Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis
Simple Summary
Abstract
1. Introduction
- Primary Objective: To retrospectively evaluate the association between CLT and thyroid tumour malignancy, acknowledging the study’s constraints in establishing causality.
- Secondary Objective: To identify potential risk factors (age, sex, tumour size, single/multifocal presentation, Bethesda category in FNAB) for thyroid tumour malignancy in patients with CLT, while recognizing the limitations of retrospective analysis.
- Tertiary Objective: To assess the prevalence and anatomical distribution of lymph node metastases (LNM) in patients with coexisting CLT and thyroid cancer, within the confines of the study design.
2. Materials and Methods
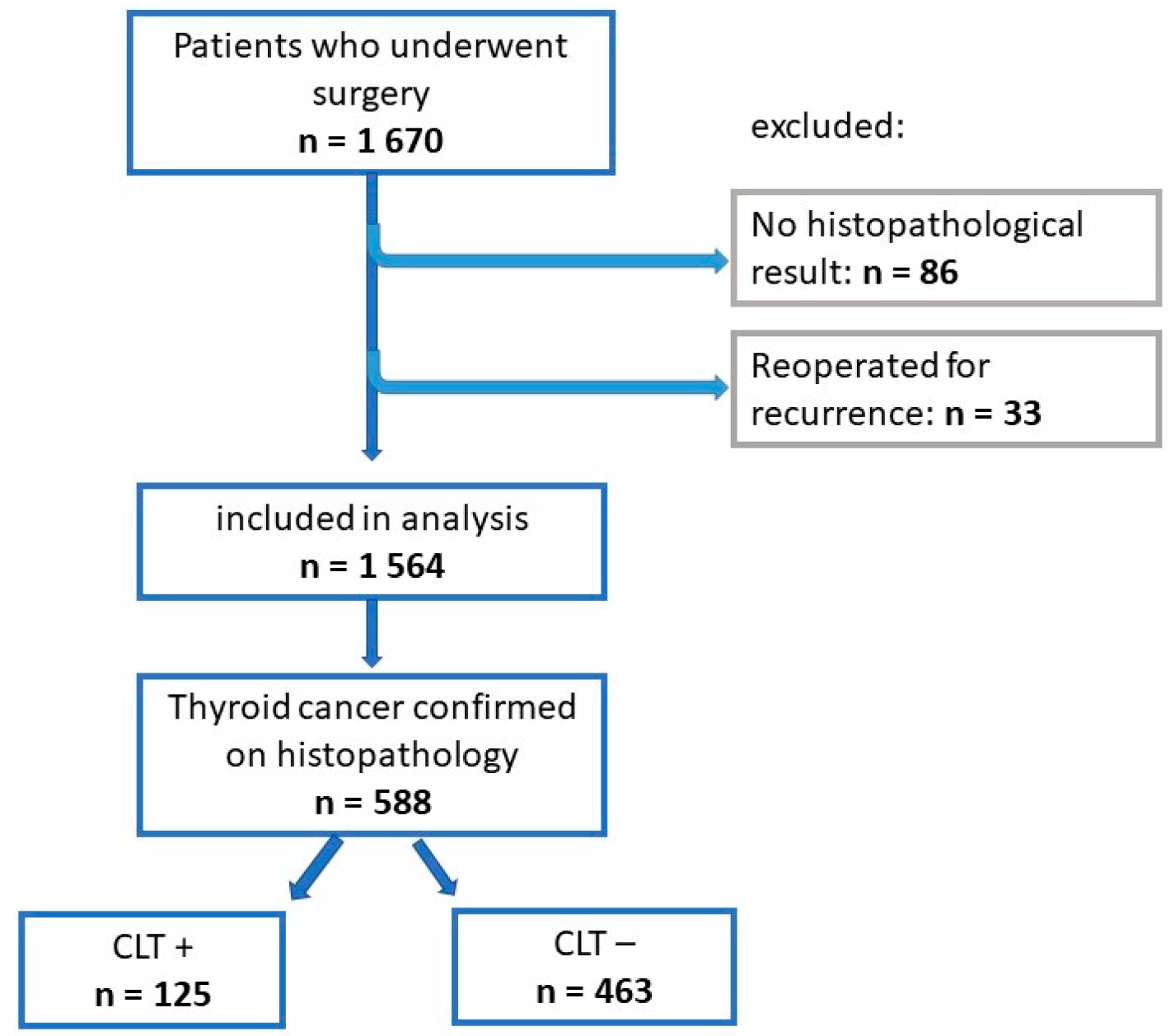
2.1. Study Group
2.2. Diagnosis and Evaluation
2.3. Statistical Analysis
3. Results
3.1. Univariate Analysis of the Entire Study Group (n = 1564)
3.2. Univariate Analysis of Patients with Malignancy CLT-Positive Compared to Malignancy CLT-Negative
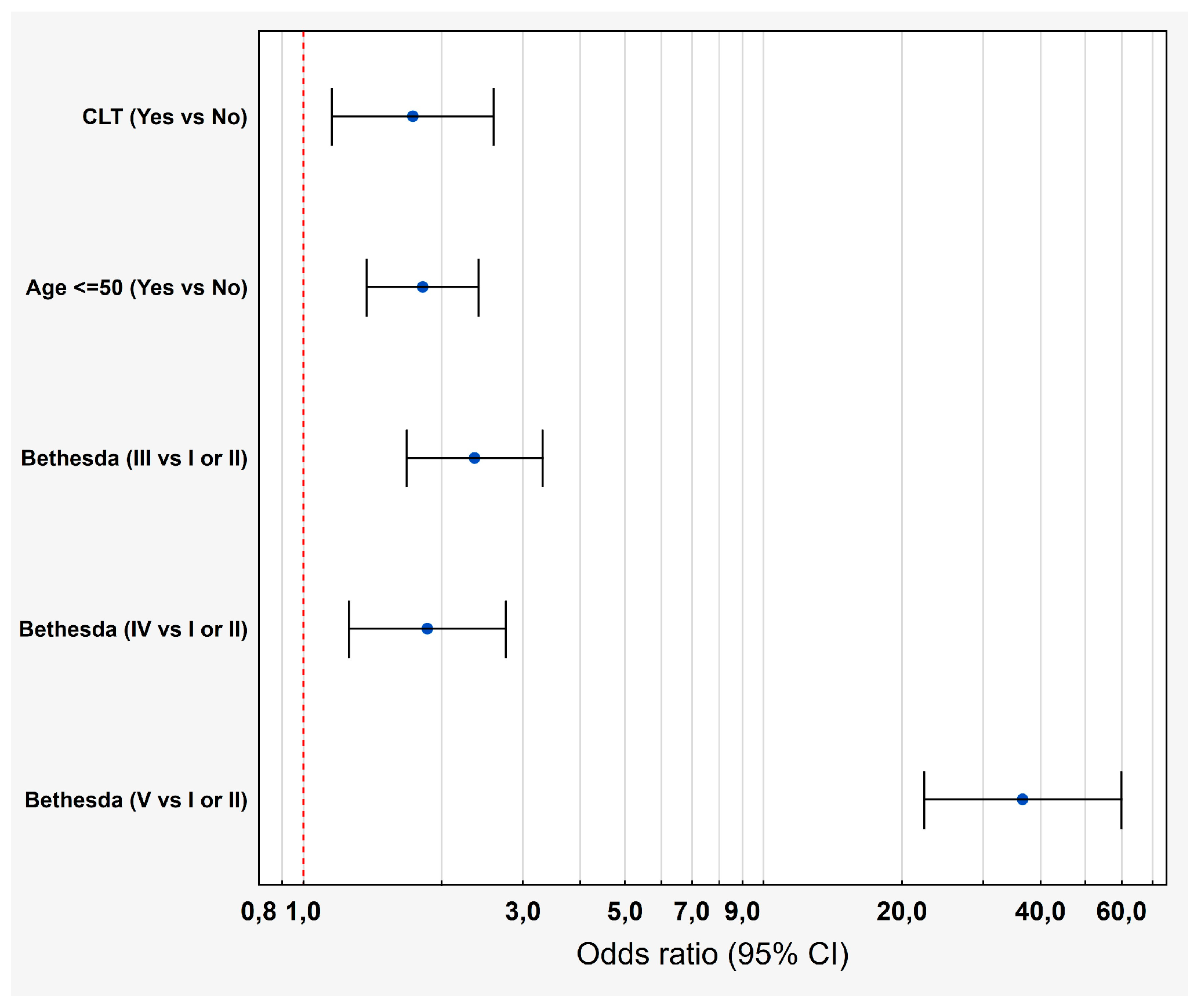
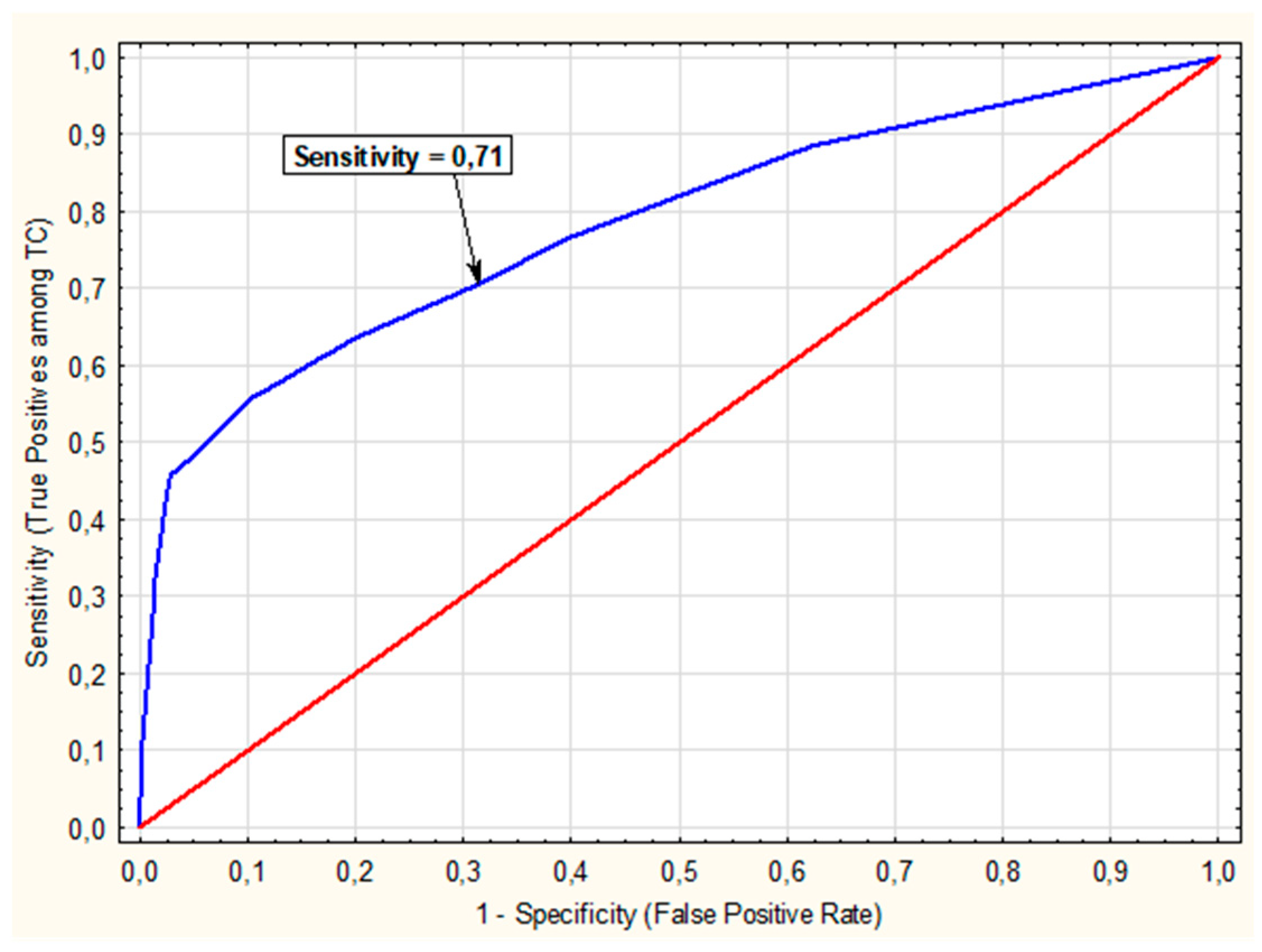
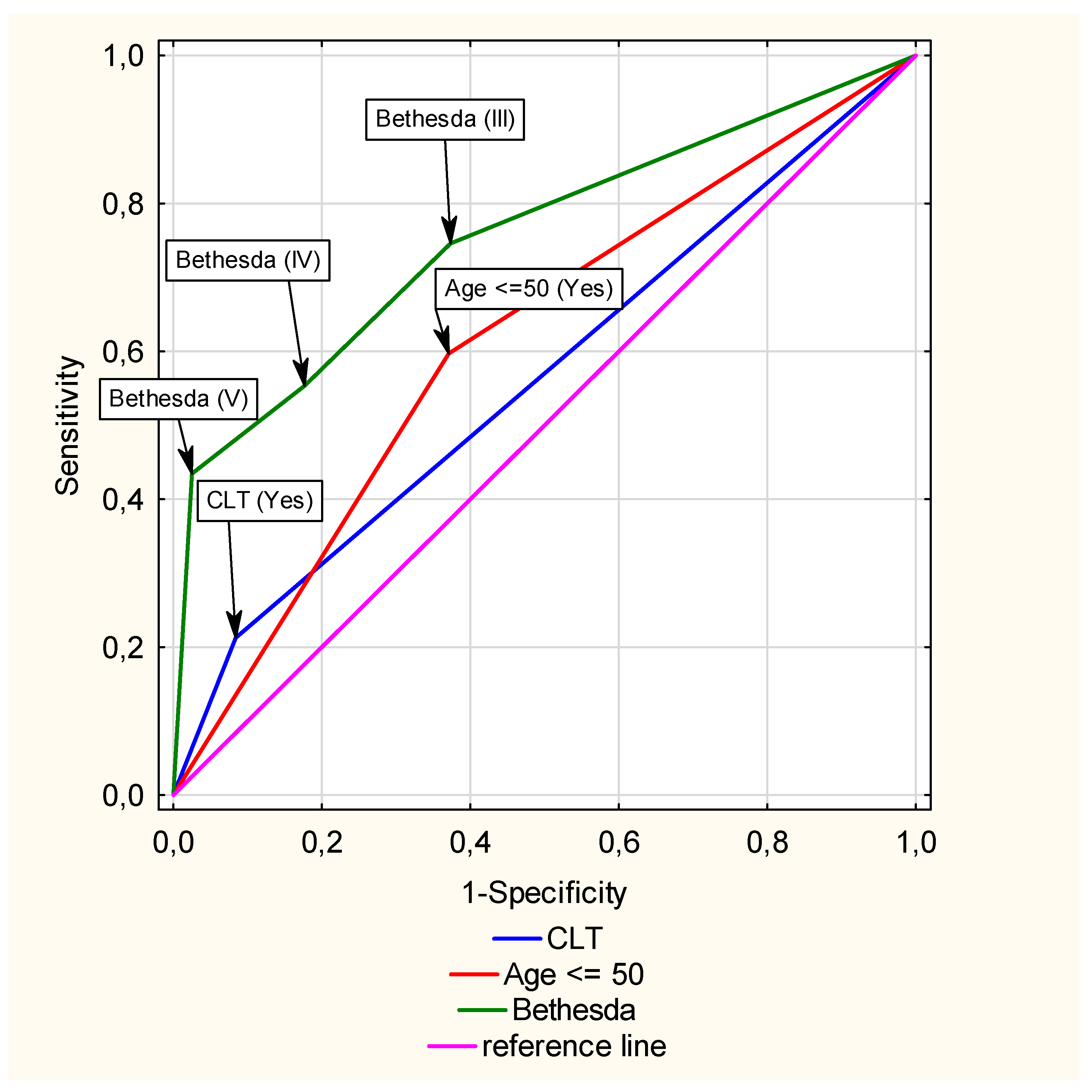
3.3. Multivariate Analysis of the Impact of CLT on the Risk of TC
3.4. Multivariate Analysis of the Impact of CLT on the Presence and Location of Lymph Node Metastases (LNM)
3.5. Multivariate Analysis of the Impact of CTL on PTC Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef]
- Tamimi, D.M. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int. J. Surg. Pathol. 2002, 10, 141–146. [Google Scholar] [CrossRef]
- Kebebew, E.; Treseler, P.A.; Ituarte, P.H.; Clark, O.H. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J. Surg. 2001, 25, 632–637. [Google Scholar] [CrossRef]
- Jackson, D.; Handelsman, R.S.; Farrá, J.C.; Lew, J.I. Increased Incidental Thyroid Cancer in Patients with Subclinical Chronic Lymphocytic Thyroiditis. J. Surg. Res. 2020, 245, 115–118. [Google Scholar] [CrossRef]
- Antonaci, A.; Consorti, F.; Mardente, S.; Giovannone, G. Clinical and biological relationship between chronic lymphocytic thyroiditis and papillary thyroid carcinoma. Oncol. Res. 2009, 17, 495–503. [Google Scholar] [CrossRef]
- Lee, I.; Kim, H.K.; Soh, E.Y.; Lee, J. The Association Between Chronic Lymphocytic Thyroiditis and the Progress of Papillary Thyroid Cancer. World J. Surg. 2020, 44, 1506–1513. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H. Autoimmune Thyroid Disease and Differentiated Thyroid Carcinoma: A Review of the Mechanisms That Explain an Intriguing and Exciting Relationship. World J. Oncol. 2024, 15, 14–27. [Google Scholar] [CrossRef]
- Consorti, F.; Loponte, M.; Milazzo, F.; Potasso, L.; Antonaci, A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto’s thyroiditis. Eur. Surg. Res. 2010, 45, 333–337. [Google Scholar] [CrossRef]
- Konturek, A.; Barczyński, M.; Wierzchowski, W.; Stopa, M.; Nowak, W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch. Surg. 2013, 398, 389–394. [Google Scholar] [CrossRef]
- Abbasgholizadeh, P.; Naseri, A.; Nasiri, E.; Sadra, V. Is Hashimoto thyroiditis associated with increasing risk of thyroid malignancies? A systematic review and meta-analysis. Thyroid. Res. 2021, 14, 26. [Google Scholar] [CrossRef]
- Resende de Paiva, C.; Grønhøj, C.; Feldt-Rasmussen, U.; von Buchwald, C. Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol. 2017, 7, 53. [Google Scholar] [CrossRef]
- Iskra, I.; Tomaš, M.I.; Crnčić, T.B.; Kukić, E.; Hadžisejdić, I.; Avirović, M.; Girotto, N. Two lymphoma histotypes and papillary thyroid carcinoma coexisting on Hashimoto ground: A case report and review of the literature. Diagn. Pathol. 2024, 19, 52. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, Z.; Xiao, Y.; He, Y.; Chen, Y.; Huang, W.; Lu, L. The Predictive Value of ACR TI-RADS Classification for Central Lymph Node Metastasis of Papillary Thyroid Carcinoma: A Retrospective Study. Int. J. Endocrinol. 2022, 2022, 4412725. [Google Scholar] [CrossRef]
- Wu, Y.; Rao, K.; Liu, J.; Han, C.; Gong, L.; Chong, Y.; Liu, Z.; Xu, X. Machine Learning Algorithms for the Prediction of Central Lymph Node Metastasis in Patients with Papillary Thyroid Cancer. Front. Endocrinol. 2020, 11, 577537. [Google Scholar] [CrossRef]
- Min, Y.; Huang, Y.; Wei, M.; Wei, X.; Chen, H.; Wang, X.; Chen, J.; Xiang, K.; Feng, Y.; Yin, G. Preoperatively Predicting the Central Lymph Node Metastasis for Papillary Thyroid Cancer Patients with Hashimoto’s Thyroiditis. Front. Endocrinol. 2021, 12, 713475. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, D.; Chen, J.; Su, W.; Sun, L.; Jiang, J.; Wang, J.; Zhou, Q. Predicting central cervical lymph node metastasis in papillary thyroid carcinoma with Hashimoto’s thyroiditis: A practical nomogram based on retrospective study. PeerJ. 2024, 12, e17108. [Google Scholar] [CrossRef]
- Lin, Y.; Cui, N.; Li, F.; Wang, Y.; Wang, B. The model for predicting the central lymph node metastasis in cN0 papillary thyroid microcarcinoma with Hashimoto’s thyroiditis. Front. Endocrinol. 2024, 15, 1330896. [Google Scholar] [CrossRef]
- Heo, D.B.; Won, H.R.; Tae, K.; Kang, Y.E.; Jeon, E.; Ji, Y.B.; Chang, J.W.; Choi, J.Y.; Yu, H.W.; Ku, E.J.; et al. Clinical impact of coexistent chronic lymphocytic thyroiditis on central lymph node metastasis in low- to intermediate-risk papillary thyroid carcinoma: The MASTER study. Surgery 2024, 175, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Pan, W.; Peng, L. Association between Hashimoto thyroiditis and clinical outcomes of papillary thyroid carcinoma: A meta-analysis. PLoS ONE 2022, 17, e0269995. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, H.; Qian, J.; Liu, Y.; Huang, Y.; Wang, X.; Liu, S.; Xu, Z.; Liu, J. Prevalence of Hashimoto Thyroiditis in Adults with Papillary Thyroid Cancer and Its Association with Cancer Recurrence and Outcomes. JAMA Netw. Open 2021, 4, e2118526. [Google Scholar] [CrossRef] [PubMed]
- Lai, V.; Yen, T.W.; Rose, B.T.; Fareau, G.G.; Misustin, S.M.; Evans, D.B.; Wang, T.S. The Effect of Thyroiditis on the Yield of Central Compartment Lymph Nodes in Patients with Papillary Thyroid Cancer. Ann. Surg. Oncol. 2015, 22, 4181–4186. [Google Scholar] [CrossRef]
- Harmantepe, A.T.; Ozdemir, K.; Bayhan, Z.; Kocer, B. The Underestimated Impact of Hashimoto Thyroiditis on Thyroid Papillary Carcinoma. Updates Surg. 2024, 76, 1085–1089. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Kim, H.J.; Lee, J.W.; Kim, J.M.; Koo, B.S. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur. Arch. Otorhinolaryngol. 2012, 269, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Wu, T.; Yang, N.; Yin, Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1021–1026. [Google Scholar] [CrossRef]
- Dedivitis, R.A.; Matos, L.L.; Souza, F.G.S.; Bogado Ortiz, J.L. Association between Thyroiditis and Multifocality in Papillary Thyroid Carcinoma. Int. Arch. Otorhinolaryngol. 2021, 25, e219–e223. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xie, X.J.; Xia, Q.; Wu, Y.J. Indicators of multifocality in papillary thyroid carcinoma concurrent with Hashimoto’s thyroiditis. Am. J. Cancer Res. 2019, 9, 1786–1795. [Google Scholar] [PubMed] [PubMed Central]
- Dong, S.; Xia, Q.; Wu, Y.J. High TPOAb Levels (>1300 IU/mL) Indicate Multifocal PTC in Hashimoto’s Thyroiditis Patients and Support Total Thyroidectomy. Otolaryngol. Head. Neck Surg. 2015, 153, 20–26. [Google Scholar] [CrossRef]
- Paparodis, R.D.; Karvounis, E.; Bantouna, D.; Chourpiliadis, C.; Chourpiliadi, H.; Livadas, S.; Imam, S.; Jaume, J.C. Incidentally Discovered Papillary Thyroid Microcarcinomas Are More Frequently Found in Patients with Chronic Lymphocytic Thyroiditis Than with Multinodular Goiter or Graves’ Disease. Thyroid 2020, 30, 531–535. [Google Scholar] [CrossRef]
- Lopes, N.M.D.; Lens, H.H.M.; da Silva Brito, W.A.; Bianchi, J.K.; Marinello, P.C.; Cecchini, R.; Armani, A.; Cecchini, A.L. Role of papillary thyroid carcinoma patients with Hashimoto thyroiditis: Evaluation of oxidative stress and inflammatory markers. Clin. Transl. Oncol. 2022, 24, 2366–2378. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Chung, Y.J.; Kim, H.S. Malignancy Rate of Bethesda Class III Thyroid Nodules Based on the Presence of Chronic Lymphocytic Thyroiditis in Surgical Patients. Front. Endocrinol. 2021, 12, 745395. [Google Scholar] [CrossRef] [PubMed]
- Vaghaiwalla, T.M.; DeTrolio, V.; Saghira, C.; Akcin, M.; Chen, C.B.; McGillicuddy, C.M.; Lew, J.I. Impact of chronic lymphocytic thyroiditis on the diagnostic and intraoperative management of papillary thyroid cancer. Surgery 2025, 179, 108937. [Google Scholar] [CrossRef]
- Kim, W.W.; Ha, T.K.; Bae, S.K. Clinical implications of the BRAF mutation in papillary thyroid carcinoma and chronic lymphocytic thyroiditis. J. Otolaryngol. Head. Neck Surg. 2018, 47, 4. [Google Scholar] [CrossRef] [PubMed]
- Janicki, L.; Patel, A.; Jendrzejewski, J.; Hellmann, A. Prevalence and Impact of BRAF mutation in patients with concomitant papillary thyroid carcinoma and Hashimoto’s thyroiditis: A systematic review with meta-analysis. Front. Endocrinol. 2023, 14, 1273498. [Google Scholar] [CrossRef]
- EUROCRINE. Available online: https://eurocrine.eu (accessed on 28 April 2024).
- Jung, C.K.; Bychkov, A.; Kakudo, K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol. Metab. 2022, 37, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Uhliarova, B.; Hajtman, A. Hashimoto’s thyroiditis—An independent risk factor for papillary carcinoma. Braz. J. Otorhinolaryngol. 2018, 84, 729–735. [Google Scholar] [CrossRef]
- Cinar, I.; Sengul, I. Coexistence of Hashimoto’s thyroiditis and papillary thyroid carcinoma revisited in thyroidology, an experience from an endemic region: Fad or future? Rev. Assoc. Med. Bras. 2024, 70, e20231380. [Google Scholar] [CrossRef]
- Dailey, M.E.; Lindsay, S.; Skahen, R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch. Surg. 1955, 70, 291–297. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U. Hashimoto’s thyroiditis as a risk factor for thyroid cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 364–371. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, H. Papillary thyroid carcinoma with Hashimoto’s thyroiditis: Impact and correlation. Front. Endocrinol. 2025, 16, 1512417. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yin, D.-T.; Wang, N.; Li, N.; Dong, G.; Peng, M.-F. Identifying and analyzing the key genes shared by papillary thyroid carcinoma and Hashimoto’s thyroiditis using bioinformatics methods. Front. Endocrinol. 2023, 14, 1140094. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Feng, Q.; Li, Q.; Ge, M. Mutation of Hashimoto’s thyroiditis and papillary thyroid carcinoma related genes and the screening of candidate genes. Front. Oncol. 2021, 11, 813802. [Google Scholar] [CrossRef] [PubMed]
- Tsyryuk, O.; Falalyeyeva, T.; Chernenko, O.; Shapochka, D.; Nechay, O.; Selesnov, O.; Penchuk, Y.; Sulaieva, O.; Kobyliak, N.; Maievskyi, O. Mechanisms of the impact of Hashimoto thyroiditis on papillary thyroid carcinoma progression: Relationship with the tumor immune microenvironment. Endocrinol. Metab. 2020, 35, 443–455. [Google Scholar] [CrossRef]
- Yang, I.; Yu, J.M.; Chung, H.S.; Kim, Y.J.; Roh, Y.K.; Choi, M.K.; Park, S.H.; Park, Y.J.; Moon, S. Hashimoto Thyroiditis and Mortality in Patients with Differentiated Thyroid Cancer: The National Epidemiologic Survey of Thyroid Cancer in Korea and Meta-Analysis. Endocrinol. Metab. 2024, 39, 140–151. [Google Scholar] [CrossRef]
- Xue, X.; Wu, D.; Yao, H.; Wang, K.; Liu, Z.; Qu, H. Mechanisms underlying the promotion of papillary thyroid carcinoma occurrence and progression by Hashimoto’s thyroiditis. Front. Endocrinol. 2025, 16, 1551271. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, G.; Huo, D.; Su, Y.; Jin, Q.; Lu, Y.; Sun, Y.; Zhang, D.; Chen, X. Impact of Hashimoto’s thyroiditis on the tumor microenvironment in papillary thyroid cancer: Insights from single-cell analysis. Front. Endocrinol. 2024, 15, 1339473. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, L.; Wang, J.; Li, F.; Xie, L. Cytokine production of papillary thyroid carcinoma coexisting with Hashimoto’s thyroiditis. Int. J. Clin. Exp. Pathol. 2017, 10, 9567–9574. [Google Scholar]
- Zhang, X.; Tian, L.; Teng, D.; Teng, W. The Relationship between Thyrotropin Serum Concentrations and Thyroid Carcinoma. Cancers 2023, 15, 5017. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Dionigi, G.; Sun, H. The relationship between subclinical hypothyroidism and invasive papillary thyroid cancer. Front. Endocrinol. 2023, 14, 1294441. [Google Scholar] [CrossRef]
- Boelaert, K. The association between serum TSH concentration and thyroid cancer. Endocr. Relat. Cancer 2009, 16, 1065–1072. [Google Scholar] [CrossRef]
- Fiore, E.; Rago, T.; Provenzale, M.A.; Scutari, M.; Ugolini, C.; Basolo, F.; Di Coscio, G.; Berti, P.; Grasso, L.; Elisei, R.; et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: Thyroid autonomy may play a protective role. Endocr. Relat. Cancer 2009, 16, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, Y.; Jiang, Y.; Fu, R.; Wang, Y.; Zhang, Q. The associations between thyroid-related hormones and the risk of thyroid cancer: An overall and dose-response meta-analysis. Front. Endocrinol. 2022, 13, 992566. [Google Scholar] [CrossRef]
- Shahrokh, M.; Alsultan, M.; Kabalan, Y. The relationship between papillary thyroid carcinoma and preoperative TSH level: A cross-sectional study from Syria. Medicine 2023, 102, e34283. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, M.Y.; Jin, S.M.; Lee, S.H. The association between serum concentration of thyroid hormones and thyroid cancer: A cohort study. Endocr. Relat. Cancer 2022, 29, 635–644. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, L.; Wang, X.; Shi, X. The relationship between thyroid peroxidase antibody and differentiated thyroid cancer: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1349041. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, X.; Cheng, F.; Zhou, B.; Zhu, X.; Zhu, L. Correlation between thyroid autoantibodies and the risk of thyroid papillary carcinoma. Gland. Surg. 2020, 9, 95055. [Google Scholar] [CrossRef]
- Livadas, S.; Karvounis, E.; Bantouna, D.; Zoupas, I.; Paparodis, R.; Imam, S.; Angelopoulos, N.; Jaume, J.C. Elevated preoperative TPO ab titers decrease risk for DTC in a linear fashion: A retrospective analysis of 1,635 cases. J. Clin. Endocrinol. Metab. 2023, 109, e347–e355. [Google Scholar] [CrossRef]
- Selek, A.; Cetinarslan, B.; Tarkun, I.; Canturk, Z.; Ustuner, B.; Akyay, Z. Thyroid autoimmunity: Is really associated with papillary thyroid carcinoma? Eur. Arch. Otorhinolaryngol. 2017, 274, 1677–1681. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Lu, G.; Li, T.; Zhang, Y.; Yu, N.; Gao, Y.; Gao, Y.; Guo, X. Clinical Relationship Between IgG4-Positive Hashimoto’s Thyroiditis and Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2016, 101, 1516–1524. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Chen, D.; Ma, C. Correlation analysis of Hashimoto’s thyroiditis with papillary thyroid carcinoma occurrence and its central lymph node metastasis: A single center experience. Front. Endocrinol. 2025, 15, 1420998. [Google Scholar] [CrossRef]
| Parameters | CLT (−) | CLT (+) | Total | p-Value |
|---|---|---|---|---|
| N = 1357 (86.76%) | N = 207 (13.23%) | N = 1564 | ||
| Age (years) £ | ||||
| Mean ± SD | 53.1 ± 14.6 | 47.6 ± 15.0 | 1564 | <0.001 |
| Median (Q1–Q3) | 54 | 46 | ||
| (41.0–65.0) | (36.0–59.0) | |||
| N | 1357 | 207 | ||
| Gender n (%) § | ||||
| Female | 1094 (80.62%) | 194 (93.72%) | 1288 | <0.001 |
| Male | 263 (19.38%) | 13 (6.28%) | 276 | |
| Bethesda category § | ||||
| I | 51 (3.76%) | 3 (1.45%) | 54 | <0.001 |
| II | 551 (40.60%) | 51 (24.64%) | 602 | |
| III | 224 (16.51%) | 29 (14.01%) | 253 | |
| IV | 153 (11.27%) | 30 (14.49%) | 183 | |
| V | 150 (11.05%) | 55 (26.57%) | 205 | |
| VI | 120 (8.84%) | 36 (17.39%) | 156 | |
| Not performed | 108 (7.96%) | 3 (1.45%) | 111 | |
| Histology § | ||||
| Malignancy | 463 (34.12%) | 125 (60.39%) | 588 | <0.001 |
| Benign thyroid disease | 894 (65.88%) | 82 (39.61%) | 976 | |
| Thyroid surgery § | ||||
| Total thyroidectomy | 1160 (85.48%) | 195 (94.20%) | 1355 | |
| Unilateral lobectomy | 182 (13.41%) | 12 (5.80%) | 194 | |
| Other surgery | 15 (1.11%) | 0 (0.00%) | 15 | |
| Lymph node surgery § | ||||
| UCLND | 202 (14.91%) | 33 (15.94%) | 235 | |
| BCLND | 442 (32.62%) | 112 (54.11% | 554 | |
| BLLND | 3 (0.22%) | 1 (0.48% | 4 | |
| CLND and one-sided LLND | 49 (3.62%) | 6 (2.90%) | 55 | |
| BCLND and BLLND | 3 (0.22%) | 2 (0.97%) | 5 | |
| One-sided LLND | 13 (0.96%) | 0 (0.00%) | 13 | |
| Extirpation of lymph nodes | 1 (0.07%) | 1 (0.48%) | 2 | |
| None | 642 (47.38%) | 52 (25.12%) | 694 |
| Parameters | Malignancy (+) CLT (−) | Malignancy (+) CLT (+) | Total | p-Value |
|---|---|---|---|---|
| N = 463 (78.75%) | N = 125 (21.25%) | N = 588 | ||
| Age (years) £ | ||||
| Mean ± SD | 49.8 ± 15.7 | 42.9 ± 14.0 | 588 | <0.001 |
| Median | 48 | 41 | ||
| (Q1–Q3) | (38.0–62.0) | (32.0–51.0) | ||
| Gender—n (%) § | ||||
| Female | 364 (78.62) | 116 (92.80) | 480 | <0.001 |
| Male | 99 (21.38%) | 9 (7.2) | 108 | |
| Diameter of the largest foci (mm) £ | ||||
| mean ± SD | 13.3 ± 12.6 | 9.7 ± 7.7 | 0.013 | |
| Median (Q1–Q3) | 9 (6.0–16.0) | 8 (5.0–11.0) | ||
| N | 443 | 117 | 560 | |
| Missing | 20 | 8 | 28 | |
| Bethesda category—n (%) § | ||||
| I | 14 (3.02) | 1 (0.80) | 15 | 0.019 |
| II | 80 (17.28) | 12 (9.60) | 92 | |
| III | 67 (14.47) | 14 (11.20) | 81 | |
| IV | 41 (8.86) | 9 (7.2) | 50 | |
| V | 130 (28.08) | 53 (42.40) | 183 | |
| VI | 113 (24.41) | 36 (28.80) | 149 | |
| Not performed | 18 (3.89) | 0 (0.0) | 18 | |
| Type of Malignancy—n (%) § | ||||
| Papillary Thyroid Cancer (PTC) | 379 (81.86) | 116 (92.80) | 495 | |
| Follicular Thyroid Cancer (FTC) | 43 (9.29) | 4 (3.20) | 47 | |
| Oncotytic Thyroid Cancer (OTC) | 12 (2.59) | 3 (2.40) | 15 | |
| Medullary Thyroid Cancer (MTC) | 24 (5.18) | 1 (0.80) | 25 | |
| Anaplastic Thyroid Cancer (ATC) | 3 (0.65) | 0 (0.00) | 3 | |
| Lymphoma | 2 (0.43) | 1 (0.80) | 3 | |
| Type of Malignancy—n (%) § | ||||
| PTC | 379 (81.86) | 116 (92.80) | 495 | 0.003 |
| Other types | 84 (18.14) | 9 (7.20) | 93 | |
| Subtype of PTC—n (%) § | ||||
| Encapsulated variant of PTC | 4 (1.06) | 2 (1.72) | 6 | 0.176 |
| Follicular variant of PTC | 98 (25.99) | 20 (17.24) | 118 | |
| Classic PTC | 247 (65.52) | 81 (69.83) | 328 | |
| Other unusual variants of PTC | 28 (7.43) | 13 (11.21) | 41 | |
| Positive FNAB (V i VI) for PTC—n (%) § | ||||
| No, False Positive | 142 (39.23) | 30 (25.86) | 172 | 0.009 |
| Yes, True Positive | 220 (60.77) | 86 (74.14) | 306 | |
| Microcarcinoma—n (%) § | ||||
| Yes (<10 mm) | 231 (49.89) | 75 (60.00) | 306 | 0.021 |
| No (≥10 mm) | 212 (45.79) | 42 (33.60) | 254 | |
| Missing | 20 (4.32) | 8 (6.4) | 28 | |
| Multifocality—n (%) § | ||||
| Yes (≥2 foci) | 97 (20.95) | 32 (25.60) | 129 | 0.248 |
| No (1 fucus) | 358 (77.32) | 90 (72.00) | 448 | |
| Missing | 8 (1.73) | 3 (2.4) | 11 | |
| NLNM—n (%) § | ||||
| Yes | 82 (21.75) | 22 (18.97) | 104 | 0.520 |
| No | 295 (78.25) | 94 (81.03) | 389 | |
| pNx + missing | 86 (18.57) | 9 (7.20) | 95 | |
| CLNM/LLNM—n (%) § | ||||
| Central LNM (pN1a) | 59 (12.74) | 20 (16.00) | 79 | 0.065 |
| Lateral LNM (pN1b) | 23 (4.97) | 2 (1.60) | 25 | |
| pN0 | 295 (63.71) | 94 (75.20) | 389 | |
| pNx + missing | 86 (18.57) | 9 (7.20) | 95 | |
| Number of metastatic LN £ | ||||
| Mean ± SD | 0.84 ± 2.84 | 0.76 ± 3.45 | 0.859 | |
| N | 455 | 120 | 575 | |
| Missing | 8 | 5 | ||
| Number of removed LN £ | ||||
| Mean ± SD | 4.75 ± 6.84 | 5.39 ± 6.17 | 0.002 | |
| N | 456 | 120 | 576 | |
| Missing | 7 | 5 | 12 | |
| Total number of metastatic lymph nodes/Total number of lymph nodes on histology £ | ||||
| Mean ± SD | 0.11 ± 0.24 | 0.08 ± 0.20 | 0.445 | |
| N | 389 | 116 | 505 | |
| Missing | 74 | 9 | 83 | |
| T—TNM classification—n (%) § | ||||
| I (pT1a + pT1b) | 352 (80.55) | 106 (87.60) | 458 | 0.155 |
| II (pT2) | 63 (14.42) | 14 (11.57) | 77 | |
| III (pT3a + pT3b) | 19 (4.35) | 1 (0.83) | 20 | |
| IV (pT4a + pT4b) | 3 (0.69) | 0 (0.00) | 3 | |
| pTx | 26 (5.62) | 4 (3.20) | 30 | |
| Total excised thyroid gland weight (gram) £ | ||||
| Mean ± SD | 37.61 ± 91.07 | 22.48 ± 21.82 | <0.001 | |
| N | 433 | 118 | 551 | |
| Missing | 30 | 7 | 37 | |
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Predictors | β | p-Value | OR (95% CI) | β | p-Value | OR (95% CI) |
| Gender (F vs. M) | −0.08 | 0.56 | 0.92 (0.71–1.21) | Not included | ||
| CLT (Yes vs. No) | 1.08 | <0.01 | 2.94 (2.18–3.97) | 0.58 | 0.01 | 1.73 (1.15–2.29) |
| Bethesda (III vs. I or II) | 0.88 | <0.01 | 2.42 (1.73–3.38) | 0.87 | <0.01 | 2.35 (1.68–3.31) |
| Bethesda (IV vs. I or II) | 0.66 | <0.01 | 1.93 (1.31–2.84) | 0.62 | <0.01 | 1.86 (1.25–2.75) |
| Bethesda (V vs. I or II) | 3.75 | <0.01 | 42.68 (26.19–69.55) | 3.60 | <0.01 | 36.58 (22.34 –59.89) |
| Age ≤ 50 (Yes vs. No) | 0.95 | <0.01 | 2.51 (2.04–3.10) | 0.60 | <0.01 | 1.82 (1.37–2.41) |
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Predictors | β | p-Value | OR | β | p-Value | OR (95% CI) |
| Gender (F vs. M) | −0.99 | <0.01 | 0.37 (0.22–0.61) | −0.80 | <0.01 | 0.45 (0.26–0.77) |
| CLT (Yes vs. No) | −0.17 | 0.52 | 0.84 (0.50–1.42) | 0.08 | 0.78 | 1.09 (0.62–1.92) |
| Microcarcinoma (Yes vs. No) | −1.38 | <0.01 | 0.25 (0.16–0.40) | −1.34 | <0.01 | 0.26 (0.16–0.43) |
| Multifocality (Yes vs. No) | 0.21 | 0.40 | 1.24 (0.76–2.03) | 0.23 | 0.40 | 1.25 (0.74–2.13) |
| Age ≤ 50 (Yes vs. No) | 0.42 | 0.08 | 1.52 (0.96–2.42) | 0.44 | 0.08 | 1.56 (0.95–2.56) |
| N-TNM Types | Predictor | Coeff. | p-Value | OR (95% CI) |
|---|---|---|---|---|
| CLNM (pN1a) vs. pNo | ||||
| CLT (Yes vs. No) | 0.19 | 0.52 | 1.21 (0.67–2.19) | |
| Age ≤ 50 | 0.56 | 0.05 | 1.75 (1.01–3.03) | |
| Gender (F vs. M) | −0.56 | 0.08 | 0.57 (0.31–1.06) | |
| Multifocality (Yes vs. No) | 0.23 | 0.43 | 1.26 (0.71–2.21) | |
| Microcarcinoma (Yes vs. No) | −1.03 | <0.001 | 0.36 (0.21–0.59) | |
| LLNM (pN1b) vs. pNo | ||||
| CLT (Yes vs. No) | −0.65 | 0.40 | 0.52 (0.11–2.41) | |
| Age ≤ 50 | 0.06 | 0.90 | 1.06 (0.43–2.61) | |
| Gender (F vs. M) | −1.45 | 0.00 | 0.23 (0.10–0.58) | |
| Multifocality (Yes vs. No) | 0.22 | 0.67 | 1.25 (0.45–3.49) | |
| Microcarcinoma (Yes vs. No) | −3.45 | <0.001 | 0.03 (0.00–0.24) |
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Predictors | β | p-Value | OR (95% CI) | β | p-Value | OR (95% CI) |
| Gender (F vs. M) | 0.24 | 0.11 | 1.27 (0.95–1.69) | not included | ||
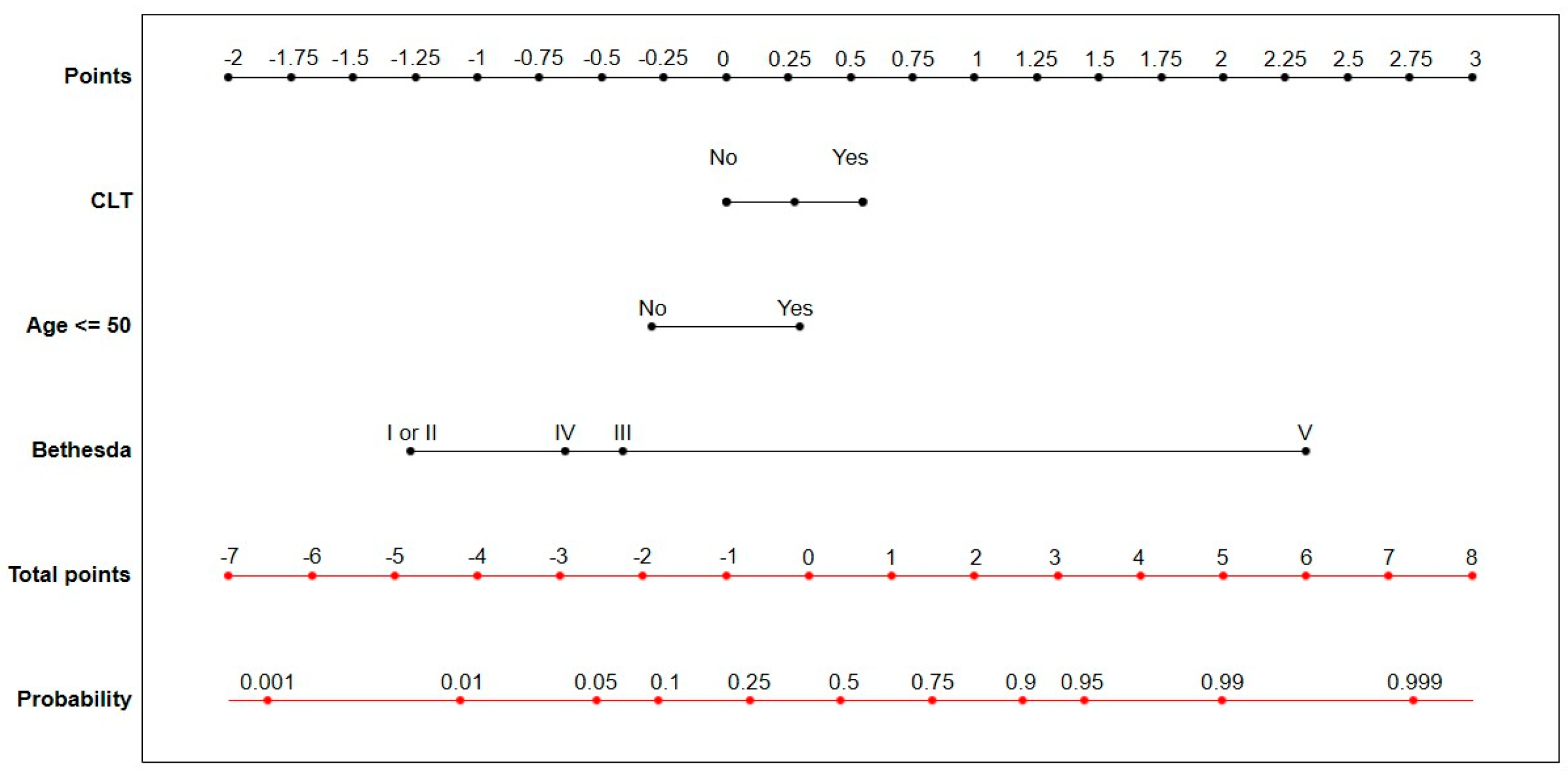
| CLT (Yes vs. No) | 1.21 | <0.01 | 3.34 (2.45–4.54) | 0.75 | 0.01 | 2.11 (1.37–3.26) |
| Bethesda (III vs. I or II) | 0.78 | <0.01 | 2.18 (1.50–3.17) | 0.75 | <0.01 | 2.12 (1.45–3.11) |
| Bethesda (IV vs. I or II) | 0.34 | 0,15 | 1.41 (0.89–2.24) | 0.26 | 0.29 | 1.29 (0.81–2,08) |
| Bethesda (V vs. I or II) | 3.92 | <0.01 | 50.50 (30.65–83.18) | 3.75 | <0.01 | 42.64 (25.72 –70.68) |
| Age ≤ 50 (Yes vs. No) | 1.06 | <0.01 | 2.89 (2.31–3.62) | 0.74 | <0.01 | 2.10 (1.53–2.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzentowska, A.; Konturek, A.; Gołkowski, F.; Merklinger-Gruchała, A.; Barczyński, M. Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis. Cancers 2025, 17, 1964. https://doi.org/10.3390/cancers17121964
Krzentowska A, Konturek A, Gołkowski F, Merklinger-Gruchała A, Barczyński M. Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis. Cancers. 2025; 17(12):1964. https://doi.org/10.3390/cancers17121964
Chicago/Turabian StyleKrzentowska, Anna, Aleksander Konturek, Filip Gołkowski, Anna Merklinger-Gruchała, and Marcin Barczyński. 2025. "Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis" Cancers 17, no. 12: 1964. https://doi.org/10.3390/cancers17121964
APA StyleKrzentowska, A., Konturek, A., Gołkowski, F., Merklinger-Gruchała, A., & Barczyński, M. (2025). Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis. Cancers, 17(12), 1964. https://doi.org/10.3390/cancers17121964







