A Retrospective Assessment of Computed Tomography-Based Body Composition and Toxicity in Ovarian Cancer Patients Treated with PARP Inhibitors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. Clinical Data Recorded
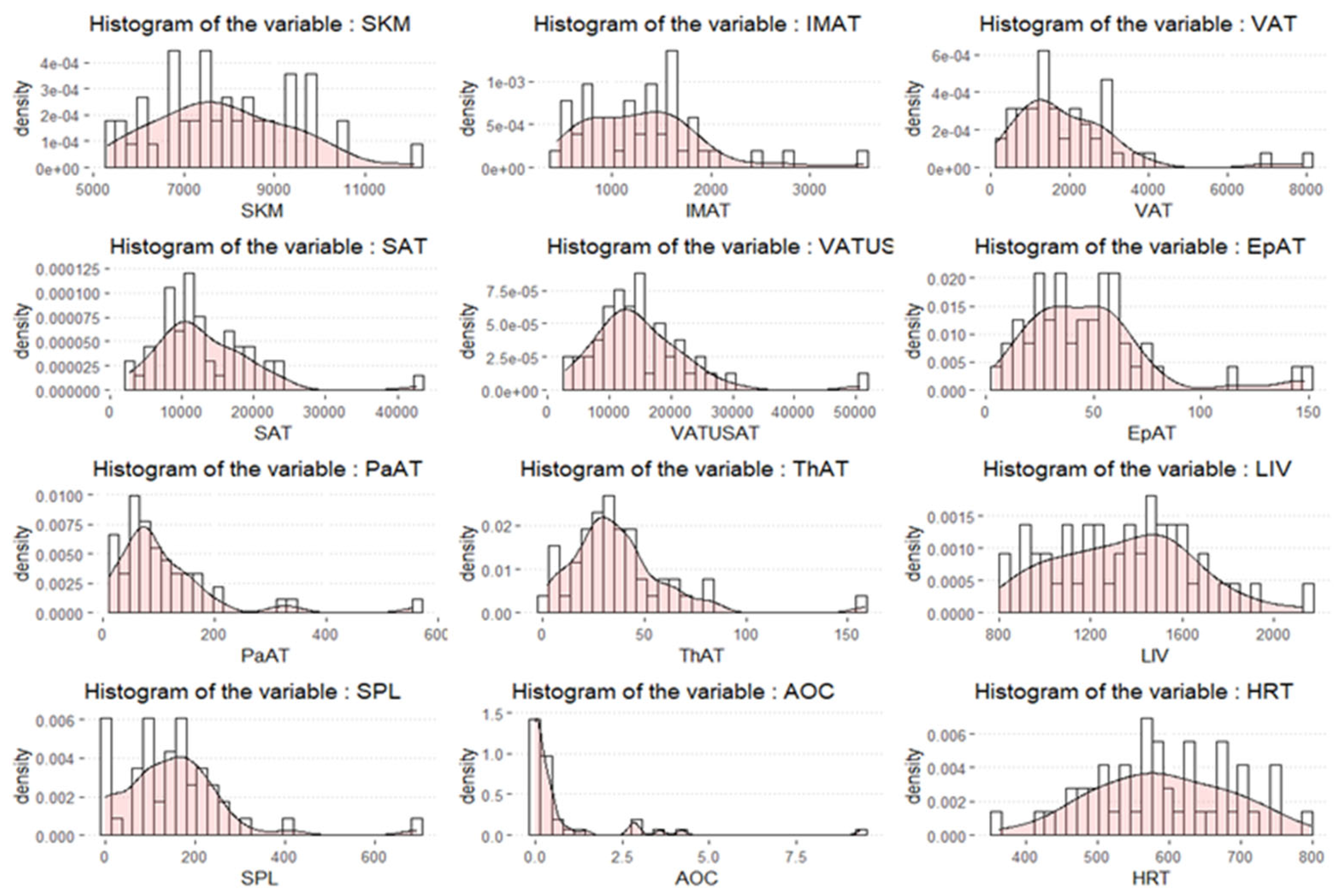
2.3. Extraction of Volumetric Body Composition Features
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Brown, J.; Barnicle, A.; Wessen, J.; Lao-Sirieix, P.; Criscione, S.W.; du Bois, A.; Lorusso, D.; Romero, I.; Petru, E.; et al. Homologous Recombination Repair Gene Mutations to Predict Olaparib Plus Bevacizumab Efficacy in the First-Line Ovarian Cancer PAOLA-1/ENGOT-ov25 Trial. JCO Precis. Oncol. 2023, 7, e2200258. [Google Scholar] [CrossRef]
- Mirza, M.R.; González-Martín, A.; Graybill, W.S.; O’mAlley, D.M.; Gaba, L.; Yap, O.W.S.; Guerra, E.M.; Rose, P.G.; Baurain, J.; Ghamande, S.A.; et al. Prospective evaluation of the tolerability and efficacy of niraparib dosing based on baseline body weight and platelet count: Results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Cancer 2023, 129, 1846–1855. [Google Scholar] [CrossRef]
- Del Campo, J.M.; Matulonis, U.A.; Malander, S.; Provencher, D.; Mahner, S.; Follana, P.; Waters, J.; Berek, J.S.; Woie, K.; Oza, A.M.; et al. Niraparib Maintenance Therapy in Patients With Recurrent Ovarian Cancer After a Partial Response to the Last Platinum-Based Chemotherapy in the ENGOT-OV16/NOVA Trial. J. Clin. Oncol. 2019, 37, 2968–2973. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; A Ledermann, J.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O'MAlley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA–MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F.; et al. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef]
- Huber, F.A.; Del Grande, F.; Rizzo, S.; Guglielmi, G.; Guggenberger, R. MRI in the assessment of adipose tissues and muscle composition: How to use it. Quant. Imaging Med. Surg. 2020, 10, 1636–1649. [Google Scholar] [CrossRef]
- Raia, G.; Del Grande, M.; Colombo, I.; Nerone, M.; Manganaro, L.; Gasparri, M.L.; Papadia, A.; Del Grande, F.; Rizzo, S. Whole-Body Composition Features by Computed Tomography in Ovarian Cancer: Pilot Data on Survival Correlations. Cancers 2023, 15, 2602. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Cao, F.; Wang, C.-B.; Dong, J.-N.; Wang, Z.-H. Nomogram of Combining CT-Based Body Composition Analyses and Prognostic Inflammation Score: Prediction of Survival in Advanced Epithelial Ovarian Cancer Patients. Acad. Radiol. 2021, 29, 1394–1403. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, T.M.; Lee, M.; Kim, H.S.; Chung, H.H.; Cho, J.Y.; Song, Y.S. Impact of CT-Determined Sarcopenia and Body Composition on Survival Outcome in Patients with Advanced-Stage High-Grade Serous Ovarian Carcinoma. Cancers 2020, 12, 559. [Google Scholar] [CrossRef]
- Del Grande, M.; Rizzo, S.; Nicolino, G.M.; Colombo, I.; Rossi, L.; Manganaro, L.; Del Grande, F. Computed Tomography–Based Body Composition in Patients With Ovarian Cancer: Association With Chemotoxicity and Prognosis. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Wood, N.; Morton, M.; Shah, S.N.; Yao, M.; Barnard, H.; Tewari, S.; Suresh, A.; Kollikonda, S.; AlHilli, M.M. Association between CT-based body composition assessment and patient outcomes during neoadjuvant chemotherapy for epithelial ovarian cancer. Gynecol. Oncol. 2022, 169, 55–63. [Google Scholar] [CrossRef]
- McSharry, V.; Glennon, K.; Mullee, A.; Brennan, D. The impact of body composition on treatment in ovarian cancer: A current insight. Expert Rev. Clin. Pharmacol. 2021, 14, 1065–1074. [Google Scholar] [CrossRef]
- Dalal, T.; Kalra, M.K.; Rizzo, S.M.R.; Schmidt, B.; Suess, C.; Flohr, T.; Blake, M.A.; Saini, S. Metallic Prosthesis: Technique to Avoid Increase in CT Radiation Dose with Automatic Tube Current Modulation in a Phantom and Patients. Radiology 2005, 236, 671–675. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Classification and regression trees. Nat. Methods 2017, 14, 757–758. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006; p. xvii. 391p. [Google Scholar]
- Bai, P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol. Cell 2015, 58, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, Y.Y.; Jang, H.; Han, J.S.; Nahmgoong, H.; Park, Y.J.; Han, S.M.; Cho, C.; Lim, S.; Noh, J.-R.; et al. SREBP1c-PARP1 axis tunes anti-senescence activity of adipocytes and ameliorates metabolic imbalance in obesity. Cell Metab. 2022, 34, 702–718.e5. [Google Scholar] [CrossRef]
- Tan, A.; Younis, A.Z.; Evans, A.; Creighton, J.V.; Coveny, C.; Boocock, D.J.; Sale, C.; Lavery, G.G.; Coutts, A.S.; Doig, C.L. PARP1 mediated PARylation contributes to myogenic progression and glucocorticoid transcriptional response. Cell Death Discov. 2023, 9, 1–13. [Google Scholar] [CrossRef]
- Bruin, M.A.C.; Sonke, G.S.; Beijnen, J.H.; Huitema, A.D.R. Pharmacokinetics and Pharmacodynamics of PARP Inhibitors in Oncology. Clin. Pharmacokinet. 2022, 61, 1649–1675. [Google Scholar] [CrossRef]
- Velev, M.; Puszkiel, A.; Blanchet, B.; de Percin, S.; Delanoy, N.; Medioni, J.; Gervais, C.; Balakirouchenane, D.; Khoudour, N.; Pautier, P.; et al. Association between Olaparib Exposure and Early Toxicity in BRCA-Mutated Ovarian Cancer Patients: Results from a Retrospective Multicenter Study. Pharmaceuticals 2021, 14, 804. [Google Scholar] [CrossRef]
- Guo, X.; Tang, J.; He, H.; Jian, L.; Qiang, O.; Xie, Y. Body composition and inflammation variables as the potential prognostic factors in epithelial ovarian cancer treated with Olaparib. Front. Oncol. 2024, 14, 1359635. [Google Scholar] [CrossRef]
- González-Martín, A.; Desauw, C.; Heitz, F.; Cropet, C.; Gargiulo, P.; Berger, R.; Ochi, H.; Vergote, I.; Colombo, N.; Mirza, M.R.; et al. Maintenance olaparib plus bevacizumab in patients with newly diagnosed advanced high-grade ovarian cancer: Main analysis of second progression-free survival in the phase III PAOLA-1/ENGOT-ov25 trial. Eur. J. Cancer 2022, 174, 221–231. [Google Scholar] [CrossRef]
- Penson, R.T.; Valencia, R.V.; Cibula, D.; Colombo, N.; Leath, C.A., III; Bidziński, M.; Kim, J.-W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020, 38, 1164–1174. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Kristeleit, R.; Lisyanskaya, A.; Fedenko, A.; Dvorkin, M.; de Melo, A.C.; Shparyk, Y.; Rakhmatullina, I.; Bondarenko, I.; Colombo, N.; Svintsitskiy, V.; et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 465–478. [Google Scholar] [CrossRef]
- Eakin, C.M.; Ewongwo, A.; Pendleton, L.; Monk, B.J.; Chase, D.M. Real world experience of poly (ADP-ribose) polymerase inhibitor use in a community oncology practice. Gynecol. Oncol. 2020, 159, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Noh, J.J.; Baek, S.H.; Kim, B.-G.; Lim, M.C.; Park, S.-Y. Real-world Experience of Niraparib in Newly-diagnosed Epithelial Ovarian Cancer. Anticancer. Res. 2021, 41, 4603–4607. [Google Scholar] [CrossRef]
- Hatch, R.V.; Patel, S.U.; Cambareri, C.; Uritsky, T.; Martin, L.P. Evaluation of the management of PARP inhibitor toxicities in ovarian and endometrial cancer within a multi-institution health-system. J. Oncol. Pharm. Pr. 2021, 28, 1102–1110. [Google Scholar] [CrossRef]
- Arend, R.C.; O’mAlley, D.M.; Banerjee, S.; McLaurin, K.; Davidson, R.; Long, G.H. Utilization of Poly(ADP-Ribose) Polymerase Inhibitors in Ovarian Cancer: A Retrospective Cohort Study of US Healthcare Claims Data. Adv. Ther. 2021, 39, 328–345. [Google Scholar] [CrossRef]
- Cecere, S.C.; Giannone, G.; Salutari, V.; Arenare, L.; Lorusso, D.; Ronzino, G.; Lauria, R.; Cormio, G.; Carella, C.; Scollo, P.; et al. Olaparib as maintenance therapy in patients with BRCA 1–2 mutated recurrent platinum sensitive ovarian cancer: Real world data and post progression outcome. Gynecol. Oncol. 2020, 156, 38–44. [Google Scholar] [CrossRef]
| N (%) | |
|---|---|
| Age at diagnosis, median (IQR) | 61 (53;68.5) |
| Histology | |
| High grade serous | 43 (90) |
| Endometrioid | 3 (6) |
| Clear Cell | 1 (2) |
| Undifferentiated | 1 (2) |
| Surgery | |
| Primary surgery | 35 (73) |
| Interval debulking surgery | 10 (21) |
| No surgery | 3 (6) |
| FIGO Stage at diagnosis | |
| IC | 2 (4) |
| IIA | 1 (2) |
| IIIA | 2 (4) |
| IIIB | 4 (8) |
| IIIC | 20 (42) |
| IV | 19 (40) |
| g/s BRCA1/2 status | |
| g/s BRCA1/2 PV | 15 (31) |
| g/s BRCA1/2 wild type | 27 (56) |
| Untested | 6 (13) |
| PARP inhibitor | |
| Olaparib | 38 (79) |
| Niraparib | 10 (21) |
| PARP inhibitor setting | |
| 1st line maintenance | 16 (33) |
| 2nd line maintenance | 24 (50) |
| 3rd line maintenance | 6 (13) |
| 4th line maintenance | 2 (4) |
| Dose reduction | |
| No | 28 (58) |
| Yes | 20 (42) |
| Reasons for dose reduction | |
| Trombocytopenia (G1-G2) | 5 |
| Trombocytopenia G3 | 1 |
| Anemia (G1-G2) | 4 |
| Anemia G3 | 1 |
| Nausea (G1-G2) | 4 |
| Fatigue | 4 |
| Disgeusia | 1 |
| Increased AST/ALT | 1 |
| Vomiting | 1 |
| Cough | 1 |
| Heart Failure | 1 |
| Renal impairment | 1 |
| Permanent discontinuation | 7 (15) |
| Anemia G2 | 3 |
| Trombocytopenia (G1-G2) | 1 |
| Fatigue G2 | 2 |
| Nausea G1 | 1 |
| Recurence/progression | |
| No | 22 (46) |
| Yes | 26 (54) |
| BMI, median (IQR) | 24 (21.5;28.7) |
| No Dose Reduction | Dose Reduction | |
|---|---|---|
| N | 28 | 20 |
| SKM (mean (SD)) | 8049.59 (1681.14) | 7639.54 (1205.84) |
| IMAT (mean (SD)) | 1304.75 (579.91) | 1310.40 (672.98) |
| VAT (mean (SD)) | 1985.72 (1444.50) | 1953.22 (1568.51) |
| SAT (mean (SD)) | 12,372.74 (5924.65) | 13,888.81 (8048.12) |
| VAT-U-SAT (mean (SD)) | 14,358.46 (7037.06) | 15,842.03 (9489.89) |
| EpAT (mean (SD)) | 48.32 (30.32) | 45.75 (29.69) |
| PaAT (mean (SD)) | 108.73 (82.84) | 108.14 (112.70) |
| ThAT (mean (SD)) | 36.61 (21.44) | 38.06 (32.09) |
| LIV (mean (SD)) | 1371.04 (266.89) | 1308.54 (345.58) |
| SPL (mean (SD)) | 142.86 (95.24) | 164.66 (150.20) |
| AOC (mean (SD)) | 0.45 (0.87) | 1.13 (2.26) |
| HRT (mean (SD)) | 597.89 (102.00) | 580.79 (89.68) |
| AgeD (mean (SD)) | 60.11 (10.88) | 60.80 (11.66) |
| BMI (mean (SD)) | 25.18 (5.42) | 25.51 (7.92) |
| Variable | Crude OR | (95%CI) | p-Value |
|---|---|---|---|
| SKM | 0.9998 | (0.9994,1.0002) | 0.3498 |
| IMAT | 1 | (0.9991,1.001) | 0.9746 |
| VAT | 1 | (0.9996,1.0004) | 0.9396 |
| SAT | 1 | (0.9999,1.0001) | 0.4528 |
| VAT-U-SAT | 1 | (1,1.0001) | 0.5319 |
| EpAT | 0.997 | (0.9774,1.017) | 0.7658 |
| PaAT | 0.9999 | (0.9939,1.006) | 0.983 |
| ThAT | 1.0022 | (0.9803,1.0245) | 0.8478 |
| LIV | 0.9993 | (0.9973,1.0012) | 0.4745 |
| SPL | 1.0015 | (0.9967,1.0064) | 0.5369 |
| AOC | 1.3652 | (0.8476,2.1989) | 0.2005 |
| HRT | 0.9981 | (0.9921,1.0042) | 0.5418 |
| Estimate | Std. Error | z Value | Pr (>|z|) | |
|---|---|---|---|---|
| SKM ≥ 8650 cm3 | −1.2993 | 0.6513 | −1.995 | 0.0461 |
| 7506 ≤ SKM < 8650 cm3 | 2.2156 | 0.8799 | 2518 | 0.0118 |
| SKM < 7506 cm3 | 0.6802 | 0.8025 | 0.8025 | 0.3966 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerone, M.; Raia, G.; Del Grande, M.; Manganaro, L.; Moscatelli, G.; Di Serio, C.; Papadia, A.; Ciliberti, E.; Trevisi, E.; Sessa, C.; et al. A Retrospective Assessment of Computed Tomography-Based Body Composition and Toxicity in Ovarian Cancer Patients Treated with PARP Inhibitors. Cancers 2025, 17, 1963. https://doi.org/10.3390/cancers17121963
Nerone M, Raia G, Del Grande M, Manganaro L, Moscatelli G, Di Serio C, Papadia A, Ciliberti E, Trevisi E, Sessa C, et al. A Retrospective Assessment of Computed Tomography-Based Body Composition and Toxicity in Ovarian Cancer Patients Treated with PARP Inhibitors. Cancers. 2025; 17(12):1963. https://doi.org/10.3390/cancers17121963
Chicago/Turabian StyleNerone, Marta, Giorgio Raia, Maria Del Grande, Lucia Manganaro, Giordano Moscatelli, Clelia Di Serio, Andrea Papadia, Esteban Ciliberti, Elena Trevisi, Cristiana Sessa, and et al. 2025. "A Retrospective Assessment of Computed Tomography-Based Body Composition and Toxicity in Ovarian Cancer Patients Treated with PARP Inhibitors" Cancers 17, no. 12: 1963. https://doi.org/10.3390/cancers17121963
APA StyleNerone, M., Raia, G., Del Grande, M., Manganaro, L., Moscatelli, G., Di Serio, C., Papadia, A., Ciliberti, E., Trevisi, E., Sessa, C., Del Grande, F., Colombo, I., & Rizzo, S. (2025). A Retrospective Assessment of Computed Tomography-Based Body Composition and Toxicity in Ovarian Cancer Patients Treated with PARP Inhibitors. Cancers, 17(12), 1963. https://doi.org/10.3390/cancers17121963







