Combined Hyperthermic Intraperitoneal Chemotherapy and Normothermic Intraperitoneal Chemotherapy Long-Term After Interval Cytoreduction in Ovarian Cancer: A Phase I Clinical Trial (BICOV1)
Simple Summary
Abstract
1. Introduction
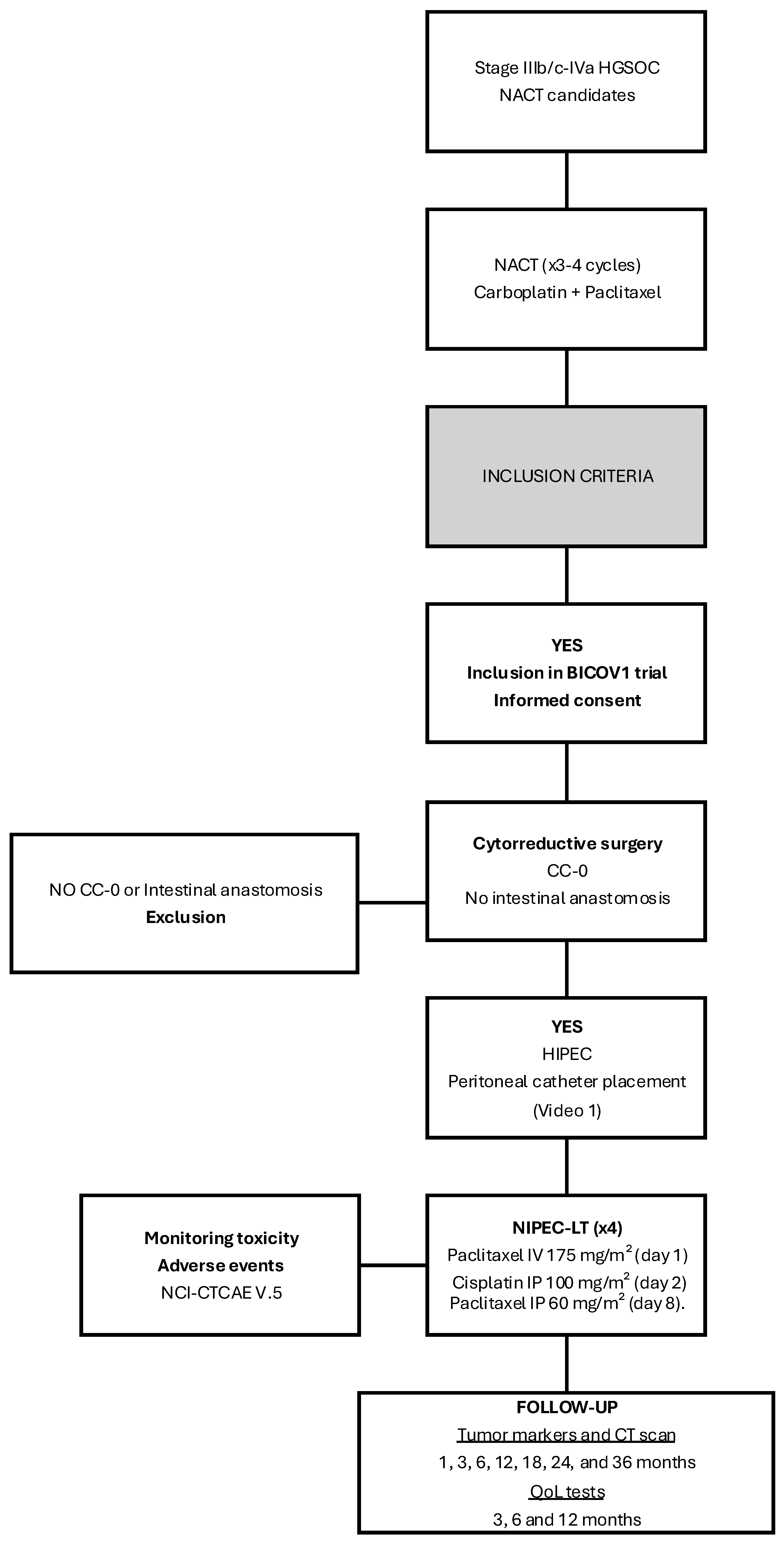
2. Material and Methods
2.1. Primary Outcome
2.2. Secondary Outcomes
2.3. Sample Size and Data Analysis
2.4. Treatment Strategy
2.4.1. Surgical Procedure
2.4.2. HIPEC Protocol and Intraperitoneal Catheter Management
2.4.3. NIPEC-LT Protocol
2.5. Ethics
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Heintz, A.P.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.Y.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. S1), S161–S192. [Google Scholar] [PubMed]
- Cascales-Campos, P.A.; Gil, J.; Gil, E.; Feliciangeli, E.; González-Gil, A.; Parrilla, J.J.; Parrilla, P. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann. Surg. Oncol. 2014, 21, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2016, 2016, CD005340. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open. 2022, 7, 100586. [Google Scholar] [CrossRef]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B.; et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J.; Ball, H.; Hakes, T.B.; et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Landrum, L.M.; Java, J.; Mathews, C.A.; Lanneau, G.S.; Copeland, L.J.; Armstrong, D.K.; Walker, J.L. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013, 130, 12–18. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Lee, S.J.; Nam, B.H.; Park, S.Y.; Seo, S.S.; Kang, S.; Yun, J.Y.; et al. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Koole, S.N.; Van Lieshout, C.; Van Driel, W.J.; Van Schagen, E.; Sikorska, K.; Kieffer, J.M.; Schagen van Leeuwen, J.H.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; et al. Cost Effectiveness of Interval Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy in Stage III Ovarian Cancer on the Basis of a Randomized Phase III Trial. J. Clin. Oncol. 2019, 37, 2041–2050. [Google Scholar] [CrossRef]
- Kim, J.H.; Chun, S.Y.; Lee, D.E.; Woo, Y.H.; Chang, S.J.; Park, S.Y.; Chang, Y.J.; Lim, M.C. Cost-effectiveness of hyperthermic intraperitoneal chemotherapy following interval cytoreductive surgery for stage III-IV ovarian cancer from a randomized controlled phase III trial in Korea (KOV-HIPEC-01). Gynecol. Oncol. 2023, 170, 19–24. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines. Ovarian Cancer Version: 1. 2024. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 24 March 2025).
- National Institute for Health and Care Excellence. Cytoreduction Surgery with Hyperthermic Intraoperative Peritoneal Chemotherapy for Peritoneal Carcinomatosis; NICE Interventional Procedures Guidance [IPG688]; NICE: London, UK, 2021; Available online: https://www.nice.org.uk/guidance/ipg688 (accessed on 23 April 2025).
- Classe, J.M.; Meeus, P.; Hudry, D.; Wernert, R.; Quenet, F.; Marchal, F.; Houvenaeghel, G.; Bats, A.S.; Lecuru, F.; Ferron, G.; et al. Hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer (CHIPOR): A randomised, open-label, phase 3 trial. Lancet Oncol. 2024, 25, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version, 5; National Institutes of Health: Bethesda, MD, USA; National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; EORTC: Brussels, Belgium, 2001; ISBN 2-930064-22-6. [Google Scholar]
- EuroQol Research Foundation. EQ-5D-5L. Available online: https://euroqol.org/information-and-support/euroqol-instruments/eq-5d-5l/ (accessed on 24 March 2025).
- Spielberger, C.D. State-Trait Anxiety Inventory: Bibliography, 2nd ed.; Consulting Psychologists Press: Palo Alto, CA, USA, 1989. [Google Scholar]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. It’s what the surgeon doesn’t see that kills the patient. J. Nippon. Med. Sch. 2000, 67, 5–8. [Google Scholar] [CrossRef]
- Oaknin, A.; Roda, D.; González-Martin, A.; Chiva, L.; García-Donas, J.; de Juan, A.; Redondo, A.; Martínez, S.; García, Y.; Catot, S.; et al. Feasibility of a modified outpatient regimen of intravenous/intraperitoneal chemotherapy in optimally debulked stage III ovarian cancer patients: A GEICO study. Int. J. Gynecol. Cancer. 2011, 21, 1048–1055. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Dellinger, T.H.; Han, E.S. State of the Science: The role of HIPEC in the treatment of ovarian cancer. Gynecol. Oncol. 2021, 160, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.-Q.; Han, I.-H.; Seow, K.-M.; Chen, K.-H. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): An Overview of the Molecular and Cellular Mechanisms of Actions and Effects on Epithelial Ovarian Cancers. Int. J. Mol. Sci. 2022, 23, 10078. [Google Scholar] [CrossRef] [PubMed]
- de Bree, E.; Tsiftsis, D.D. Experimental and pharmacokinetic studies in intraperitoneal cemotherapy: From laboratory bench to bedside. Recent. Results Cancer Res. 2007, 169, 53–73. [Google Scholar] [PubMed]
- Chen, T.; Guo, J.; Han, C.; Yang, M.; Cao, X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol. 2009, 182, 1449–1459. [Google Scholar] [CrossRef]
| INCLUSION CRITERIA |
|
| EXCLUSION CRITERIA |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gil, A.; Gil-Gómez, E.; Olivares-Ripoll, V.; Cerezuela Fernández de Palencia, Á.; Martínez-García, J.; Sánchez-Martínez, D.; Guijarro-Campillo, A.R.; Cascales-Campos, P.A. Combined Hyperthermic Intraperitoneal Chemotherapy and Normothermic Intraperitoneal Chemotherapy Long-Term After Interval Cytoreduction in Ovarian Cancer: A Phase I Clinical Trial (BICOV1). Cancers 2025, 17, 1957. https://doi.org/10.3390/cancers17121957
González-Gil A, Gil-Gómez E, Olivares-Ripoll V, Cerezuela Fernández de Palencia Á, Martínez-García J, Sánchez-Martínez D, Guijarro-Campillo AR, Cascales-Campos PA. Combined Hyperthermic Intraperitoneal Chemotherapy and Normothermic Intraperitoneal Chemotherapy Long-Term After Interval Cytoreduction in Ovarian Cancer: A Phase I Clinical Trial (BICOV1). Cancers. 2025; 17(12):1957. https://doi.org/10.3390/cancers17121957
Chicago/Turabian StyleGonzález-Gil, Alida, Elena Gil-Gómez, Vicente Olivares-Ripoll, Álvaro Cerezuela Fernández de Palencia, Jerónimo Martínez-García, Domingo Sánchez-Martínez, Alberto Rafael Guijarro-Campillo, and Pedro Antonio Cascales-Campos. 2025. "Combined Hyperthermic Intraperitoneal Chemotherapy and Normothermic Intraperitoneal Chemotherapy Long-Term After Interval Cytoreduction in Ovarian Cancer: A Phase I Clinical Trial (BICOV1)" Cancers 17, no. 12: 1957. https://doi.org/10.3390/cancers17121957
APA StyleGonzález-Gil, A., Gil-Gómez, E., Olivares-Ripoll, V., Cerezuela Fernández de Palencia, Á., Martínez-García, J., Sánchez-Martínez, D., Guijarro-Campillo, A. R., & Cascales-Campos, P. A. (2025). Combined Hyperthermic Intraperitoneal Chemotherapy and Normothermic Intraperitoneal Chemotherapy Long-Term After Interval Cytoreduction in Ovarian Cancer: A Phase I Clinical Trial (BICOV1). Cancers, 17(12), 1957. https://doi.org/10.3390/cancers17121957






