression. Oncol. Res. 2014, 22, 301–309. [Google Scholar] [CrossRef]
- González-Duarte, R.J.; Cázares-Ordoñez, V.; Romero-Córdoba, S.; Diaz, L.; Ortiz, V.; Freyre-Gonzalez, J.A.; Hidalgo-Miranda, A.; Larrea, F.; Avila, E. Calcitriol increases Dicer expression and modifies the microRNAs signature in SiHa cervical cancer cells. Biochem. Cell Biol. 2015, 93, 376–384. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Xie, W.; Yuan, L.; Sheng, X. The role of vitamin D in ovarian cancer: Epidemiology, molecular mechanism and prevention. J. Ovarian Res. 2018, 11, 71. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Tang, J.; Kasiappan, R.; Jinwal, U.; Li, P.; Hann, S.; Nicosia, S.V.; Wu, J.; Zhang, X.; et al. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol. Cell Endocrinol. 2011, 338, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.F.; Gao, S.H.; Wang, P.; Zhang, H.M.; Liu, L.Z.; Ye, M.X.; Zhou, G.M.; Zhang, Z.L.; Li, B.Y. 1α,25(OH)2D3 Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2016, 17, 1285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Bao, J.; Li, P.; Nicosia, S.V.; Bai, W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J. Biol. Chem. 2004, 279, 53213–53221. [Google Scholar] [CrossRef]
- Thill, M.; Woeste, A.; Reichert, K.; Fisher, D.; Rody, A.; Friedrich, M.; Koster, F. Vitamin D inhibits ovarian cancer cell line proliferation in combination with celecoxib and suppresses cyclooxygenase-2 expression. Anticancer. Res. 2015, 35, 1197–1203. [Google Scholar]
- Dovnik, A.; Dovnik, N.F. Vitamin D and Ovarian Cancer: Systematic Review of the Literature with a Focus on Molecular Mechanisms. Cells 2020, 9, 335. [Google Scholar] [CrossRef]
- Lungchukiet, P.; Sun, Y.; Kasiappan, R.; Quarni, W.; Nicosia, S.V.; Zhang, X.; Bai, W. Suppression of epithelial ovarian cancer invasion into the omentum by 1α,25-dihydroxyvitamin D3 and its receptor. J. Steroid. Biochem. Mol. Biol. 2015, 148, 138–147. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Cashman, K.D. Global differences in vitamin D status and dietary intake: A review of the data. Endocr. Connect. 2022, 11, e210282. [Google Scholar] [CrossRef]
- McKenna, M.J. Differences in vitamin D status between countries in young adults and the elderly. Am. J. Med. 1992, 93, 69–77. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Pugliese, G.; Garcia-Velasquez, E.; DE Los Angeles Carignano, M.; Savastano, S.; Colao, A.; Muscogiuri, G. Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol. 2021, 46, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.; Noriega-Mejía, B.J.; González-Macías, J.; Cortes-Hernandez, U.; Garcia-Quiroz, J.; Garcia-Becerra, R.; Diaz, L. The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 8665. [Google Scholar] [CrossRef] [PubMed]
- Punnonen, R.; Gillespy, M.; Hahl, M.; Koskinen, T.; Notelovitz, M. Serum 25-OHD, vitamin A and vitamin E concentrations in healthy Finnish and Floridian women. Int. J. Vitam. Nutr. Res. 1988, 58, 37–39. [Google Scholar] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Garland, C.F.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am. J. Prev. Med. 2006, 31, 512–514. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-analysis: Circulating vitamin D and ovarian cancer risk. Gynecol. Oncol. 2011, 121, 369–375. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef]
- Grant, W.B. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003, 164, 371–377. [Google Scholar] [CrossRef]
- Lefkowitz, E.S.; Garland, C.F. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int. J. Epidemiol. 1994, 23, 1133–1136. [Google Scholar] [CrossRef]
- Freedman, D.M.; Dosemeci, M.; McGlynn, K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: A composite death certificate based case-control study. Occup. Env. Med. 2002, 59, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martinez, E.; Lazcano-Ponce, E.C.; Gonzalez Lira-Lira, G.; Escudero-De los Rios, P.; Hernandez-Avila, M. Nutritional determinants of epithelial ovarian cancer risk: A case-control study in Mexico. Oncology 2002, 63, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bakhru, A.; Mallinger, J.B.; Buckanovich, R.J.; Griggs, J.J. Casting light on 25-hydroxyvitamin D deficiency in ovarian cancer: A study from the NHANES. Gynecol. Oncol. 2010, 119, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Walentowicz-Sadlecka, M.; Grabiec, M.; Sadlecki, P.; Gotowska, M.; Walentowicz, P.; Krintus, M.; Mankowska-Cyl, A.; Sypniewska, G. 25(OH)D3 in patients with ovarian cancer and its correlation with survival. Clin. Biochem. 2012, 45, 1568–1572. [Google Scholar] [CrossRef]
- Webb, P.M.; de Fazio, A.; Protani, M.M.; Ibiebele, T.I.; Nagle, C.M.; Brand, A.H.; Blomfield, P.I.; Grant, P.; Perrin, L.C.; Neale, R.E.; et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am. J. Clin. Nutr. 2015, 102, 109–114. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359, j4761. [Google Scholar] [CrossRef]
- Sajo, E.A.; Okunade, K.S.; Olorunfemi, G.; Rabiu, K.A.; Anorlu, R.I. Serum vitamin D deficiency and risk of epithelial ovarian cancer in Lagos, Nigeria. E Cancer Med. Sci. 2020, 14, 1078. [Google Scholar] [CrossRef]
- Xu, J.; Chen, K.; Zhao, F.; Huang, D.; Zhang, H.; Fu, Z.; Xu, J.; Wu, Y.; Lin, H.; Zhou, Y.; et al. Association between vitamin D/calcium intake and 25-hydroxyvitamin D and risk of ovarian cancer: A dose-response relationship meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 417–429. [Google Scholar] [CrossRef]
- Zheng, W.; Danforth, K.N.; Tworoger, S.S.; Goodman, M.T.; Arslan, A.A.; Patel, A.V.; McCullough, M.L.; Weinstein, S.J.; Kolonel, L.N.; Purdue, M.P.; et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 70–80. [Google Scholar] [CrossRef]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Is ultraviolet B irradiance inversely associated with incidence rates of endometrial cancer: An ecological study of 107 countries. Prev. Med. 2007, 45, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UVB irradiance and smoking. Int. J. Cancer 2007, 120, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Lindqvist, P.G.; Geppert, B.; Olsson, H. A population-based cohort study on sun habits and endometrial cancer. Br. J. Cancer 2009, 101, 537–540. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Bandera, E.V.; Moore, D.F.; Kushi, L.H. Vitamin D and calcium intake in relation to risk of endometrial cancer: A systematic review of the literature. Prev. Med. 2008, 46, 298–302. [Google Scholar] [CrossRef]
- Zeleniuch-Jacquotte, A.; Gallicchio, L.; Hartmuller, V.; Helzlsouer, K.J.; McCullough, M.L.; Setiawan, V.W.; Shu, X.O.; Weinstein, S.J.; Weiss, J.M.; Arslan, A.A. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 36–46. [Google Scholar] [CrossRef]
- Yu, W.; Cline, M.; Maxwell, L.G.; Berrigan, D.; Rodriguez, G.; Warri, A.; Hilakivi-Clarke, L. Dietary vitamin D exposure prevents obesity-induced increase in endometrial cancer in Pten+/− mice. Cancer Prev. Res. 2010, 3, 1246–1258. [Google Scholar] [CrossRef]
- Dong, H.; Chen, S.; Liang, X.; Cai, Q.; Zhang, X.; Xie, J.; Sun, Z. Vitamin D and Its Receptors in Cervical Cancer. J. Cancer 2024, 15, 926–938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vuolo, L.; Di Somma, C.; Faggiano, A.; Colao, A. Vitamin D and cancer. Front. Endocrinol. 2012, 3, 58. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Mahmoodi, S.; Samimi, M.; Gilasi, H.R.; Bahmani, F.; Soltani, A.; Esfahani, M.S.; Asemi, Z. Long-Term Vitamin D Supplementation and the Effects on Recurrence and Metabolic Status of Cervical Intraepithelial Neoplasia Grade 2 or 3: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Nutr. Metab. 2018, 72, 151–160. [Google Scholar] [CrossRef]
- Avila, E.; García-Becerra, R.; Rodríguez-Rasgado, J.A.; Diaz, L.; Ordaz-Rosado, D.; Zugel, U.; Steinmeyer, A.; Barrera, D.; Halhali, A.; Larrea, F.; et al. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010, 30, 2667–2672. [Google Scholar] [PubMed]
- Christopher, K.L.; Wiggins, A.T.; Van Meter, E.M.; Means, R.T., Jr.; Hayslip, J.W.; Roach, J.P. Differences in vitamin D nutritional status between newly diagnosed cancer patients from rural or urban settings in Kentucky. Nutr. Cancer 2013, 65, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Lamberg-Allardt, C.; Kärkkäinen, M.; Outila, T.; Salamatullah, Q.; Shamim, A.A. Vitamin D deficiency: A concern in premenopausal Bangladeshi women of two socio-economic groups in rural and urban region. Eur. J. Clin. Nutr. 2002, 56, 51–56. [Google Scholar] [CrossRef]
- Nurbazlin, M.; Chee, W.S.; Rokiah, P.; Tan, A.T.; Chew, Y.Y.; Nusaibach, A.R.; Chan, S.P. Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 391–399. [Google Scholar] [CrossRef]
- Maddah, M.; Sharami, S.H.; Neyestani, T.R. Vitamin D insufficiency among postmenopausal women in urban and rural areas in Guilan, Northern Iran. J. Nutr. Elder. 2009, 28, 386–393. [Google Scholar] [CrossRef]
- Bojar, I.; Raczkiewicz, D.; Sarecka-Hujar, B. Depression, Metabolic Syndrome, Serum TSH, and Vitamin D Concentrations in Rural and Urban Postmenopausal Women. Medicina 2020, 56, 511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anwar, S.; Iqbal, M.P.; Azam, I.; Habib, A.; Bhutta, S.; Soofi, S.B.; Bhutta, Z.A. Urban and rural comparison of vitamin D status in Pakistani pregnant women and neonates. J. Obs. Gynaecol. 2016, 36, 318–323. [Google Scholar] [CrossRef]
- Praveen, P.A.; Singh, A.; Lakshmy, R.; Amarchand, R.; Berry, P.; Krishnan, A.; Prabhakaran, D.; Tandon, N.; Roy, A. Prevalence and Correlates of Vitamin D Deficiency among Adult Population in Urban and Rural Areas of the National Capital Region of Delhi, India. WHO South-East Asia J. Public Heal. 2023, 12, 104–109. [Google Scholar] [CrossRef]
- Chávez-Courtois, M.; Godínez-Martínez, E.; Muñoz-Manrique, C.; Negrete-Martínez, V.; González-Leyva, C.P.; Tolentino-Dolores, M.; Suárez-Rico, B.; Estrada-Gutierrez, G.; Perichart-Perera, O. Vitamin D Status and Its Determinants in Mexican Pregnant Women from a Rural and an Urban Area: A Comparative Study. Int. J. Environ. Res. Public Health 2021, 18, 4571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, F.; Wei, H.; Wang, K.; Tan, L.; Zhang, W.; Ding, L.; Liu, T.; Shan, Z.; Zhu, M. High prevalence of vitamin D deficiency and influencing factors among urban and rural residents in Tianjin, China. Arch. Osteoporos. 2018, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Zębalski, M.A.; Parysek, K.; Krzywon, A.; Nowosielski, K. LUNA EMG as a Marker of Adherence to Prehabilitation Programs and Its Effect on Postoperative Outcomes among Patients Undergoing Cytoreductive Surgery for Ovarian Cancer and Suspected Ovarian Tumors. Cancers 2024, 16, 2493. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, L.; Jia, S.; Lin, T.; Liu, G.; Yue, J.; Huang, X. Effects of Circulating 25(OH)D Status in Advanced Colorectal Cancer Patients Undergoing Chemotherapy: A Systematic Review. Anticancer. Res. 2021, 41, 5903–5912. [Google Scholar] [CrossRef] [PubMed]
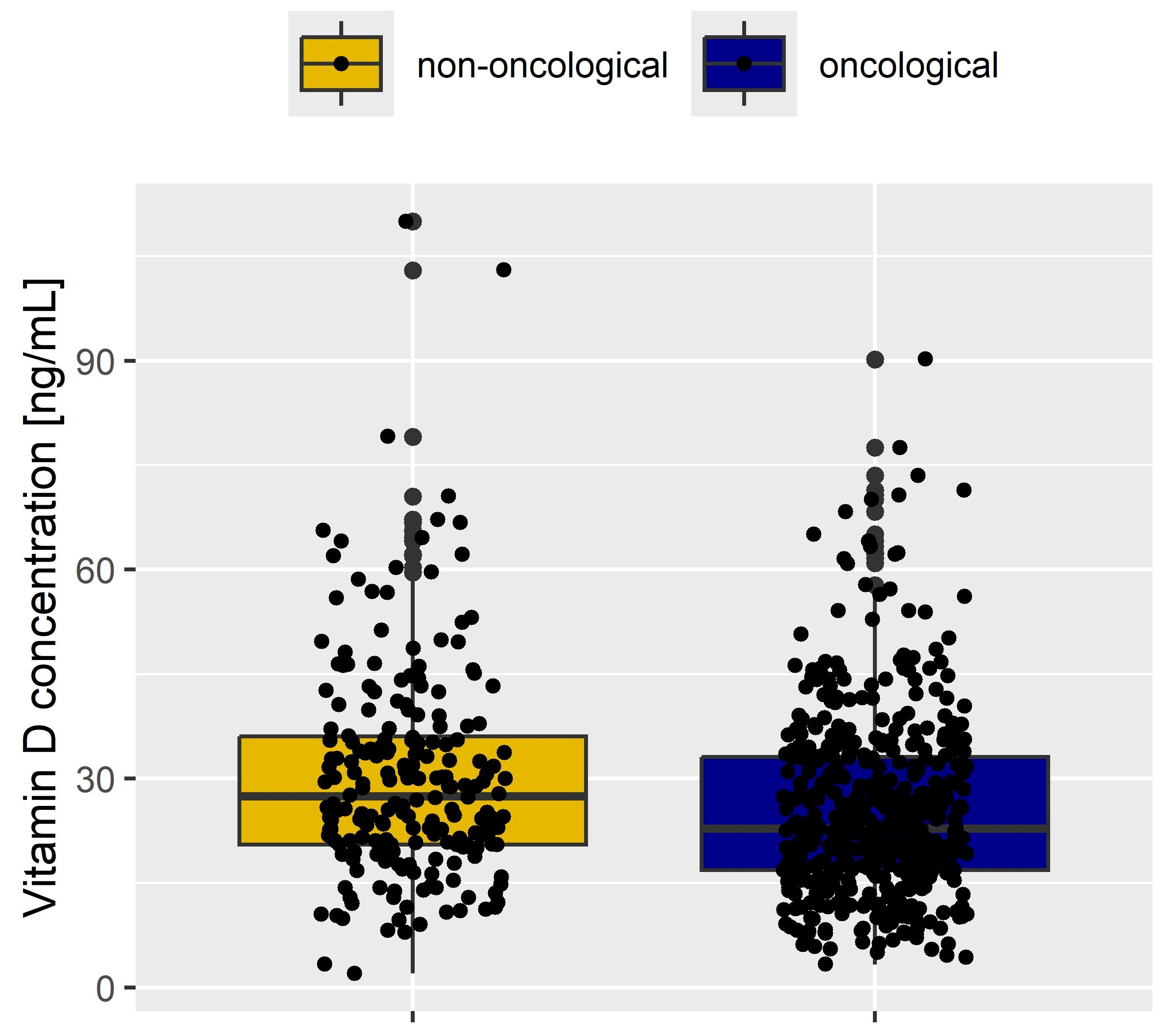
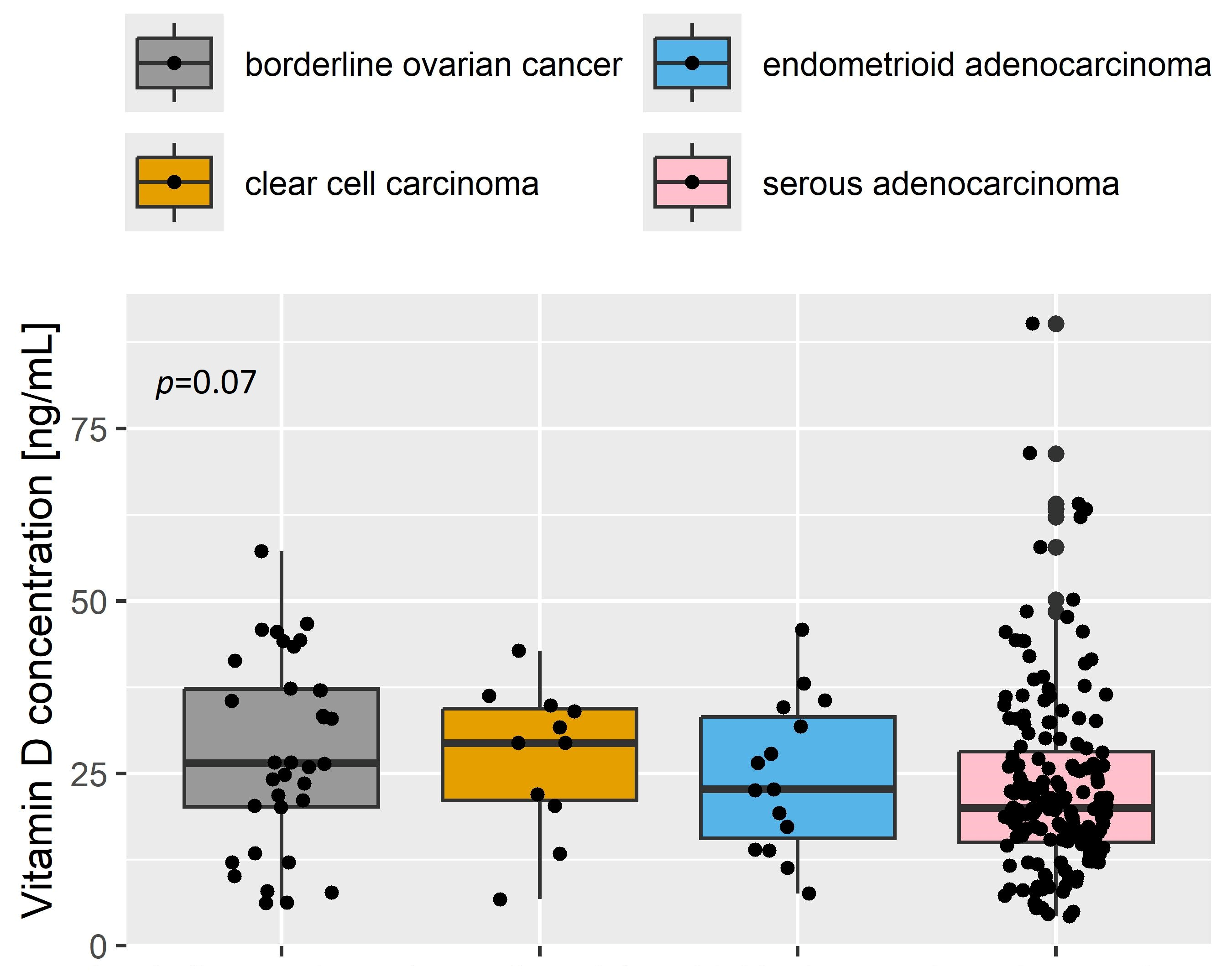
| Characteristic | Oncological Patients (n Total = 472) | Non-Oncological Patients (n Total = 214) | p-Value |
|---|---|---|---|
| Age [years], (SD) | 56 (14) | 56 (14) | p > 0.05 |
| BMI [kg/m2], (SD) | 27 (6) | 27 (6) | p > 0.05 |
| City inhabitant, n/n total (%) | 407/472 (86) | 189/214 (88) | p > 0.05 |
| Diabetes, n/n total (%) | 86/472 (18) | 26/214 (12) | p > 0.05 |
| Hypertension, n/n total (%) | 197/472 (42) | 81/214 (38) | p > 0.05 |
| Ischemic heart disease, n/n total (%) | 33/472 (7) | 13/214 (6) | p > 0.05 |
| Smoking, n/n total (%) | 103/472 (22) | 47/214 (22) | p > 0.05 |
| Use of oral contraceptives, n/n total (%) | 23/472 (4.8) | 13/214 (6.1) | p > 0.05 |
| Characteristic | Number of Patients |
|---|---|
| Oncological patients, n/n total (%) | 472/686 (68.8) |
| Ovarian cancer, n/n total of oncological patients (%) | 283/472 (60) |
| Endometrial cancer, n/n total of oncological patients (%) | 135/472 (29) |
| Cervical cancer, n/n total of oncological patients (%) | 54/472 (11) |
| Non-oncological patients, n/n total (%) | 214/686 (31.2) |
| Uterine fibroid, n/n total of non-oncologic patients (%) | 52/214 (24.3) |
| Benign ovarian lesions, n/n total of non-oncologic patients (%) | 162/214 (75.7) |
| Serous adenoma, n/n total of benign ovarian lesions (%) | 22/162 (13.6) |
| Mucous adenoma, n/n total of benign ovarian lesions (%) | 12/162 (7.4) |
| Teratoma, n/n total of benign ovarian lesions (%) | 11/162 (6.8) |
| Fibroma, n/n total of benign ovarian lesions (%) | 3/162 (1.9) |
| Thecoma, n/n total of benign ovarian lesions (%) | 2/162 (1.2) |
| Benign Brenner tumor, n/n total of benign ovarian lesions (%) | 3/162 (1.9) |
| Sertoli-Leydig cell tumor, n/n total of benign ovarian lesions (%) | 2/162 (1.2) |
| Endometrial cyst, n/n total of benign ovarian lesions (%) | 16/162 (9.9) |
| Hemorrhagic cyst, n/n total of benign ovarian lesions (%) | 14/162 (8.6) |
| Serous cyst, n/n total of benign ovarian lesions (%) | 77/162 (47.5) |
| Type of Cancer vs. Non-Oncological Patients | Number of Patients | Vitamin D Concentration (Median [IQR], ng/mL | p-Value |
|---|---|---|---|
| Ovarian cancer vs. non-oncological patients | 283 | 22 (16, 32) | |
| vs. | vs. | <0.01 | |
| 214 | 28 (21, 36) | ||
| Endometrial cancer vs. non-oncological patients | 135 | 24 (18, 35) | |
| vs. 214 | vs. 28 (21, 36) | <0.01 | |
| Cervical cancer vs. non-oncological patients | 54 | 26 (20, 31) | |
| vs. 214 | vs. 28 (21, 36) | 0.1 |
| Characteristic | Number of Patients | Non-Oncological Patients from Countryside, n = 25 | Oncological Patients from Countryside, n = 65 | Non-Oncological Patients from City, n = 189 | Oncological Patients from City, n = 408 | p-Value |
|---|---|---|---|---|---|---|
| Vitamin D concentration, median [IQR] (ng/mL) | 686 | 29 (22, 36) | 24 (19, 32) | 27 (21, 36) | 23 (17, 33) | <0.001 |
| Characteristic | Beginning of Prehabilitation Program | End of Prehabilitation Program | Change * | p-Value |
|---|---|---|---|---|
| Vitamin D concentration, median [IQR] (ng/mL) | 23 (17, 33) | 35 (28, 46) | 8 (1, 18) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zębalski, M.A.; Zębalska, P.; Krzywon, A.; Nowosielski, K. Vitamin D Concentration Among Women with Gynecological Cancers. Cancers 2025, 17, 1987. https://doi.org/10.3390/cancers17121987
Zębalski MA, Zębalska P, Krzywon A, Nowosielski K. Vitamin D Concentration Among Women with Gynecological Cancers. Cancers. 2025; 17(12):1987. https://doi.org/10.3390/cancers17121987
Chicago/Turabian StyleZębalski, Marcin Adam, Patrycja Zębalska, Aleksandra Krzywon, and Krzysztof Nowosielski. 2025. "Vitamin D Concentration Among Women with Gynecological Cancers" Cancers 17, no. 12: 1987. https://doi.org/10.3390/cancers17121987
APA StyleZębalski, M. A., Zębalska, P., Krzywon, A., & Nowosielski, K. (2025). Vitamin D Concentration Among Women with Gynecological Cancers. Cancers, 17(12), 1987. https://doi.org/10.3390/cancers17121987







