Perioperative Systemic Therapy in Rare, Chemosensitive Subtypes of Retroperitoneal Sarcoma: A Hospital-Based Propensity Score-Matched Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
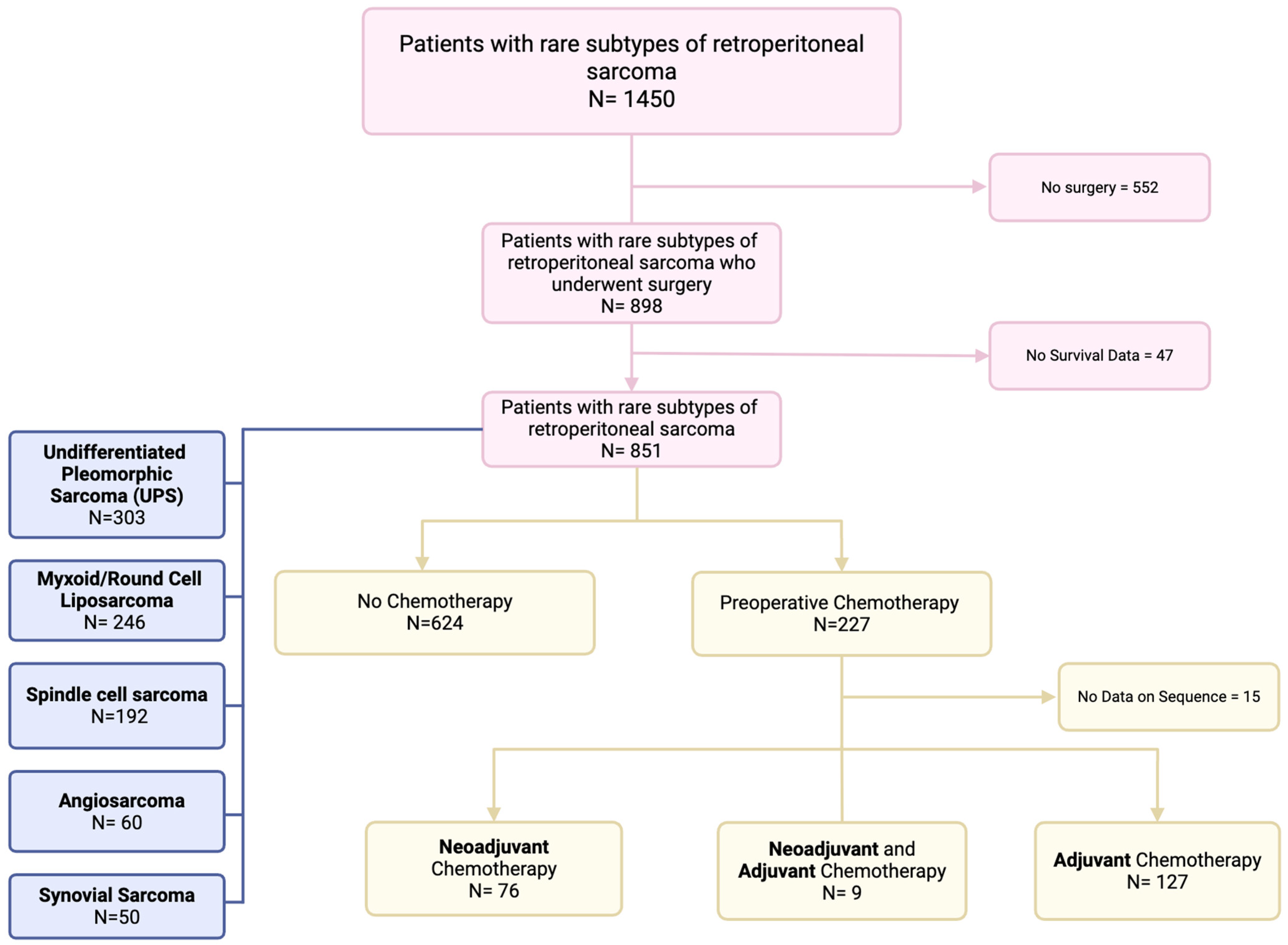
2.1. Data Source and Study Population
2.2. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Pathologic Characteristics
3.2. Multivariable Analysis
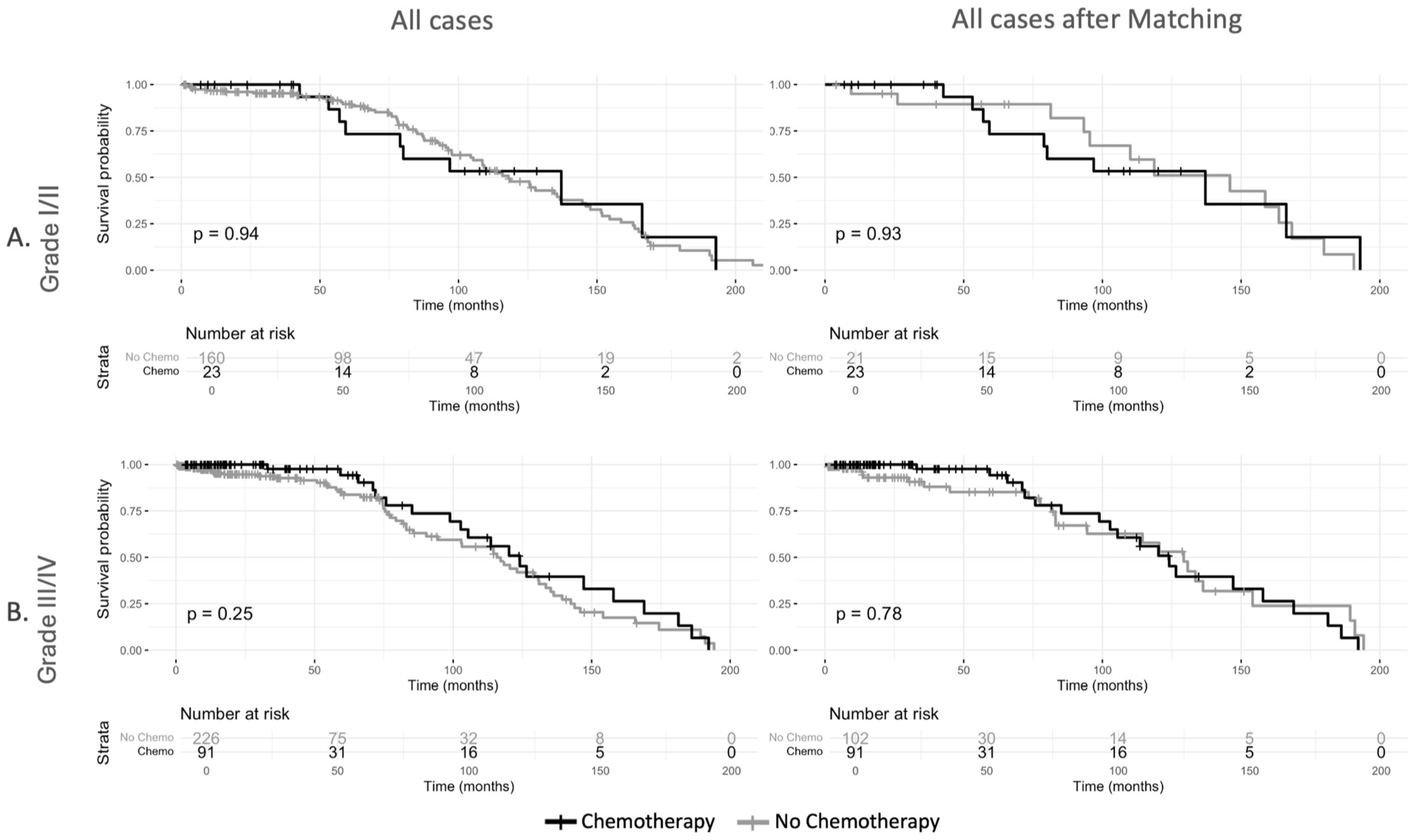
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leffall, L.D., Jr. Retroperitoneal sarcoma. Ann. Surg. 1991, 214, 1. [Google Scholar]
- Schmitz, E.; Nessim, C. Retroperitoneal Sarcoma Care in 2021. Cancers 2022, 14, 1293. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Zlotecki, R.A.; Hochwald, S.N.; Hemming, A.W.; Grobmyer, S.R.; Cance, W.G. Retroperitoneal soft tissue sarcoma. Cancer 2005, 104, 669–675. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Almond, L.M.; Gronchi, A.; Strauss, D.; Jafri, M.; Ford, S.; Desai, A. Neoadjuvant and adjuvant strategies in retroperitoneal sarcoma. Eur. J. Surg. Oncol. 2018, 44, 571–579. [Google Scholar] [CrossRef]
- Datta, J.; Ecker, B.L.; Neuwirth, M.G.; Geha, R.C.; Fraker, D.L.; Roses, R.E.; Karakousis, G.C. Contemporary reappraisal of the efficacy of adjuvant chemotherapy in resected retroperitoneal sarcoma: Evidence from a nationwide clinical oncology database and review of the literature. Surg. Oncol. 2017, 26, 117–124. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Xiao, M.; Wu, S.; Min, L.; Luo, C. Adjuvant therapy for retroperitoneal sarcoma: A meta-analysis. Radiat. Oncol. 2021, 16, 196. [Google Scholar] [CrossRef]
- Miura, J.T.; Charlson, J.; Gamblin, T.C.; Eastwood, D.; Banerjee, A.; Johnston, F.M.; Turaga, K.K. Impact of chemotherapy on survival in surgically resected retroperitoneal sarcoma. Eur. J. Surg. Oncol. 2015, 41, 1386–1392. [Google Scholar] [CrossRef]
- Angele, M.K.; Albertsmeier, M.; Prix, N.J.; Hohenberger, P.; Abdel-Rahman, S.; Dieterle, N.; Schmidt, M.; Mansmann, U.; Bruns, C.J.; Issels, R.D.; et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: A subgroup analysis of a randomized phase-III multicenter study. Ann. Surg. 2014, 260, 749–754. [Google Scholar] [CrossRef]
- Zhou, D.D.; Connolly, E.A.; Mar, J.; Lazarakis, S.; Grimison, P.S.; Connor, J.; Gyorki, D.E.; Hong, A.M. A systematic review of the role of chemotherapy in retroperitoneal sarcoma by the Australia and New Zealand sarcoma association clinical practice guidelines working party. Cancer Treat. Rev. 2024, 122, 102663. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Lambdin, J.; Ryan, C.; Gregory, S.; Cardona, K.; Hernandez, J.M.; van Houdt, W.J.; Gronchi, A. A Randomized Phase III Study of Neoadjuvant Chemotherapy Followed by Surgery Versus Surgery Alone for Patients with High-Risk Retroperitoneal Sarcoma (STRASS2). Ann. Surg. Oncol. 2023, 30, 4573–4575. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Rubin, D. The Central Role of the Propensity Score in Observational Studies For Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Tortorello, G.N.; Li, E.H.; Sharon, C.E.; Ma, K.L.; Maki, R.G.; Miura, J.T.; Fraker, D.L.; DeMatteo, R.P.; Karakousis, G.C. Neoadjuvant Chemotherapy in Retroperitoneal Sarcoma: A National Cohort Study. Ann. Surg. Oncol. 2023, 30, 6886–6893. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Gortzak, E.; Azzarelli, A.; Buesa, J.; Bramwell, V.H.; van Coevorden, F.; van Geel, A.N.; Ezzat, A.; Santoro, A.; Oosterhuis, J.W.; van Glabbeke, M.; et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur. J. Cancer 2001, 37, 1096–1103. [Google Scholar] [CrossRef]
- Frustaci, S.; Gherlinzoni, F.; De Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef]
- Vlenterie, M.; Litière, S.; Rizzo, E.; Marréaud, S.; Judson, I.; Gelderblom, H.; Le Cesne, A.; Wardelmann, E.; Messiou, C.; Gronchi, A.; et al. Outcome of chemotherapy in advanced synovial sarcoma patients: Review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur. J. Cancer 2016, 58, 62–72. [Google Scholar] [CrossRef]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef]
- Eilber, F.C.; Brennan, M.F.; Eilber, F.R.; Eckardt, J.J.; Grobmyer, S.R.; Riedel, E.; Forscher, C.; Maki, R.G.; Singer, S. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann. Surg. 2007, 246, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef]
- Avancès, C.; Mottet, N.; Mahatmat, A.; Chapuis, E.; Serre, I.; Culine, S. Prognostic factors for first recurrence in patients with retroperitoneal sarcoma. Urol. Oncol. 2006, 24, 94–96. [Google Scholar] [CrossRef]
- Anaya, D.A.; Lahat, G.; Wang, X.; Xiao, L.; Pisters, P.W.; Cormier, J.N.; Hunt, K.K.; Feig, B.W.; Lev, D.C.; Pollock, R.E. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann. Oncol. 2010, 21, 397–402. [Google Scholar] [CrossRef]
- Ardoino, I.; Miceli, R.; Berselli, M.; Mariani, L.; Biganzoli, E.; Fiore, M.; Collini, P.; Stacchiotti, S.; Casali, P.G.; Gronchi, A. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer 2010, 116, 2429–2436. [Google Scholar] [CrossRef]
- Gronchi, A.; Miceli, R.; Shurell, E.; Eilber, F.C.; Eilber, F.R.; Anaya, D.A.; Kattan, M.W.; Honoré, C.; Lev, D.C.; Colombo, C.; et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: Histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J. Clin. Oncol. 2013, 31, 1649–1655. [Google Scholar] [CrossRef]
- Borghi, A.; Gronchi, A. Sarculator: How to improve further prognostication of all sarcomas. Curr. Opin. Oncol. 2024, 36, 253–262. [Google Scholar] [CrossRef]
- Squires, M.H.; Ethun, C.G.; Donahue, E.E.; Benbow, J.H.; Anderson, C.J.; Jagosky, M.H.; Manandhar, M.; Patt, J.C.; Kneisl, J.S.; Salo, J.C.; et al. Extremity Soft Tissue Sarcoma: A Multi-Institutional Validation of Prognostic Nomograms. Ann. Surg. Oncol. 2022, 29, 3291–3301. [Google Scholar] [CrossRef]
- de Bree, E.; Michelakis, D.; Heretis, I.; Kontopodis, N.; Spanakis, K.; Lagoudaki, E.; Tolia, M.; Zografakis-Sfakianakis, M.; Ioannou, C.; Mavroudis, D. Retroperitoneal Soft Tissue Sarcoma: Emerging Therapeutic Strategies. Cancers 2023, 15, 5469. [Google Scholar] [CrossRef]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.-Y.; Tendero, O.; Beveridge, R.D. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]


| Overall N = 851 | No Chemotherapy N = 624 | Chemotherapy N = 227 | p-Value * | |
|---|---|---|---|---|
| Age | 62 (51, 71) | 64 (55, 73) | 56 (42, 64) | <0.001 |
| Sex | 0.9 | |||
| Male | 503 (59%) | 368 (59%) | 135 (59%) | |
| Female | 348 (41%) | 256 (41%) | 92 (41%) | |
| Race | 0.5 | |||
| White | 727 (85%) | 535 (86%) | 192 (85%) | |
| Black | 75 (8.8%) | 51 (8.2%) | 24 (11%) | |
| Other | 34 (4.0%) | 25 (4.0%) | 9 (4.0%) | |
| Histology | <0.001 | |||
| Angiosarcoma | 60 (7.1%) | 33 (5.3%) | 27 (12%) | |
| Undifferentiated pleomorphic sarcoma | 303 (36%) | 216 (35%) | 87 (38%) | |
| Myxoid/round cell liposarcoma | 246 (29%) | 217 (35%) | 29 (13%) | |
| Spindle cell sarcoma | 192 (23%) | 147 (24%) | 45 (20%) | |
| Synovial sarcoma | 50 (5.9%) | 11 (1.8%) | 39 (17%) | |
| Grade | <0.001 | |||
| I | 96 (15%) | 90 (18%) | 6 (4.1%) | |
| II | 87 (14%) | 70 (14%) | 17 (11%) | |
| III | 161 (25%) | 115 (23%) | 46 (31%) | |
| IV | 156 (24%) | 111 (23%) | 45 (30%) | |
| Tumor size | 0.006 | |||
| <10 cm | 205 (24%) | 156 (25%) | 49 (22%) | |
| 10–20 cm | 348 (41%) | 240 (38%) | 108 (48%) | |
| >20 cm | 252 (30%) | 200 (32%) | 52 (23%) | |
| Radiotherapy | 284 (33%) | 210 (34%) | 74 (33%) | 0.8 |
| Chemotherapy sequence | ||||
| Neoadjuvant chemotherapy | 76 (8.9%) | - | 76 (33%) | |
| Adjuvant chemotherapy | 127 (14.9%) | - | 127 (56%) | |
| Neoadjuvant and adjuvant chemotherapy | 9 (1.5%) | - | 9 (4.0%) | |
| Surgical margin | 0.5 | |||
| No residual tumor R0 | 400 (47%) | 298 (48%) | 102 (45%) | |
| Residual tumor R1or R2 | 129 (15%) | 89 (14%) | 40 (18%) | |
| Microscopic residual tumor R1 | 131 (15%) | 92 (15%) | 39 (17%) | |
| Macroscopic residual tumor R2 | 33 (3.9%) | 24 (3.8%) | 9 (4.0%) |
| aHR * [95%-CI] | p-Value | |
|---|---|---|
| Age (Reference: <60 years) | ||
| Age (60–79 years) | 0.56 [0.32–0.97] | 0.04 |
| Age (≥80 years) | 0.18 [(0.04–0.82] | 0.03 |
| Sex (Reference: Female) | ||
| Sex (Male) | 0.96 [0.56–1.66] | 0.9 |
| Race (Reference: White) | ||
| Race (Black) | 1.42 [0.59–3.45] | 0.44 |
| Race (Other/Unknown) | 2.66 [0.62–11.35] | 0.19 |
| Tumor Size (Reference: <10 cm) | ||
| Tumor Size (10–20 cm) | 1.09 [0.56–2.1] | 0.80 |
| Tumor Size (>20 cm) | 0.82 [0.39–1.71] | 0.59 |
| Grade (Reference: Grade I/II) | ||
| Grade (III/IV) | 2.52 [1.35–4.69] | 0.004 |
| Margin (Reference: R0) | ||
| Margin (R1) | 1.50 [0.78–2.87] | 0.22 |
| Margin (R2) | 1.17 [0.42–3.29] | 0.76 |
| Radiotherapy (Reference: No Radiotherapy) | ||
| Radiotherapy (Yes) | 1.22 [0.70–2.12] | 0.48 |
| aHR* [95%-CI] | p-Value | |
|---|---|---|
| Chemotherapy (Reference: No Chemotherapy) | ||
| Chemotherapy | 0.89 [0.55–1.43] | 0.63 |
| Age (Reference: <60 years) | ||
| Age (60–79 years) | 1.55 [1.04–2.32] | 0.03 |
| Age (≥80 years) | 5.05 [1.85–13.79] | 0.002 |
| Sex (Reference: Female) | ||
| Sex (Male) | 0.92 [0.63–1.34] | 0.65 |
| Tumor Size (Reference: <10 cm) | ||
| Tumor size (10–20 cm) | 0.77 [0.49–1.23] | 0.28 |
| Tumor size (>20 cm) | 0.63 [0.36–1.10] | 0.1 |
| Grade (Reference: Grade I/II) | ||
| Grade (III/IV) | 0.93 [0.60–1.43] | 0.73 |
| Margin (Reference: R0) | ||
| Margin (R1) | 0.86 [0.50–1.50] | 0.6 |
| Margin (R2) | 2.77 [1.18–6.53] | 0.02 |
| Radiotherapy (Reference: No Radiotherapy) | ||
| Radiotherapy | 1.20 [0.80–1.79] | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesler, B.; Forountani, L.; Ganjouei, A.A.; Studerus, L.; Kettelhack, C.; Krasniqi, F.; Kasenda, B.; Müller, B.P.; Adam, M.A.; Wilhelm, A. Perioperative Systemic Therapy in Rare, Chemosensitive Subtypes of Retroperitoneal Sarcoma: A Hospital-Based Propensity Score-Matched Analysis. Cancers 2025, 17, 1931. https://doi.org/10.3390/cancers17121931
Wiesler B, Forountani L, Ganjouei AA, Studerus L, Kettelhack C, Krasniqi F, Kasenda B, Müller BP, Adam MA, Wilhelm A. Perioperative Systemic Therapy in Rare, Chemosensitive Subtypes of Retroperitoneal Sarcoma: A Hospital-Based Propensity Score-Matched Analysis. Cancers. 2025; 17(12):1931. https://doi.org/10.3390/cancers17121931
Chicago/Turabian StyleWiesler, Benjamin, Laleh Forountani, Amir Ashraf Ganjouei, Lara Studerus, Christoph Kettelhack, Fatime Krasniqi, Benjamin Kasenda, Beat P. Müller, Mohamed A. Adam, and Alexander Wilhelm. 2025. "Perioperative Systemic Therapy in Rare, Chemosensitive Subtypes of Retroperitoneal Sarcoma: A Hospital-Based Propensity Score-Matched Analysis" Cancers 17, no. 12: 1931. https://doi.org/10.3390/cancers17121931
APA StyleWiesler, B., Forountani, L., Ganjouei, A. A., Studerus, L., Kettelhack, C., Krasniqi, F., Kasenda, B., Müller, B. P., Adam, M. A., & Wilhelm, A. (2025). Perioperative Systemic Therapy in Rare, Chemosensitive Subtypes of Retroperitoneal Sarcoma: A Hospital-Based Propensity Score-Matched Analysis. Cancers, 17(12), 1931. https://doi.org/10.3390/cancers17121931





