From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia
Simple Summary
Abstract
1. Introduction
2. Methods
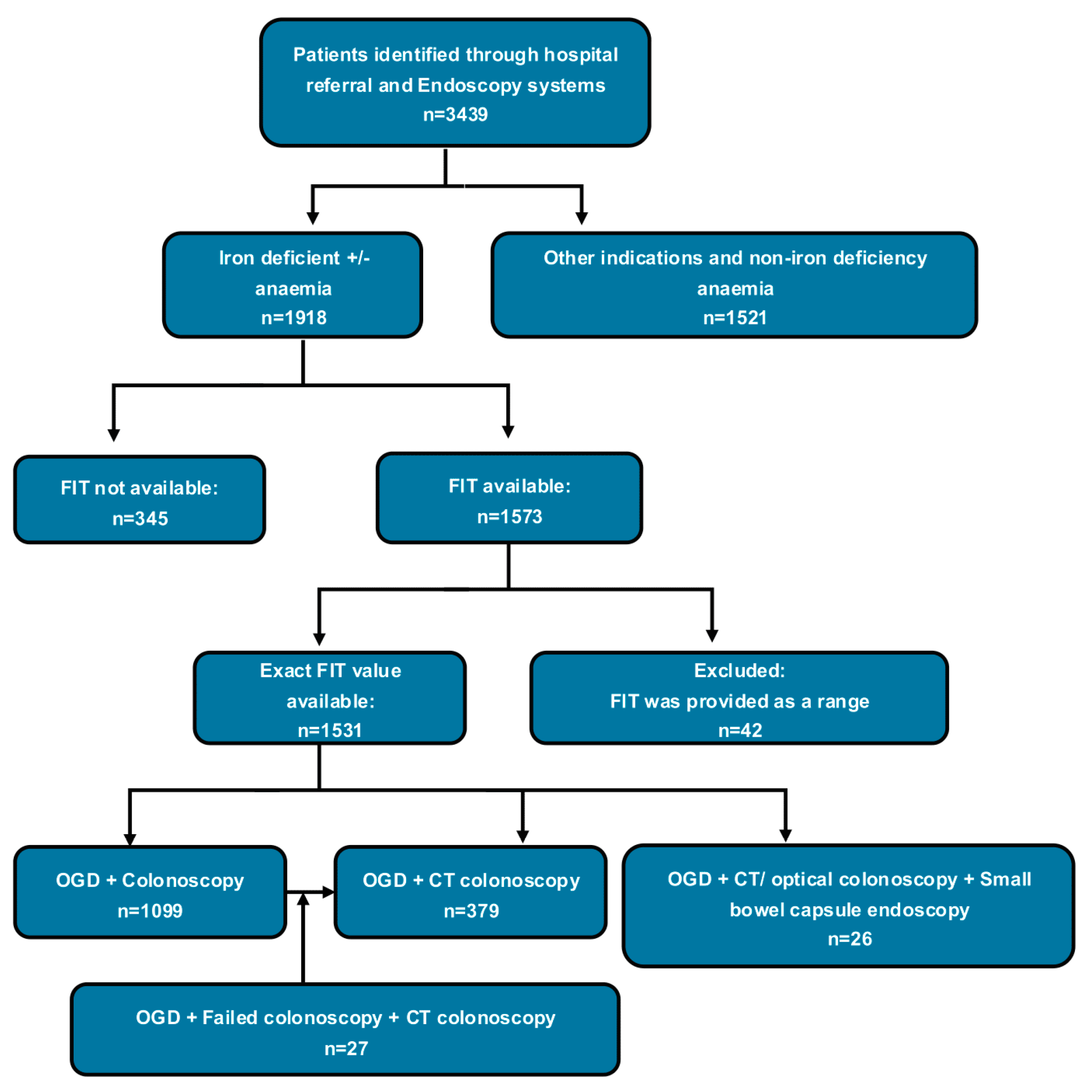
2.1. Study Design and Participants
2.2. Outcome Measures
2.3. Statistical Analysis
- (1)
- A threshold-based analysis was conducted using the Cumulative Sum Control (CUSUM) Charts by incrementally increasing FIT values (0.1 µg/g) and calculating the cumulative number of urgent colonoscopies at each level. Sharp increases (≥5 cases) were identified as “jump points”, indicating potential inflection thresholds. Corresponding conversion rates were calculated as the proportion of urgent cases at or below each FIT threshold [16].
- (2)
- A cost–benefit analysis was performed to estimate the net benefit per patient across varying FIT thresholds, incorporating the trade-offs between true positives, true negatives, false positives, and false negatives using predefined clinical cost and outcome values (see Table S2 in the Supplementary Materials) [17,18].
- (3)
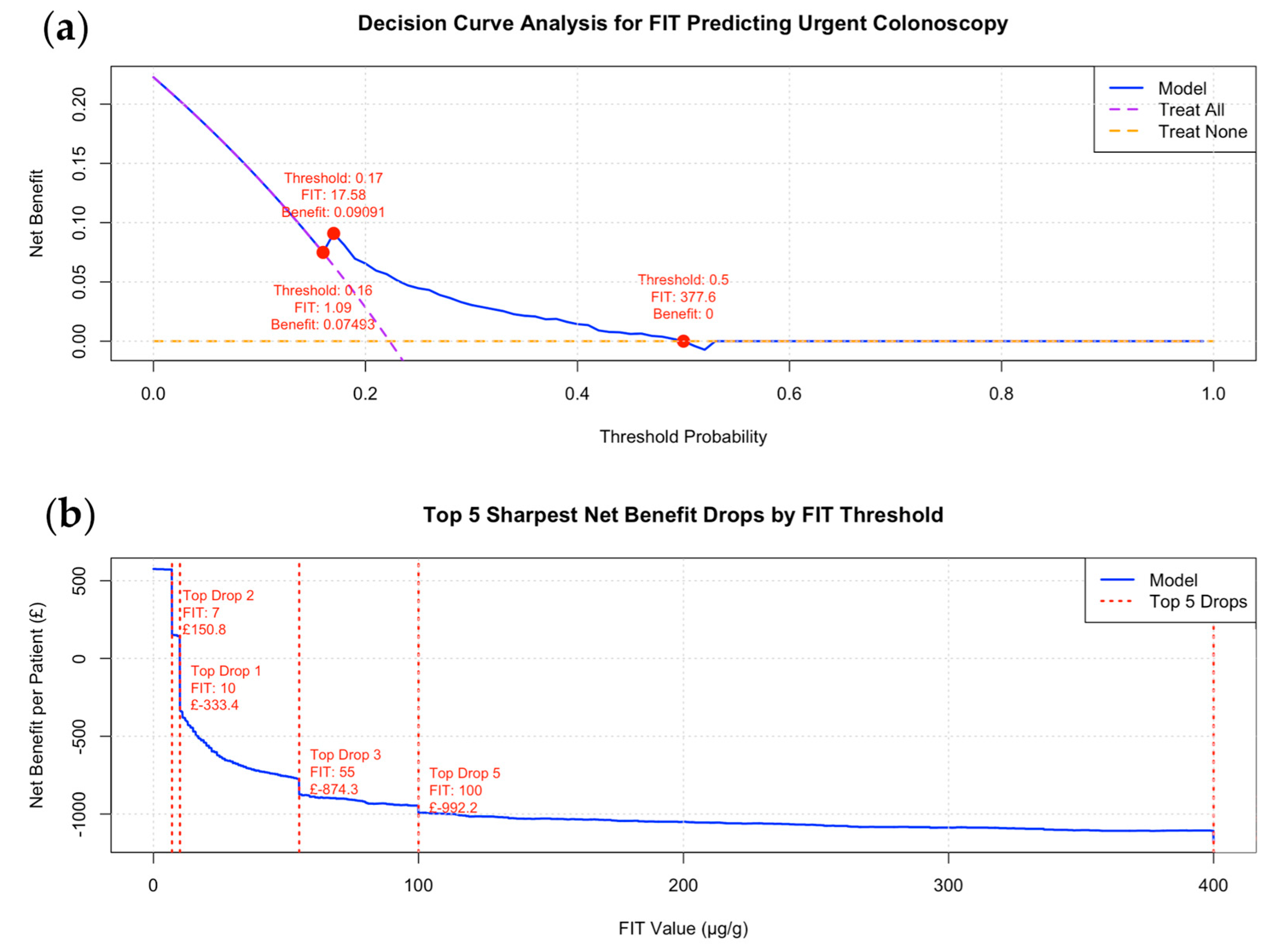
- A decision curve analysis (DCA) was conducted using a logistic regression model, with the binary outcome variable “Urgent Colonoscopy” modelled as a function of continuous FIT values. Predicted probabilities were used to calculate the net benefit across threshold probabilities ranging from 0 to 1 (in 0.01 increments), classifying patients as high or low risk based on each threshold [19,20,21,22].
2.4. Ethical Approval
3. Results
3.1. The Accuracy of FIT in Predicting CRC, Polyp, and CCC (Urgent)
3.2. Threshold-Based Analysis

3.3. Decision Curve and Cost–Benefit Analysis for FIT Thresholds to Minimise CCC
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BSG | British Society of Gastroenterology |
| CCC | CCE-to-Colonoscopy Conversion |
| CCE | Colon Capsule Endoscopy |
| COVID-19 | Coronavirus Disease 2019 |
| CR | Completion Rate |
| CRC | Colorectal Cancer |
| CT | Computed Tomography |
| CTC | CT Colonography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| GI | Gastrointestinal |
| NHS | National Health Service |
| NPV | Negative Predicted Value |
| PCE | Panenteric Capsule Endoscopy |
References
- Cilona, A.; Zullo, A.; Hassan, C.; Ridola, L.; Annese, M. Is faecal-immunochemical test useful in patients with iron deficiency anaemia and without overt bleeding? Dig. Liver Dis. 2011, 43, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Alquist, D. American Gastroenterological Association medical position statement: Evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology 2000, 118, 197–201. [Google Scholar] [CrossRef]
- James, M.W.; Chen, C.M.; Goddard, W.P.; Scott, B.B.; Goddard, A.F. Risk factors for gastrointestinal malignancy in patients with iron-deficiency anaemia. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef]
- Romeo, S.; Neri, B.; Mossa, M.; Riccioni, M.E.; Scucchi, L.; Sena, G.; Potenza, S.; Petruzziello, C.; Biancone, L. Diagnostic yield of small bowel capsule endoscopy in obscure gastrointestinal bleeding: A real-world prospective study. Intern. Emerg. Med. 2022, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Leen, R.; O’morain, C.; McNamara, D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy 2014, 46, 473–478. [Google Scholar]
- Turvill, J.; Haritakis, M.; Pygall, S.; Bryant, E.; Cox, H.; Forshaw, G.; Musicha, C.; Allgar, V.; Logan, R.; McAlindon, M. Multicentre Study of 10,369 Symptomatic Patients Comparing the Diagnostic Accuracy of Colon Capsule Endoscopy, Colonoscopy and CT Colonography. Aliment. Pharmacol. Ther. 2025, 61, 1532–1544. [Google Scholar] [CrossRef]
- MacLeod, C.; Hudson, J.; Brogan, M.; Cotton, S.; Treweek, S.; MacLennan, G.; Watson, A.J.M. ScotCap—A large observational cohort study. Color. Dis. 2022, 24, 411–421. [Google Scholar] [CrossRef]
- Healthcare Improvement Scotland. Colon Capsule Endoscopy for Detection of Colorectal Polyps and Cancer. Innovative Medical Technology Overview. 2024. Available online: https://shtg.scot/media/2430/20240109-cce-update-v10.pdf (accessed on 1 April 2025).
- Alihosseini, S.; Aryankhesal, A.; Sabermahani, A. Second-generation colon capsule endoscopy for detection of colorectal polyps: A meta-analysis. Med. J. Islam. Repub. Iran. 2020, 34, 81. [Google Scholar] [CrossRef]
- Mollers, T.; Schwab, M.; Gildein, L.; Hoffmeister, M.; Albert, J.; Brenner, H.; Jager, S. Second-generation colon capsule endoscopy for detection of colorectal polyps: Systematic review and meta-analysis of clinical trials. Endosc. Int. Open 2021, 9, E562–E571. [Google Scholar] [CrossRef]
- (NICE), N.I.F.H.A.C.E. PillCam COLON 2 for Investigation of the Colon Through Direct Visualisation. Available online: https://www.nice.org.uk/guidance/gid-dg10083/documents/final-scope-2 (accessed on 23 March 2025).
- Lei, I.I.; Robertson, A.; Koulaouzidis, A.; Arasaradnam, R.; The International Capsule Endoscopy Research Group. Evaluation of Colon Capsule Utilisation in Europe-CAPTURE EU Survey Findings. J. Clin. Med. 2024, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Joint Advisory Group on Gastrointestinal Endoscopy. Guide to Meeting the Quality and Safety Standards. 2021. Available online: https://www.thejag.org.uk/CMS/UploadedDocuments/Scheme/Scheme5/Guidance/191107%20-%20guidance%20-%20JAG%20quality%20and%20safety%201.0%20final.pdf (accessed on 1 April 2025).
- Lei, I.I.; Parisi, I.; Bhandare, A.; Perez, F.P.; Lee, T.; Shehkar, C.; McStay, M.; Anderson, S.; Watson, A.; Conlin, A.; et al. Factors predicting conversion from colon capsule endoscopy to conventional optical endoscopy-findings from the CESCAIL study. BMC Gastroenterol. 2025, 25, 363. [Google Scholar] [CrossRef]
- Grigg, O.A.; Farewell, V.T.; Spiegelhalter, D.J. Use of risk-adjusted CUSUM and RSPRT charts for monitoring in medical contexts. Stat. Methods Med. Res. 2003, 12, 147–170. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.; Oudre, L.; Vayatis, N. Selective review of offline change point detection methods. Signal Process. 2020, 167, 107299. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Making 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Vickers, A.J.; van Calster, B.; Steyerberg, E.W. A simple, step-by-step guide to interpreting decision curve analysis. Diagn. Progn. Res. 2019, 3, 18. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016, 352, i6. [Google Scholar] [CrossRef]
- Champely, S. pwr: Basic Functions for Power Analysis, R Package Version 1.3-0. 2020. Available online: https://cran.r-project.org/web/packages/pwr/pwr.pdf (accessed on 3 April 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: Display and Analyze ROC Curves. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles. In R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Council, M.R. Is My Study Research? NHS Health Research Authority: London, UK, 2022. [Google Scholar]
- Petersen, M.M.; Kleif, J.; Jorgensen, L.N.; Hendel, J.W.; Seidelin, J.B.; Madsen, M.R.; Vilandt, J.; Brandsborg, S.; Rasmussen, J.S.; Andersen, L.M.; et al. Optimizing Screening for Colorectal Cancer: An Algorithm Combining Fecal Immunochemical Test, Blood-Based Cancer-Associated Proteins and Demographics to Reduce Colonoscopy Burden. Clin. Color. Cancer 2023, 22, 199–210. [Google Scholar] [CrossRef]
- Afzal, A.; Aranan, Y.S.; Roberts, T.; Covington, J.; Vidal, L.; Ahmed, S.; Gill, T.; Francis, N. Diagnostic accuracy of the faecal immunochemical test and volatile organic compound analysis in detecting colorectal polyps: Meta-analysis. BJS Open 2024, 9, zrae154. [Google Scholar] [CrossRef] [PubMed]
- Turvill, J.L.; Turnock, D.; Cottingham, D.; Haritakis, M.; Jeffery, L.; Girdwood, A.; Hearfield, T.; Mitchell, A.; Keding, A. The Fast Track FIT study: Diagnostic accuracy of faecal immunochemical test for haemoglobin in patients with suspected colorectal cancer. Br. J. Gen. Pract. 2021, 71, e643–e651. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Burke, C.A.; Aggarwal, M.; Macaron, C.; Singh, A.; Kim, M.K.; Regueiro, M.; Amit, B.; Chahal, P.; Garg, S. Sessile serrated polyp detection rates after fecal immunochemical test or multitarget stool DNA test: Systematic review and meta-analysis. Endosc. Int. Open 2024, 12, E474–E487. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Weigl, K.; Hoffmeister, M.; Brenner, H. Fecal immunochemical tests in combination with blood tests for colorectal cancer and advanced adenoma detection-systematic review. United Eur. Gastroenterol. J. 2018, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chandrapalan, S.; Khasawneh, F.; Singh, B.; Lewis, S.; Turvill, J.; Persaud, K.; Arasaradnam, R.P. A Multi-Centre Study to Risk Stratify Colorectal Polyp Surveillance Patients Utilising Volatile Organic Compounds and Faecal Immunochemical Test. Cancers 2022, 14, 4951. [Google Scholar] [CrossRef]
- Spada, C.; Piccirelli, S.; Hassan, C.; Ferrari, C.; Toth, E.; Gonzalez-Suarez, B.; Keuchel, M.; McAlindon, M.; Finta, A.; Rosztoczy, A.; et al. AI-assisted capsule endoscopy reading in suspected small bowel bleeding: A multicentre prospective study. Lancet Digit. Health 2024, 6, e345–e353. [Google Scholar] [CrossRef]
- Moen, S.; Vuik, F.E.R.; Kuipers, E.J.; Spaander, M.C.W. Artificial Intelligence in Colon Capsule Endoscopy—A Systematic Review. Diagnostics 2022, 12, 1994. [Google Scholar] [CrossRef]
- Nadimi, E.S.; Braun, J.M.; Schelde-Olesen, B.; Khare, S.; Gogineni, V.C.; Blanes-Vidal, V.; Baatrup, G. Towards full integration of explainable artificial intelligence in colon capsule endoscopy’s pathway. Sci. Rep. 2025, 15, 5960. [Google Scholar] [CrossRef]
- Lei, I.I.; Parsons, N.; Koulaouzidis, A.; Alexander, R.; Wenzek, H.; Laiz, P.; Watson, A.; White, E.; Arasaradna, R. P165 Accelerating cutting edge breakthroughs: Capsule endoscopy delivery at scale through enhanced AI analysis (CESCAIL) study—A comprehensive interim analysis. Gut 2024. [Google Scholar] [CrossRef]
- Lei, I.I.; Tompkins, K.; White, E.; Watson, A.; Parsons, N.; Noufaily, A.; Segui, S.; Wenzek, H.; Badreldin, R.; Conlin, A.; et al. Study of capsule endoscopy delivery at scale through enhanced artificial intelligence-enabled analysis (the CESCAIL study). Color. Dis. 2023, 25, 1498–1505. [Google Scholar] [CrossRef]
- Holleran, G.E.; Barry, S.A.; Thornton, O.J.; Dobson, M.J.; McNamara, D.A. The use of small bowel capsule endoscopy in iron deficiency anaemia: Low impact on outcome in the medium term despite high diagnostic yield. Eur. J. Gastroenterol. Hepatol. 2013, 25, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, A.; Rondonotti, E.; Giannakou, A.; Plevris, J.N. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: A systematic review. Gastrointest. Endosc. 2012, 76, 983–992. [Google Scholar] [CrossRef] [PubMed]


| Number of included patients | 1531 |
| Age (mean) | 65.4 ± 14.3 |
| Sex | |
| Male | 664 (43.4) |
| Female | 867 (56.6) |
| Indications | |
| Asymptomatic IDA | 757 (49.4) |
| FIT positive | 250 (16.3) |
| Dyspepsia | 29 (1.9) |
| Rectal bleeding | 49 (3.2) |
| Dysphagia | 24 (1.6) |
| Change in bowel habit | 52 (3.4) |
| Weight loss | 80 (5.2) |
| Abnormal imaging | 18 (1.2) |
| Abdominal pain | 55 (3.6) |
| Diarrhoea | 18 (1.2) |
| Other * | 199 (13) |
| Smoking | |
| Smoker | 79 (5.2) |
| Non-smoker | 447 (29.2) |
| Ex-smoker | 211 (13.8) |
| Missing data | 794 (51.8) |
| FIT level (μg/g) | |
| Median | 16 |
| Mean | 77.8 ± 122.5 |
| Bowel preparation | |
| Adequate | 641 (41.9) |
| Poor | 40 (2.6) |
| Missing data | 850 (55.5) |
| Haemoglobin | 112.2 ± 19.1 |
| Significant Findings | |
| Normal | 630 (41.1) |
| Polyp | 534 (34.9) |
| Diverticulosis (Only) | 123 (8.0) |
| CRC | 103 (6.7) |
| Angioectasia | 15 (1.0) |
| IBD | 47 (3.1) |
| Lymphoma | 4 (0.3) |
| Other findings ** | 75 (4.9) |
| Number patients with advanced polyps (≥10 mm) | 211 (13.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, I.I.; O’Connell, N.; Adu-Darko, M.A.; Parambil, J.; Suresh, V.; Mc Donnell, K.; Newville, J.; Chaplin, K.; Siyambalapityage, D.; Khan, A.; et al. From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia. Cancers 2025, 17, 1951. https://doi.org/10.3390/cancers17121951
Lei II, O’Connell N, Adu-Darko MA, Parambil J, Suresh V, Mc Donnell K, Newville J, Chaplin K, Siyambalapityage D, Khan A, et al. From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia. Cancers. 2025; 17(12):1951. https://doi.org/10.3390/cancers17121951
Chicago/Turabian StyleLei, Ian Io, Nicola O’Connell, Michael Agyekum Adu-Darko, Jessiya Parambil, Vishnupriya Suresh, Kiara Mc Donnell, Jessie Newville, Kirsten Chaplin, Deekshi Siyambalapityage, Asad Khan, and et al. 2025. "From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia" Cancers 17, no. 12: 1951. https://doi.org/10.3390/cancers17121951
APA StyleLei, I. I., O’Connell, N., Adu-Darko, M. A., Parambil, J., Suresh, V., Mc Donnell, K., Newville, J., Chaplin, K., Siyambalapityage, D., Khan, A., Muhammad, U., Emil, J., Abbas, M., Kanji, Z., Khalil, O., Alam, H., Bennett, A., Soanes, H., Bhattacharyya, A., ... Arasaradnam, R. P. (2025). From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia. Cancers, 17(12), 1951. https://doi.org/10.3390/cancers17121951









