Intra-Arterial Melphalan Chemotherapy for Retinoblastoma in a Developing Nation: Real-World Outcomes and Prognostic Factors
Simple Summary
Abstract
1. Introduction
2. Methods
- Inclusionand exclusion criteria
- Clinical Characteristics and Definitions
- Previous treatment
- Protocol for Intra-Arterial Melphalan
- Interventional Radiology Technique for IAC
- Statistical Analysis
3. Results
3.1. Demographic and Clinical Features
3.2. Tumor Features and Number of Injections
3.3. Treatment Outcomes and Eye Salvage
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, A.C.; de Magalhães-Barbosa, M.C.; Moreira, J.P.L.; Colombini, G.N.U.I.; Prata-Barbosa, A. Incidence of Retinoblastoma in Children and Adolescents in Brazil: A Population-Based Study. Front. Pediatr. 2022, 10, 1048792. [Google Scholar] [CrossRef] [PubMed]
- Yousef, Y.A.; Mohammad, M.; Al-Nawaiseh, I.; Mahafza, H.; Halalsheh, H.; Mehyar, M.; AlJabaria, R.; Al-Rawashdeh, K.; Jaradat, I.; Sultan, I.; et al. Retinoblastoma and Uveal Melanoma in Jordan: Incidence, Demographics, and Survival (2011–2020). Ophthalmic Genet. 2022, 17, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ancona-Lezama, D.; Dalvin, L.A.; Shields, C.L. Modern treatment of retinoblastoma: A 2020 review. Indian J Ophthalmol. 2020, 68, 2356–2365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaliki, S.; Vempuluru, V.S.; Mohamed, A.; Al-Jadiry, M.F.; Bowman, R.; Chawla, B.; Hamid, S.A.; Ji, X.; Kapelushnik, N.; Kebudi, R.; et al. Retinoblastoma in Asia: Clinical Presentation and Treatment Outcomes in 2112 Patients from 33 Countries. Ophthalmology 2024, 131, 468–477. [Google Scholar] [CrossRef]
- Tomar, A.S.; Finger, P.T.; Gallie, B.; Kivelä, T.T.; Mallipatna, A.; Zhang, C.; Zhao, J.; Wilson, M.W.; Brenna, R.C.; Burges, M.; et al. Global Retinoblastoma Treatment Outcomes: Association with National Income Level. Ophthalmology 2021, 128, 740–753. [Google Scholar] [CrossRef]
- Schaiquevich, P.; Francis, J.H.; Cancela, M.B.; Carcaboso, A.M.; Chantada, G.L.; Abramson, D.H. Treatment of Retinoblastoma: What Is the Latest and What Is the Future? Front. Oncol. 2022, 12, 822330. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Al-Nawaiseh, I.; Mehyar, M.; Sultan, I.; Al-Hussaini, M.; Jaradat, I.; Mohammad, M.; AlJabari, R.; Abu-Yaghi, N.; Rodriguez-Galindo, C.; et al. How Telemedicine and Centralized Care Changed the Natural History of Retinoblastoma in a Developing Country: Analysis of 478 Patients. Ophthalmology 2021, 128, 130–137. [Google Scholar] [CrossRef]
- Abramson, D.H.; Shields, C.L.; Munier, F.L.; Chantada, G.L. Treatment of Retinoblastoma in 2015: Agreement and Disagreement. JAMA Ophthalmol. 2015, 133, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kaneko, A. Management of intraocular retinoblastoma and ocular prognosis. Int. J. Clin. Oncol. 2004, 9, 1e6. [Google Scholar] [CrossRef]
- Abramson, D.H.; Marr, B.P.; Dunkel, I.J.; Brodie, S.; Zabor, E.C.; Driscoll, S.J.; Gobin, Y.P. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br. J. Ophthalmol. 2012, 96, 499–502. [Google Scholar] [CrossRef]
- Abramson, D.H.; Marr, B.P.; Francis, J.H.; Dunkel, I.J.; Fabius, A.W.; Brodie, S.E.; Mondesire-Crump, I.; Gobin, Y.P. Simultaneous bilateral ophthalmic artery chemosurgery for bilateral retinoblastoma (tandem therapy). PLoS ONE 2016, 11, e0156806. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Mittal, P.; Palkonda, V.A.R.; Chattannavar, G.; Mohan, S.; Jajapuram, S.D.; Mohamed, A. Bilateral advanced (group D or E) intraocular retinoblastoma: Outcomes in 72 Asian Indian patients. Eye 2019, 33, 1297–1304. [Google Scholar] [CrossRef]
- Daniels, A.B.; Patel, S.N.; Milam, R.W.; Kohanim, S.; Friedman, D.L.; Koyama, T. Effect of intravenous chemotherapy regimen on globe salvage success rates for retinoblastoma based on disease class-A meta-analysis. Cancers 2021, 13, 2216. [Google Scholar] [CrossRef]
- Shields, C.L.; Bianciotto, C.G.; Jabbour, P.; Ramasubramanian, A.; Lally, S.E.; Griffin, G.C.; Rosenwasser, R.; Shields, J.A. Intra-arterial chemotherapy for retinoblastoma: Report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch. Ophthalmol. 2011, 129, 1399–1406. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamane, T.; Mohri, M.; Kaneko, A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: The longterm prognosis. Ophthalmology 2011, 118, 2081–2087. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Al-Dahmash, S.; Rojanaporn, D.; Leahey, A.; Griffin, G.; Jabbour, P.; Shields, J. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina 2013, 33, 2103–2109. [Google Scholar] [CrossRef]
- Berry, J.L.; Kogachi, K.; Murphree, A.L.; Jubran, R.; Kim, J. A review of recurrent retinoblastoma: Children’s Hospital Los Angeles classification and treatment guidelines. Int. Ophthalmol. Clin. 2019, 59, 65–75. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Soliman, S.E.; Astudillo, P.P.P.; Durairaj, P.; Dimaras, H.; Chan, H.S.L.; Héon, E.; Gallie, B.L.; Shaikh, F. Intra-arterial Chemotherapy for Retinoblastoma: A Systematic Review. JAMA Ophthalmol. 2016, 134, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.F.; Macedo, C.R.P.D.; Fonseca, J.R.F.; Morales, B.; Mangeon, M.K.; Miranda, B.A.; Casaroli-Marano, R.; Sallum, J.M.F. Intra-arterial chemotherapy for retinoblastoma, outcomes analysis in 357 eyes: 13 years of experience in a referral center in Brazil. Ophthalmol. Retin. 2025, in press. [Google Scholar]
- Ancona-Lezama, D.; Dalvin, L.A.; Lucio-Alvarez, J.A.; Jabbour, P.; Shields, C.L. Ophthalmic vascular events after intra-arterial chemotherapy for retinoblastoma: Real-World Comparison Between Primary and Secondary Treatments. Retina 2019, 39, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Linn Murphree, A. Intraocular retinoblastoma: The case for a new group classification. Ophthalmol. Clin. N. Am. 2005, 18, 41–53. [Google Scholar] [CrossRef]
- Mallipatna, A.C.; Gallie, B.L.; Chévez-Barrios, P.; Singh, A.D.; Gombos, D.S.; Hungerford, J.; Shields, C.L.; Demirci, H.; Eagle, R.C., Jr.; Wilson, M.W. Retinoblastoma. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., Eds.; Springer: New York, NY, USA, 2017; pp. 819–831. [Google Scholar]
- Muen, W.J.; Kingston, J.E.; Robertson, F.; Brew, S.; Sagoo, M.S.; Reddy, M.A. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology 2012, 119, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jing, C.; Hua, X.; Liang, T.; Li, J.; Zhao, P.; Ji, X. Outcome of salvage intra-arterial chemotherapy for recurrent retinoblastoma. Eye 2022, 36, 2106–2110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shields, C.L.; Manjandavida, F.P.; Lally, S.E.; Pieretti, G.; Arepalli, S.A.; Caywood, E.H.; Jabbour, P.; Shields, J.A. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: Outcomes based on the international classification of retinoblastoma. Ophthalmology 2014, 121, 1453–1460. [Google Scholar] [CrossRef]
- Abramson, D.H.; Daniels, A.B.; Marr, B.P.; Francis, J.H.; Brodie, S.E.; Dunkel, I.J.; Gobin, Y.P. Intra-Arterial Chemotherapy (Ophthalmic Artery Chemosurgery) for Group D Retinoblastoma. PLoS ONE 2016, 11, e0146582. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.H.; Fabius, A.W.; Issa, R.; Francis, J.H.; Marr, B.P.; Dunkel, I.J.; Gobin, Y.P. Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. PLoS ONE 2015, 10, e0145436. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.H.; Abramson, D.H.; Gobin, Y.P.; Marr, B.P.; Tendler, I.; Brodie, S.E.; Dunkel, I.J. Efficacy and toxicity of second-course ophthalmic artery chemosurgery for retinoblastoma. Ophthalmology 2015, 122, 1016–1022. [Google Scholar]
- Shields, C.L.; Lally, S.E.; Leahey, A.M.; Jabbour, P.M.; Caywood, E.H.; Schwendeman, R.; Shields, J.A. Targeted retinoblastoma management: When to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr. Opin. Ophthalmol. 2014, 25, 374–385. [Google Scholar]
- Gobin, Y.P.; Dunkel, I.J.; Marr, B.P.; Brodie, S.E.; Abramson, D.H. Intra-arterial chemotherapy for the management of reti-noblastoma: Four-year experience. Arch. Ophthalmol. 2011, 129, 732–737. [Google Scholar]
- Klufas, M.A.; Gobin, Y.P.; Marr, B.; Brodie, S.E.; Dunkel, I.J.; Abramson, D.H. Intra-arterial chemotherapy as a treatment for intraocular retinoblastoma: Alternatives to direct ophthalmic artery catheterization. AJNR Am. J. Neuroradiol. 2012, 33, 1608–1614. [Google Scholar]
- Abramson, D.H.; Brodie, S.E.; Dunkel, I.J. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma: Five-year two-institution experience. PLoS ONE 2012, 7, e34120. [Google Scholar]
- Reddy, M.A.; Naeem, Z.; Duncan, C.; Robertson, F.; Herod, J.; Rennie, A.; Liasis, A.; Thompson, D.A.; Sagoo, M. Reduction of severe visual loss and complications following intra-arterial chemotherapy (IAC) for refractory retinoblastoma. Br. J. Ophthalmol. 2017, 101, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Ammanuel, S.; Alexander, M.D.; Damato, B.; Cooke, D.L.; Halbach, V.V.; Amans, M.R.; Dowd, M.R.; Higashida, R.T.; Hetts, S.W. Improved procedural safety following protocol changes for selective ophthalmic arterial infusion of chemotherapy for treatment of ocular retinoblastoma. Interv. Neuroradiol. 2018, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, C.; Bartolini, B.; Marie, G.; Beck-Popovic, M.; Saliou, G.; Munier, F.L. Risk Factors for Acute Choroidal Ischemia after Intra-arterial Melphalan for Retinoblastoma: The Role of the Catheterization Approach. Ophthalmology 2021, 128, 754–764. [Google Scholar] [CrossRef]
- Daniels, A.B.; Froehler, M.T.; Nunnally, A.H.; Pierce, J.M.; Bozic, I.; Stone, C.A.; Santapuram, P.R.; Tao, Y.K.; Boyd, K.L.; Himmel, L.E.; et al. Rabbit Model of Intra-Arterial Chemotherapy Toxicity Demonstrates Retinopathy and Vasculopathy Related to Drug and Dose, Not Procedure or Approach. Investig. Ophthalmol. Vis. Sci. 2019, 60, 954–964. [Google Scholar] [CrossRef]
- Dalvin, L.A.; Ancona-Lezama, D.; Lucio-Alvarez, J.A.; Masoomian, B.; Jabbour, P.; Shields, C.L. Ophthalmic vascular events after primary unilateral intra-arterial chemotherapy for retinoblastoma in early and recent eras. Ophthalmology 2018, 125, 1803–1811. [Google Scholar] [CrossRef]
- Boddu, S.R.; Abramson, D.H.; Marr, B.P.; Francis, J.H.; Gobin, Y.P. Selective ophthalmic artery chemosurgery (SOAC) for retinoblastoma: Fluoroscopic time and radiation dose parameters. A baseline study. J. Neurointerv. Surg. 2017, 9, 1107–1112. [Google Scholar] [CrossRef]
- Guasti, A.; Leonini, S.; Bertelli, E.; Baldi, R.; Gennari, P.; Cioni, S.; Vallone, I.M.; Romano, D.G.; Casseri, T.; Guerrini, L.; et al. Intra-arterial chemotherapy for retinoblastoma: The dosimetric impact. Neuroradiology 2019, 61, 1083–1091. [Google Scholar] [CrossRef]
- Opitz, M.; Bos, D.; Deuschl, C.; Radbruch, A.; Zensen, S.; Sirin, S.; Forsting, M.; Bechrakis, N.; Biewald, E.; Bornfeld, N.; et al. Estimation of radiation exposure of children undergoing superselective intra-arterial chemotherapy for retinoblastoma treatment: Assessment of local diagnostic reference levels as a function of age, sex, and interventional success. Neuroradiology 2021, 63, 391–398. [Google Scholar] [CrossRef]
- Manjandavida, F.P.; Stathopoulos, C.; Zhang, J.; Honavar, S.G.; Shields, C.L. Intra-arterial chemotherapy in retinoblastoma—A paradigm change. Indian J. Ophthalmol. 2019, 67, 740–754, Erratum in Indian J. Ophthalmol. 2019, 67, 1385. [Google Scholar] [CrossRef] [PubMed]
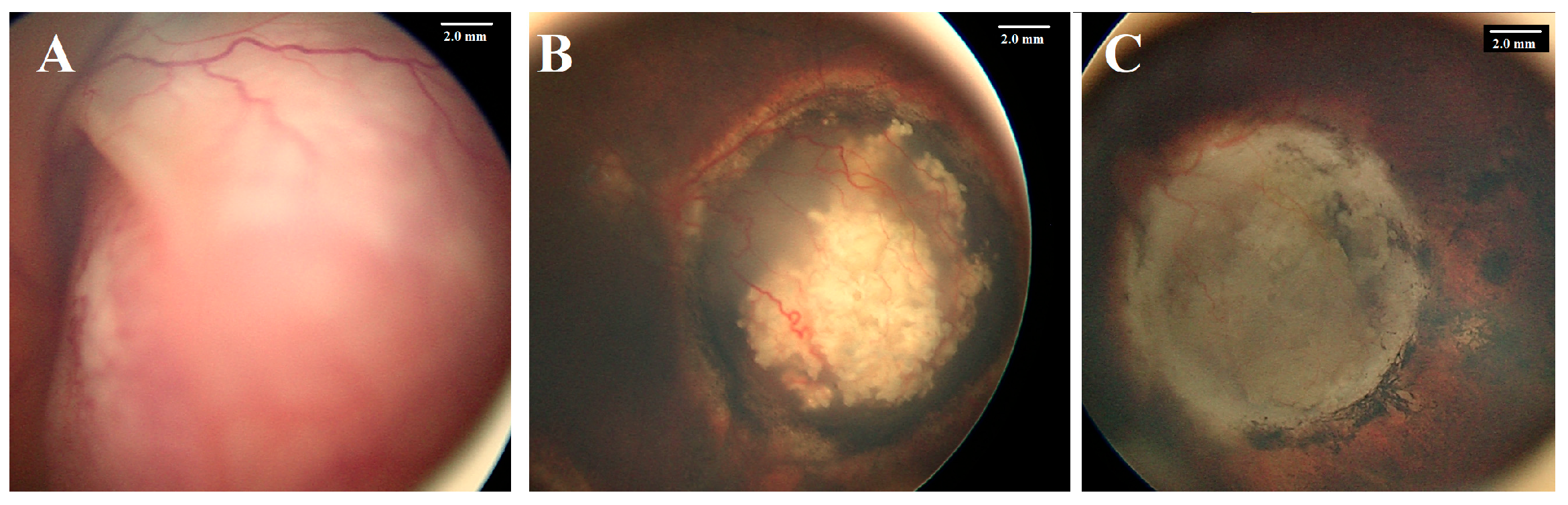
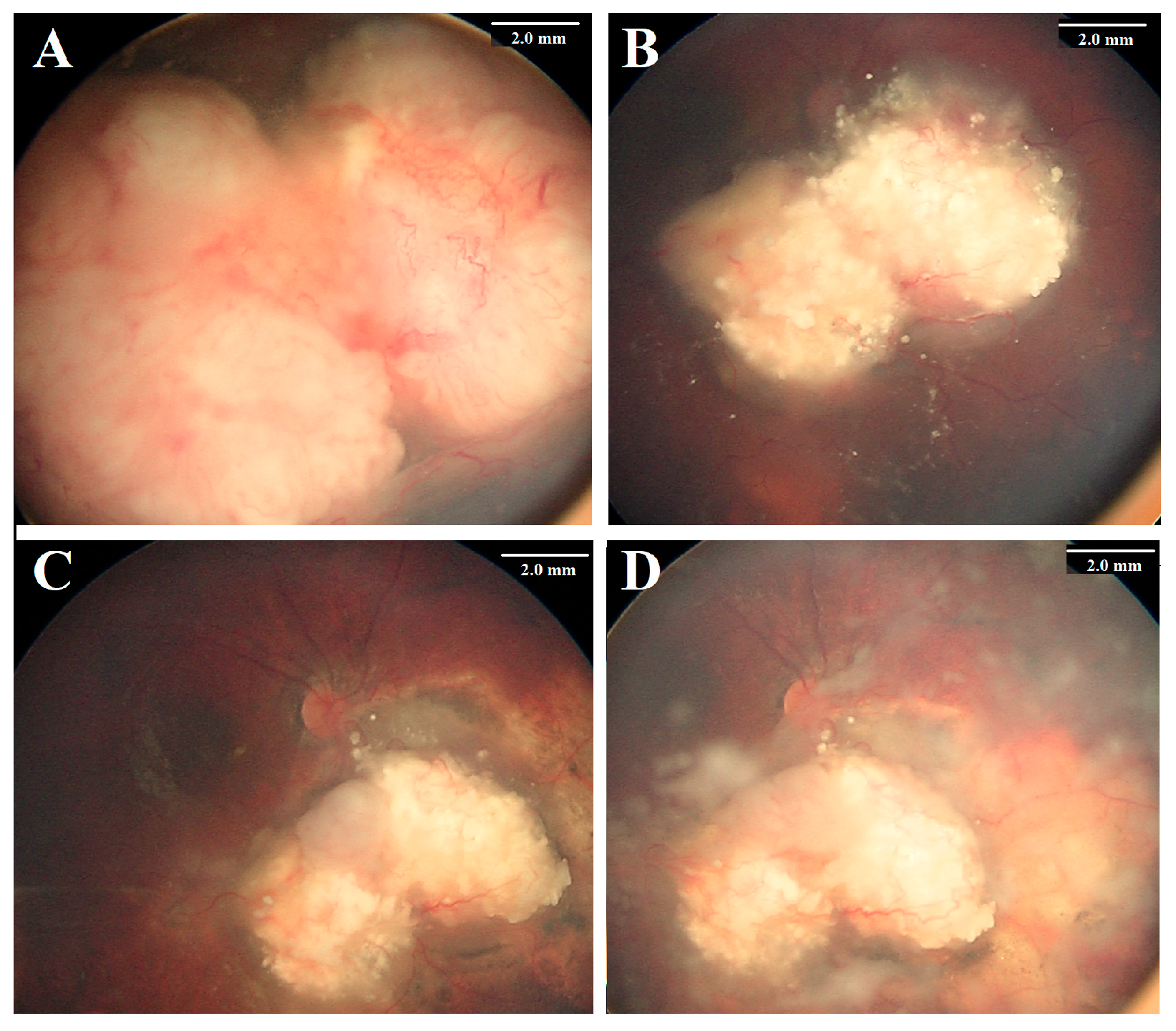
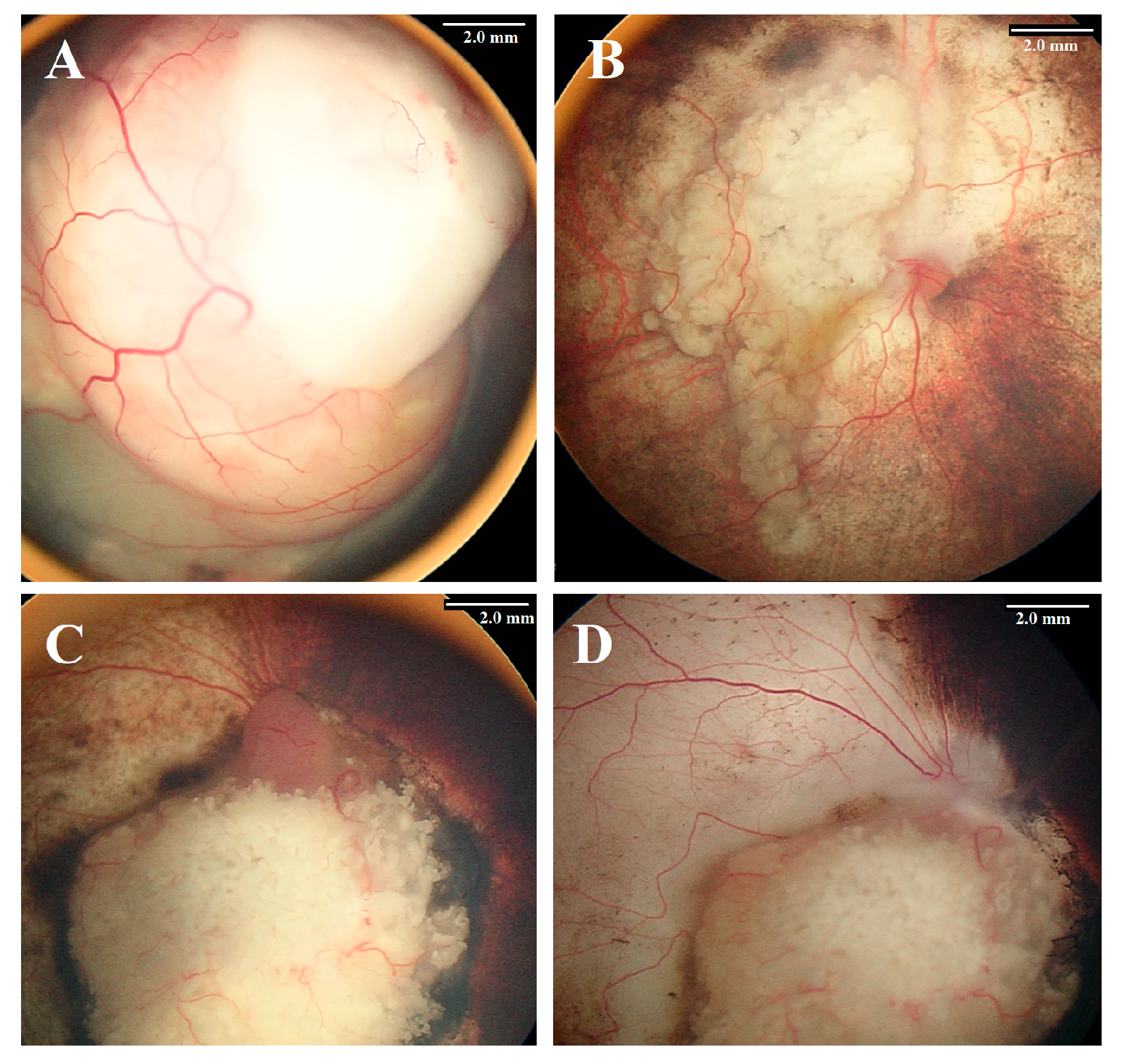
| Feature | Number | % | Eye Salvage | % | p Value | |
|---|---|---|---|---|---|---|
| Number | 20 Eyes; 67 Injections | 11 | 55% | |||
| Age at diagnosis | median = 19, mean = 21, range 1–72 Months | |||||
| Age at the first injection | ≤12 months | 4 | 20% | 3 | 75% | 0.37 |
| >12 months | 16 | 80% | 8 | 50% | ||
| Gender | Male | 7 | 35% | 3 | 43% | 0.42 |
| Female | 13 | 65% | 8 | 61% | ||
| Family history | Positive | 1 | 5% | 1 | 100% | 0.35 |
| Negative | 19 | 95% | 10 | 53% | ||
| Laterality | Unilateral | 11 | 55% | 6 | 54% | 0.48 |
| Bilateral | 9 | 45% | 5 | 55% | ||
| Side | Right | 11 | 55% | 7 | 64% | 0.40 |
| Left | 9 | 45% | 4 | 44% | ||
| Indication | Primary | 6 | 35% | 5 | 71% | 0.047 |
| Secondary | 14 | 65% | 6 | 46% | ||
| IIRC stage | C | 6 | 30% | 5 | 83% | 0.047 |
| D | 13 | 70% | 6 | 46% | ||
| E # | 1 | 5% | 0 | 0% | ||
| TNM stage | cT2a | 2 | 10% | 2 | 100% | 0.478 |
| cT2b | 17 | 85% | 9 | 67% | ||
| cT3c | 1 | 5% | 0 | 0% | ||
| Subretinal seeds | No | 6 | 30% | 4 | 67% | 0.337 |
| Yes | 14 | 70% | 7 | 50% | ||
| Vitreous seeds | No | 12 | 60% | 9 | 75% | 0.046 |
| Yes | 8 | 40% | 3 | 38% | ||
| Number of injections | ≤3 cycles | 15 | 75% | 10 | 67% | 0.034 |
| >3 cycles | 5 | 25% | 1 | 20% | ||
| Outcome (20 Patients) | Number | Percentage |
|---|---|---|
| Enucleation | 9 | 45% |
| High-risk pathological features | 0 | 0% |
| Orbital recurrence | 0 | 0% |
| Distant metastasis | 1 # | 5% |
| Systemic Complication (20 Patients) | 5 | 25% |
| Neutropenia | 4 | 20% |
| Skin erythema | 1 | 5% |
| Procedure-related Complications (67 procedures) | 15 | 22% |
| Failure of procedure | 5 | 7% |
| Ophthalmic arterial spasm | 4 | 6% |
| Strokes | 2 | 3% |
| Bronchospasm | 4 $ | 6% |
| Ocular Complications (20 Eyes) | 5 | 25% |
| Subretinal hemorrhage | 2 | 10% |
| Tumor hemorrhage | 3 * | 15% |
| Optic atrophy | 2 | 10% |
| Retinal detachment | 2 | 10% |
| Severe choroidal and retinal ischemia | 2 | 10% |
| Peripheral chorioretinal atrophy | 3 | 15% |
| Oculomotor palsy | 1 | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, Y.A.; Mohammad, M.; Al-Jabari, O.; Halawa, F.; Al-Fahoum, L.; Halalsheh, H.; Khzouz, J.; Al-Hussaini, M.; Jaradat, I.; Mehyar, M.; et al. Intra-Arterial Melphalan Chemotherapy for Retinoblastoma in a Developing Nation: Real-World Outcomes and Prognostic Factors. Cancers 2025, 17, 1955. https://doi.org/10.3390/cancers17121955
Yousef YA, Mohammad M, Al-Jabari O, Halawa F, Al-Fahoum L, Halalsheh H, Khzouz J, Al-Hussaini M, Jaradat I, Mehyar M, et al. Intra-Arterial Melphalan Chemotherapy for Retinoblastoma in a Developing Nation: Real-World Outcomes and Prognostic Factors. Cancers. 2025; 17(12):1955. https://doi.org/10.3390/cancers17121955
Chicago/Turabian StyleYousef, Yacoub A., Mona Mohammad, Odai Al-Jabari, Farah Halawa, Lama Al-Fahoum, Hadeel Halalsheh, Jakub Khzouz, Maysa Al-Hussaini, Imad Jaradat, Mustafa Mehyar, and et al. 2025. "Intra-Arterial Melphalan Chemotherapy for Retinoblastoma in a Developing Nation: Real-World Outcomes and Prognostic Factors" Cancers 17, no. 12: 1955. https://doi.org/10.3390/cancers17121955
APA StyleYousef, Y. A., Mohammad, M., Al-Jabari, O., Halawa, F., Al-Fahoum, L., Halalsheh, H., Khzouz, J., Al-Hussaini, M., Jaradat, I., Mehyar, M., Rejdak, R., Toro, M. D., Haboob, H., & Al-Nawaiseh, I. (2025). Intra-Arterial Melphalan Chemotherapy for Retinoblastoma in a Developing Nation: Real-World Outcomes and Prognostic Factors. Cancers, 17(12), 1955. https://doi.org/10.3390/cancers17121955








