Current Status and Future Applications of Robotic Surgery in Upper Gastrointestinal Surgery: A Narrative Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Minimally Invasive Surgical Approaches for Upper Gastrointestinal Cancer
3.1. RAS for EC
3.2. Evidence for MIS in GC
3.3. RAS for GC
3.4. Effect of Complications on the Prognosis of Upper Gastrointestinal Cancer
4. Current Status and Future Applications of Robot-Assisted Surgery
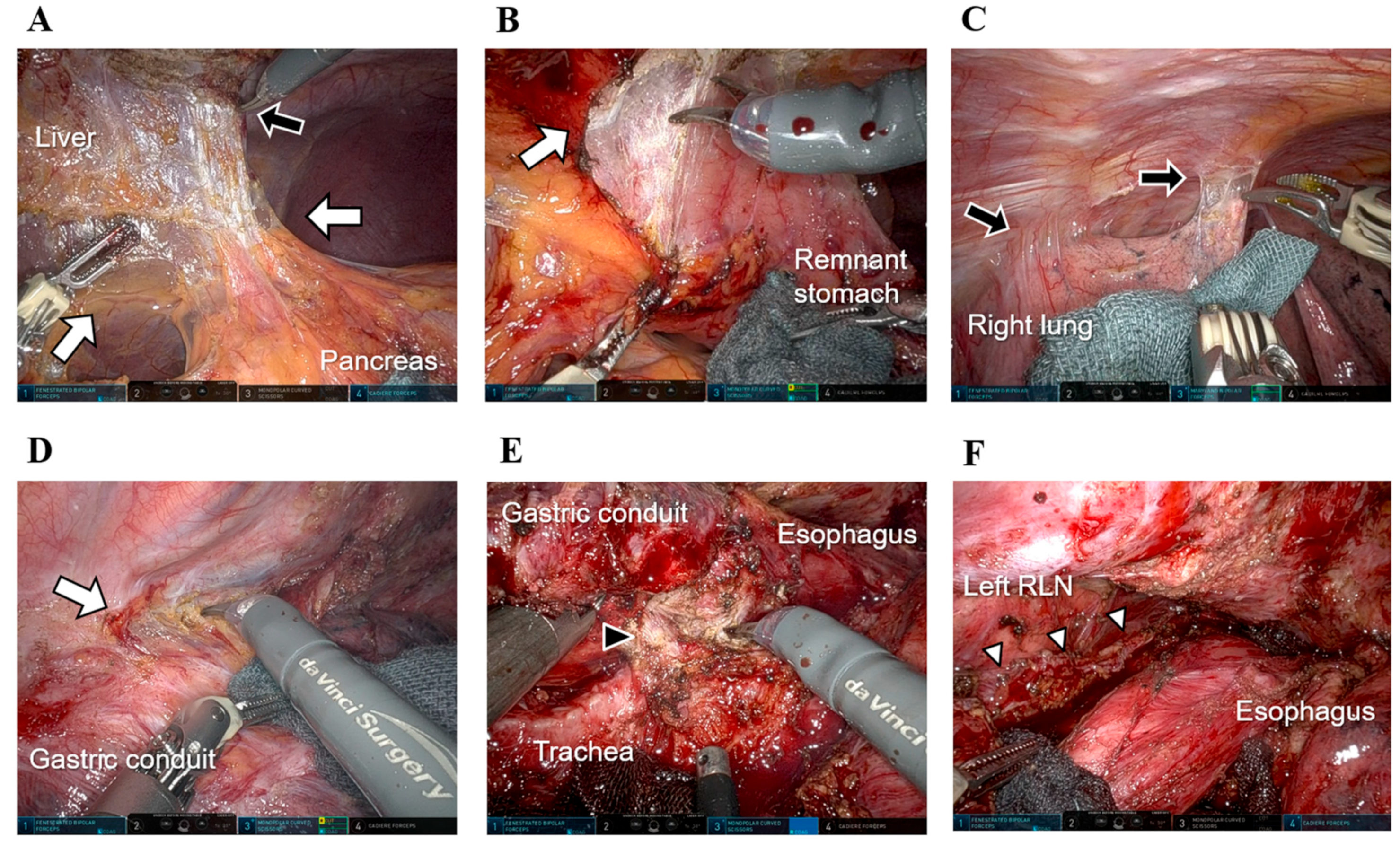
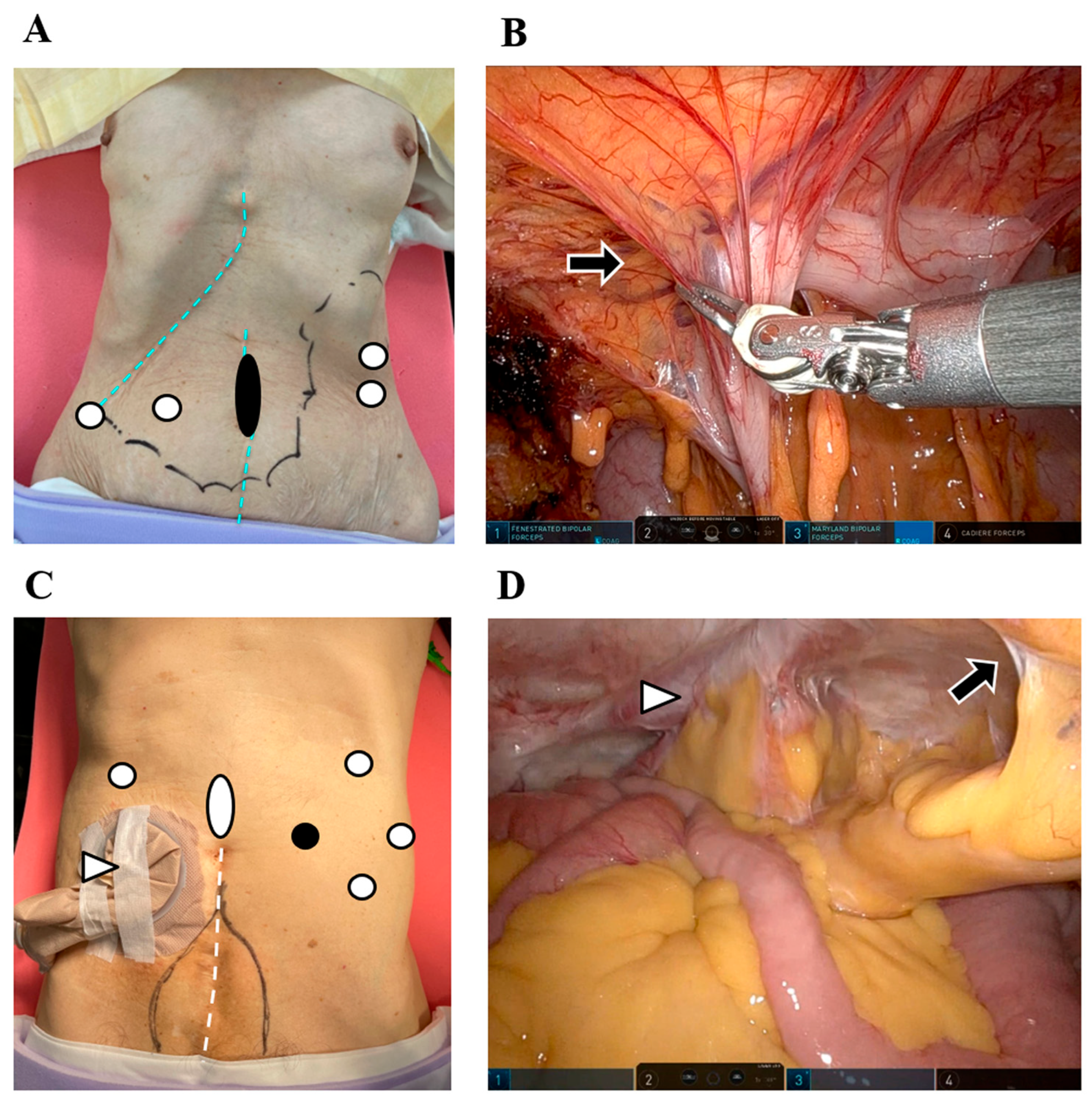
4.1. Potential of Robotic Surgery for Remnant GC
4.2. Potential of Robotic Surgery for Conversion and Salvage Surgery
4.3. RAS in Polysurgery Cases
5. Challenges and Developments in RAS Systems
Limitations and Barriers to RAS Implementation
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| C–D | Clavien–Dindo |
| DOS | Docetaxel-enhanced SOX |
| DVSS | DaVinci Surgical System |
| EC | Esophageal cancer |
| FLOT4 | 5-fluorouracil-leucovorin-oxaliplatin-docetaxel |
| GC | Gastric cancer |
| LG | Laparoscopic gastrectomy |
| LD | Linear dichroism |
| LDG | Laparoscopic distal gastrectomy |
| LN | Lymph node |
| MIE | Minimally invasive esophagectomy |
| MIS | Minimally invasive surgery |
| MST | Median survival time |
| NA | Not available |
| NAC | Neoadjuvant chemotherapy |
| NR | Not reported |
| OG | Open gastrectomy |
| OE | Open esophagectomy |
| ODG | Open distal gastrectomy |
| PF | Pancreatic fistula |
| PG | Proximal gastrectomy |
| PFS | Progression-free survival |
| PSM | Propensity score matching |
| RCT | Randomized controlled trial |
| RLN | Recurrent laryngeal nerve |
| RLNP | Recurrent laryngeal nerve paralysis |
| RAMIE | Robot-assisted thoracoscopic esophagectomy |
| RAS | Robot-assisted surgery |
| RFS | Relapse-free survival |
| RG | Robotic gastrectomy |
| SOX | S-1 plus oxaliplatin |
| TG | Total gastrectomy |
| SSSI | Superficial surgical site infection |
References
- Dikken, J.L.; van Sandick, J.W.; Allum, W.H.; Johansson, J.; Jensen, L.S.; Putter, H.; Coupland, V.H.; Wouters, M.W.; Lemmens, V.E.; van de Velde, C.J.; et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br. J. Surg. 2013, 100, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Barbosa, J.P.; Perry, I.; Barbosa, J. Short-term outcomes of robot-assisted versus conventional minimally invasive esophagectomy for esophageal cancer: A systematic review and meta-analysis of 18,187 patients. J. Robot Surg. 2024, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Tagkalos, E.; Goense, L.; Hoppe-Lotichius, M.; Ruurda, J.P.; Babic, B.; Hadzijusufovic, E.; Kneist, W.; van der Sluis, P.C.; Lang, H.; van Hillegersberg, R.; et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: A propensity-matched analysis. Dis. Esophagus 2020, 33, doz060. [Google Scholar] [CrossRef]
- Sheng, S.; Zhao, T.; Wang, X. Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer: A network meta-analysis. Medicine 2018, 97, e11817. [Google Scholar] [CrossRef] [PubMed]
- Mederos, M.A.; de Virgilio, M.J.; Shenoy, R.; Ye, L.; Toste, P.A.; Mak, S.S.; Booth, M.S.; Begashaw, M.M.; Wilson, M.; Gunnar, W.; et al. Comparison of Clinical Outcomes of Robot-Assisted, Video-Assisted, and Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e2129228. [Google Scholar] [CrossRef]
- Suda, K.; Yamamoto, H.; Nishigori, T.; Obama, K.; Yoda, Y.; Hikage, M.; Shibasaki, S.; Tanaka, T.; Kakeji, Y.; Inomata, M.; et al. Safe implementation of robotic gastrectomy for gastric cancer under the requirements for universal health insurance coverage: A retrospective cohort study using a nationwide registry database in Japan. Gastric Cancer 2022, 25, 438–449. [Google Scholar] [CrossRef]
- Ojima, T.; Nakamura, M.; Hayata, K.; Kitadani, J.; Katsuda, M.; Takeuchi, A.; Tominaga, S.; Nakai, T.; Nakamori, M.; Ohi, M.; et al. Short-term Outcomes of Robotic Gastrectomy vs Laparoscopic Gastrectomy for Patients with Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 954–963. [Google Scholar] [CrossRef]
- Brassetti, A.; Ragusa, A.; Tedesco, F.; Prata, F.; Cacciatore, L.; Iannuzzi, A.; Bove, A.M.; Anceschi, U.; Proietti, F.; D’Annunzio, S.; et al. Robotic Surgery in Urology: History from PROBOT((R)) to HUGO(TM). Sensors 2023, 23, 7104. [Google Scholar] [CrossRef]
- Dalager, T.; Jensen, P.T.; Eriksen, J.R.; Jakobsen, H.L.; Mogensen, O.; Sogaard, K. Surgeons’ posture and muscle strain during laparoscopic and robotic surgery. Br. J. Surg. 2020, 107, 756–766. [Google Scholar] [CrossRef]
- Vining, C.C.; Skowron, K.B.; Hogg, M.E. Robotic gastrointestinal surgery: Learning curve, educational programs and outcomes. Updat. Surg. 2021, 73, 799–814. [Google Scholar] [CrossRef]
- Flynn, J.; Larach, J.T.; Kong, J.C.H.; Waters, P.S.; Warrier, S.K.; Heriot, A. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: A systematic review. Colorectal. Dis. 2021, 23, 2806–2820. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Jiang, D.; Liu, X.B.; Xing, W.Q.; Liu, S.L.; Chen, P.N.; Li, P.; Ma, Y.X. Perioperative Outcomes and Learning Curve of Robot-Assisted McKeown Esophagectomy. J. Gastrointest. Surg. 2023, 27, 17–26. [Google Scholar] [CrossRef]
- Wong, S.W.; Ang, Z.H.; Yang, P.F.; Crowe, P. Robotic colorectal surgery and ergonomics. J. Robot Surg. 2022, 16, 241–246. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, H.S.; Kim, B.S.; Kwon, S.J. The impact of old age on surgical outcomes of totally laparoscopic gastrectomy for gastric cancer. Surg. Endosc. 2013, 27, 3990–3997. [Google Scholar] [CrossRef]
- Qian, F.; Yu, P.W.; Hao, Y.X.; Sun, G.; Tang, B.; Shi, Y.; Zhao, Y.L.; Lan, Y.Z.; Luo, H.X.; Mo, A. Laparoscopy-assisted resection for gastric stump cancer and gastric stump recurrent cancer: A report of 15 cases. Surg. Endosc. 2010, 24, 3205–3209. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, B.S.; Lee, I.S.; Lee, S.; Yook, J.H.; Kim, B.S. Laparoscopic gastrectomy in patients with previous gastrectomy for gastric cancer: A report of 17 cases. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 177–182. [Google Scholar] [CrossRef]
- Nagai, E.; Nakata, K.; Ohuchida, K.; Miyasaka, Y.; Shimizu, S.; Tanaka, M. Laparoscopic total gastrectomy for remnant gastric cancer: Feasibility study. Surg. Endosc. 2014, 28, 289–296. [Google Scholar] [CrossRef]
- Son, S.Y.; Lee, C.M.; Jung, D.H.; Lee, J.H.; Ahn, S.H.; Park, D.J.; Kim, H.H. Laparoscopic completion total gastrectomy for remnant gastric cancer: A single-institution experience. Gastric Cancer 2015, 18, 177–182. [Google Scholar] [CrossRef]
- Tsunoda, S.; Okabe, H.; Tanaka, E.; Hisamori, S.; Harigai, M.; Murakami, K.; Sakai, Y. Laparoscopic gastrectomy for remnant gastric cancer: A comprehensive review and case series. Gastric Cancer 2016, 19, 287–292. [Google Scholar] [CrossRef]
- Otsuka, R.; Hayashi, H.; Sakata, H.; Uesato, M.; Hayano, K.; Murakami, K.; Kano, M.; Fujishiro, T.; Toyozumi, T.; Semba, Y.; et al. Short-term clinical outcomes of laparoscopic gastrectomy for remnant gastric cancer: A single-institution experience and systematic review of the literature. Ann. Gastroenterol. Surg. 2019, 3, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Booka, E.; Kaihara, M.; Mihara, K.; Nishiya, S.; Handa, K.; Ito, Y.; Shibutani, S.; Egawa, T.; Nagashima, A. Laparoscopic total gastrectomy for remnant gastric cancer: A single-institution experience. Asian J. Endosc. Surg. 2019, 12, 58–63. [Google Scholar] [CrossRef]
- Kaihara, M.; Matsuda, S.; Booka, E.; Saida, F.; Takashima, J.; Kasai, H.; Mihara, K.; Nagashima, A.; Egawa, T. Laparoscopic completion gastrectomy in elderly patients with remnant gastric cancer: A case series. Surg. Case Rep. 2019, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Ikebe, M.; Shin, Y.; Kagawa, M.; Mano, Y.; Nakanoko, T.; Nakashima, Y.; Uehara, H.; Sugiyama, M.; Iguchi, T.; et al. Laparoscopic Total Gastrectomy for Remnant Gastric Cancer: A Single-institution Experience and Systematic Literature Review. Vivo 2020, 34, 1987–1992. [Google Scholar] [CrossRef]
- Kitadani, J.; Ojima, T.; Nakamura, M.; Hayata, K.; Katsuda, M.; Takeuchi, A.; Tominaga, S.; Fukuda, N.; Motobayashi, H.; Nakai, T.; et al. Safety and feasibility of laparoscopic gastrectomy for remnant gastric cancer compared with open gastrectomy: Single-center experience. Medicine 2021, 100, e23932. [Google Scholar] [CrossRef]
- Alhossaini, R.M.; Altamran, A.A.; Cho, M.; Roh, C.K.; Seo, W.J.; Choi, S.; Son, T.; Kim, H.I.; Hyung, W.J. Lower rate of conversion using robotic-assisted surgery compared to laparoscopy in completion total gastrectomy for remnant gastric cancer. Surg. Endosc. 2020, 34, 847–852. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, J.J.; Yu, P.W.; Zhao, Y.L.; Shi, Y.; Luo, Z.Y.; Wu, B.; Wang, J.J.; Qian, F. Robotic total gastrectomy for carcinoma in the remnant stomach: A comparison with laparoscopic total gastrectomy. Gastroenterol. Rep. 2021, 9, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Song, Q.Y.; Li, X.G.; Xie, T.Y.; Lu, Y.X.; Zhang, B.L.; Li, S.; Wang, X.X. 3D laparoscopic-assisted vs open gastrectomy for carcinoma in the remnant stomach: A retrospective cohort study. World J. Gastrointest. Surg. 2022, 14, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, R.; Tsunoda, S.; Okamura, R.; Yamashita, Y.; Hata, H.; Kinjo, Y.; Miki, A.; Kanaya, S.; Yamamoto, M.; Matsuo, K.; et al. Comparison of Minimally Invasive Surgery with Open Surgery for Remnant Gastric Cancer: A Multi-institutional Cohort Study. Ann. Surg. Oncol. 2023, 30, 3605–3614. [Google Scholar] [CrossRef]
- Umeki, Y.; Shibasaki, S.; Suzuki, K.; Serizawa, A.; Akimoto, S.; Nakauchi, M.; Tanaka, T.; Inaba, K.; Uyama, I.; Suda, K. Laparoscopic gastrectomy for remnant gastric cancer: A single-center retrospective study. Surg. Oncol. 2023, 51, 101988. [Google Scholar] [CrossRef]
- Zhong, Q.; Wu, D.; Jiang, Y.M.; He, Q.L.; Dang, X.Y.; Xu, D.B.; Sun, Y.Q.; Su, G.Q.; Guo, K.Q.; Cai, L.S.; et al. The safety, feasibility, and oncological outcomes of laparoscopic completion total gastrectomy for remnant gastric cancer: A prospective study with 3-year follow-up (FUGES-004 study). Int. J. Surg. 2024, 110, 3382–3391. [Google Scholar] [CrossRef]
- Warner, S.; Chang, Y.H.; Paripati, H.; Ross, H.; Ashman, J.; Harold, K.; Day, R.; Stucky, C.C.; Rule, W.; Jaroszewski, D. Outcomes of minimally invasive esophagectomy in esophageal cancer after neoadjuvant chemoradiotherapy. Ann. Thorac. Surg. 2014, 97, 439–445. [Google Scholar] [CrossRef]
- Defize, I.L.; van der Horst, S.; Bulbul, M.; Haj Mohammad, N.; Mook, S.; Meijer, G.J.; Brosens, L.A.A.; Ruurda, J.P.; van Hillegersberg, R. Salvage Robot-Assisted Minimally Invasive Esophagectomy (RAMIE) for T4b Esophageal Cancer After Definitive Chemoradiotherapy. Ann. Surg. Oncol. 2021, 28, 2730–2738. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Sugihara, K.; et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann. Surg. 2014, 260, 259–266. [Google Scholar] [CrossRef]
- Sano, T.; Sasako, M.; Mizusawa, J.; Yamamoto, S.; Katai, H.; Yoshikawa, T.; Nashimoto, A.; Ito, S.; Kaji, M.; Imamura, H.; et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann. Surg. 2017, 265, 277–283. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Doki, Y.; Mizusawa, J.; Terashima, M.; Katai, H.; Yoshikawa, T.; Kimura, Y.; Takiguchi, S.; Nishida, Y.; Fukushima, N.; et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): A phase 3, open-label, randomised controlled trial. Lancet. Gastroenterol. Hepatol. 2018, 3, 460–468. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Kurokawa, Y.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N. Engl. J. Med. 2008, 359, 453–462. [Google Scholar] [CrossRef]
- Katai, H.; Mizusawa, J.; Katayama, H.; Takagi, M.; Yoshikawa, T.; Fukagawa, T.; Terashima, M.; Misawa, K.; Teshima, S.; Koeda, K.; et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 2017, 20, 699–708. [Google Scholar] [CrossRef]
- Katai, H.; Mizusawa, J.; Katayama, H.; Morita, S.; Yamada, T.; Bando, E.; Ito, S.; Takagi, M.; Takagane, A.; Teshima, S.; et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): A multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet. Gastroenterol. Hepatol. 2020, 5, 142–151. [Google Scholar] [CrossRef]
- Katai, H.; Mizusawa, J.; Katayama, H.; Kunisaki, C.; Sakuramoto, S.; Inaki, N.; Kinoshita, T.; Iwasaki, Y.; Misawa, K.; Takiguchi, N.; et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 2019, 22, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared with Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann. Surg. 2016, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients with Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Honda, M.; Kumamaru, H.; Kodera, Y.; Kakeji, Y.; Hiki, N.; Etoh, T.; Miyata, H.; Yamashita, Y.; Seto, Y.; et al. Surgical outcomes of laparoscopic distal gastrectomy compared to open distal gastrectomy: A retrospective cohort study based on a nationwide registry database in Japan. Ann. Gastroenterol. Surg. 2017, 2, 55–64. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Xue, Y.; Suo, J.; Tao, K.; He, X.; et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, H.; Hu, Y.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg. 2022, 157, 9–17. [Google Scholar] [CrossRef]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy with D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Hur, H.; Hyung, W.J.; Park, Y.K.; Lee, H.J.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: 5-Year Outcomes of the KLASS-02 Randomized Clinical Trial. JAMA Surg. 2022, 157, 879–886. [Google Scholar] [CrossRef]
- Etoh, T.; Ohyama, T.; Sakuramoto, S.; Tsuji, T.; Lee, S.W.; Yoshida, K.; Koeda, K.; Hiki, N.; Kunisaki, C.; Tokunaga, M.; et al. Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial. JAMA Surg. 2023, 158, 445–454. [Google Scholar] [CrossRef]
- Kinoshita, T.; Uyama, I.; Terashima, M.; Noshiro, H.; Nagai, E.; Obama, K.; Tamamori, Y.; Nabae, T.; Honda, M.; Abe, T.; et al. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage II/III Gastric Cancer: A Multicenter Cohort Study in Japan (LOC-A Study). Ann. Surg. 2019, 269, 887–894. [Google Scholar] [CrossRef]
- Hiki, N.; Katai, H.; Mizusawa, J.; Nakamura, K.; Nakamori, M.; Yoshikawa, T.; Kojima, K.; Imamoto, H.; Ninomiya, M.; Kitano, S.; et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: A multicenter phase II trial (JCOG0703). Gastric Cancer 2018, 21, 155–161. [Google Scholar] [CrossRef]
- Osugi, H.; Takemura, M.; Higashino, M.; Takada, N.; Lee, S.; Kinoshita, H. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br. J. Surg. 2003, 90, 108–113. [Google Scholar] [CrossRef]
- Biere, S.S.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamamoto, H.; Baba, H.; Miyata, H.; Watanabe, M.; Toh, Y.; Matsubara, H.; Kakeji, Y.; Seto, Y. Can Minimally Invasive Esophagectomy Replace Open Esophagectomy for Esophageal Cancer? Latest Analysis of 24,233 Esophagectomies From the Japanese National Clinical Database. Ann. Surg. 2020, 272, 118–124. [Google Scholar] [CrossRef]
- Hikage, M.; Fujiya, K.; Kamiya, S.; Tanizawa, Y.; Bando, E.; Notsu, A.; Mori, K.; Terashima, M. Robotic Gastrectomy Compared with Laparoscopic Gastrectomy for Clinical Stage I/II Gastric Cancer Patients: A Propensity Score-Matched Analysis. World J. Surg. 2021, 45, 1483–1494. [Google Scholar] [CrossRef]
- Uyama, I.; Suda, K.; Nakauchi, M.; Kinoshita, T.; Noshiro, H.; Takiguchi, S.; Ehara, K.; Obama, K.; Kuwabara, S.; Okabe, H.; et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: A multi-institutional prospective single-arm study. Gastric Cancer 2019, 22, 377–385. [Google Scholar] [CrossRef]
- Makuuchi, R.; Terashima, M.; Terada, M.; Mizusawa, J.; Kita, R.; Tokunaga, M.; Omori, T.; Ojima, T.; Ehara, K.; Watanabe, M.; et al. Randomized controlled phase III trial to investigate superiority of robot-assisted gastrectomy over laparoscopic gastrectomy for clinical stage T1-4aN0-3 gastric cancer patients (JCOG1907, MONA LISA study): A study protocol. BMC Cancer 2023, 23, 987. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Haj Mohammad, N.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.M.; van der Horst, S.; Kingma, B.F.; Goense, L.; van der Sluis, P.C.; Ruurda, J.P.; van Hillegersberg, R. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open esophagectomy: Long-term follow-up of a randomized clinical trial. Dis. Esophagus 2020, 33, doaa079. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Seto, Y.; Aikou, S.; Takahashi, T. Efficacy evaluation of subtotal and total gastrectomies in robotic surgery for gastric cancer compared with that in open and laparoscopic resections: A meta-analysis. PLoS ONE 2014, 9, e103312. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, Z.; Zhao, J.; Liu, J.; Zhang, S.; Zhao, K.; Feng, X.; Li, J. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: A randomized clinical trial. J. Surg. Oncol. 2016, 113, 397–404. [Google Scholar] [CrossRef]
- Pan, H.F.; Wang, G.; Liu, J.; Liu, X.X.; Zhao, K.; Tang, X.F.; Jiang, Z.W. Robotic Versus Laparoscopic Gastrectomy for Locally Advanced Gastric Cancer. Surg. Laparosc. Endosc. Percutan. Tech. 2017, 27, 428–433. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, C.H.; Xu, B.B.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. Assessment of Robotic Versus Laparoscopic Distal Gastrectomy for Gastric Cancer: A Randomized Controlled Trial. Ann. Surg. 2021, 273, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Han, S.U.; Yang, H.K.; Kim, Y.W.; Lee, H.J.; Ryu, K.W.; Park, J.M.; An, J.Y.; Kim, M.C.; Park, S.; et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann. Surg. 2016, 263, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Okabe, H.; Obama, K.; Tsunoda, S.; Matsuo, K.; Tanaka, E.; Hisamori, S.; Sakai, Y. Feasibility of robotic radical gastrectomy using a monopolar device for gastric cancer. Surg. Today 2019, 49, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Makuuchi, R.; Miki, Y.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Late phase II study of robot-assisted gastrectomy with nodal dissection for clinical stage I gastric cancer. Surg. Endosc. 2016, 30, 3362–3367. [Google Scholar] [CrossRef]
- Parisi, A.; Reim, D.; Borghi, F.; Nguyen, N.T.; Qi, F.; Coratti, A.; Cianchi, F.; Cesari, M.; Bazzocchi, F.; Alimoglu, O.; et al. Minimally invasive surgery for gastric cancer: A comparison between robotic, laparoscopic and open surgery. World J. Gastroenterol. 2017, 23, 2376–2384. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, H.T.; Yu, J.P.; Su, L.; Guo, C.A.; Chen, P.; Yan, L.; Li, K.; Ma, Y.W.; Wang, L.; et al. Severity and incidence of complications assessed by the Clavien-Dindo classification following robotic and laparoscopic gastrectomy for advanced gastric cancer: A retrospective and propensity score-matched study. Surg. Endosc. 2019, 33, 3341–3354. [Google Scholar] [CrossRef]
- Ryan, S.; Tameron, A.; Murphy, A.; Hussain, L.; Dunki-Jacobs, E.; Lee, D.Y. Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma: Propensity-Matched Analysis. Surg. Innov. 2020, 27, 26–31. [Google Scholar] [CrossRef]
- Shibasaki, S.; Suda, K.; Nakauchi, M.; Nakamura, K.; Kikuchi, K.; Inaba, K.; Uyama, I. Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: A single-center, retrospective and propensity score-matched analysis. World J. Gastroenterol. 2020, 26, 1172–1184. [Google Scholar] [CrossRef]
- Shimoike, N.; Nishigori, T.; Yamashita, Y.; Kondo, M.; Manaka, D.; Kadokawa, Y.; Itami, A.; Kanaya, S.; Hosogi, H.; Satoh, S.; et al. Safety assessment of robotic gastrectomy and analysis of surgical learning process: A multicenter cohort study. Gastric Cancer 2022, 25, 817–826. [Google Scholar] [CrossRef]
- Omori, T.; Yamamoto, K.; Hara, H.; Shinno, N.; Yamamoto, M.; Fujita, K.; Kanemura, T.; Takeoka, T.; Akita, H.; Wada, H.; et al. Comparison of robotic gastrectomy and laparoscopic gastrectomy for gastric cancer: A propensity score-matched analysis. Surg. Endosc. 2022, 36, 6223–6234. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, S.; Kong, Y.; Shen, S.; Niu, Z.; Zhang, J.; Chen, D.; Jiang, H.; Lv, L.; Liu, X.; et al. Short- and long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer by the same surgical team: A propensity score matching analysis. Surg. Endosc. 2022, 36, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liao, H.; Jiang, Q.; Liu, D.; Li, T. Surgical and oncological outcomes of robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer: A propensity score-matched analysis of 1164 patients. World J. Surg. Oncol. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zhou, Y.B.; Li, T.Y.; Li, J.P.; Zhou, Z.W.; She, J.J.; Hu, J.K.; Qian, F.; Shi, Y.; Tian, Y.L.; et al. Robotic Gastrectomy Versus Laparoscopic Gastrectomy for Gastric Cancer: A Multicenter Cohort Study of 5402 Patients in China. Ann. Surg. 2023, 277, e87–e95. [Google Scholar] [CrossRef]
- Lu, J.; Li, T.Y.; Zhang, L.; Wang, Z.K.; She, J.J.; Jia, B.Q.; Qin, X.G.; Ren, S.Y.; Yao, H.L.; Huang, Z.N.; et al. Comparison of Short-term and Three-year Oncological Outcomes Between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: A Large Multicenter Cohort Study. Ann. Surg. 2024, 279, 808–817. [Google Scholar] [CrossRef]
- Weksler, B.; Sullivan, J.L. Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann. Thorac. Surg. 2017, 104, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Rizk, N.P.; Goldman, D.A.; Sima, C.; Tan, K.S.; Bains, M.S.; Adusumilli, P.S.; Molena, D.; Bott, M.; Atkinson, T.; et al. Early Quality of Life Outcomes After Robotic-Assisted Minimally Invasive and Open Esophagectomy. Ann. Thorac. Surg. 2019, 108, 920–928. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Gan, Q.; Xiang, J.; Jin, R.; Chen, K.; Che, J.; Hang, J.; Li, H. Early Outcomes of Robot-Assisted Versus Thoracoscopic-Assisted Ivor Lewis Esophagectomy for Esophageal Cancer: A Propensity Score-Matched Study. Ann. Surg. Oncol. 2019, 26, 1284–1291. [Google Scholar] [CrossRef]
- Yun, J.K.; Chong, B.K.; Kim, H.J.; Lee, I.S.; Gong, C.S.; Kim, B.S.; Lee, G.D.; Choi, S.; Kim, H.R.; Kim, D.K.; et al. Comparative outcomes of robot-assisted minimally invasive versus open esophagectomy in patients with esophageal squamous cell carcinoma: A propensity score-weighted analysis. Dis. Esophagus 2020, 33, doz071. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.; Yi, J.; Hua, R.; Chen, H.; Tan, L.; Li, H.; He, Y.; Guo, X.; Sun, Y.; et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: The RAMIE Trial. Ann. Surg. 2022, 275, 646–653. [Google Scholar] [CrossRef]
- Gong, L.; Jiang, H.; Yue, J.; Duan, X.; Tang, P.; Ren, P.; Zhao, X.; Liu, X.; Zhang, X.; Yu, Z. Comparison of the short-term outcomes of robot-assisted minimally invasive, video-assisted minimally invasive, and open esophagectomy. J. Thorac. Dis. 2020, 12, 916–924. [Google Scholar] [CrossRef]
- Fujita, T.; Sato, K.; Ozaki, A.; Akutsu, T.; Fujiwara, H.; Kojima, T.; Daiko, H. Propensity-Matched Analysis of the Short-Term Outcome of Robot-Assisted Minimally Invasive Esophagectomy Versus Conventional Thoracoscopic Esophagectomy in Thoracic Esophageal Cancer. World J. Surg. 2022, 46, 1926–1933. [Google Scholar] [CrossRef]
- Ninomiya, I.; Okamoto, K.; Yamaguchi, T.; Saito, H.; Terai, S.; Moriyama, H.; Kinoshita, J.; Fushida, S. Optimization of robot-assisted thoracoscopic esophagectomy in the lateral decubitus position. Esophagus 2021, 18, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Mori, K.; Aikou, S. Robotic surgery for esophageal cancer: Merits and demerits. Ann. Gastroenterol. Surg. 2017, 1, 193–198. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Sairenji, M.; Arai, K.; Kinoshita, T.; Nashimoto, A.; Hiratsuka, M.; Japan Clinical Oncology, G. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol. 2006, 7, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Suda, K.; Hisamori, S.; Obama, K.; Terashima, M.; Uyama, I. Robotic gastrectomy for gastric cancer: Systematic review and future directions. Gastric Cancer 2023, 26, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Hayata, K.; Kitadani, J.; Yamaue, H. Robotic curative para-aortic lymph node dissection via INfra-mesocolon for gastric cancer: Robotic CAVING approach. Surg. Oncol. 2021, 39, 101658. [Google Scholar] [CrossRef]
- Kinoshita, T.; Okayama, T. Is splenic hilar lymph node dissection necessary for proximal gastric cancer surgery? Ann. Gastroenterol. Surg. 2021, 5, 173–182. [Google Scholar] [CrossRef]
- Elliott, J.A.; Doyle, S.L.; Murphy, C.F.; King, S.; Guinan, E.M.; Beddy, P.; Ravi, N.; Reynolds, J.V. Sarcopenia: Prevalence, and Impact on Operative and Oncologic Outcomes in the Multimodal Management of Locally Advanced Esophageal Cancer. Ann. Surg. 2017, 266, 822–830. [Google Scholar] [CrossRef]
- Saeki, H.; Tsutsumi, S.; Tajiri, H.; Yukaya, T.; Tsutsumi, R.; Nishimura, S.; Nakaji, Y.; Kudou, K.; Akiyama, S.; Kasagi, Y.; et al. Prognostic Significance of Postoperative Complications After Curative Resection for Patients with Esophageal Squamous Cell Carcinoma. Ann. Surg. 2017, 265, 527–533. [Google Scholar] [CrossRef]
- Kataoka, K.; Takeuchi, H.; Mizusawa, J.; Igaki, H.; Ozawa, S.; Abe, T.; Nakamura, K.; Kato, K.; Ando, N.; Kitagawa, Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann. Surg. 2017, 265, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Fukagawa, T.; Haga, Y.; Oba, K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann. Gastroent. Surg. 2017, 1, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Miyata, H.; Sugimura, K.; Motoori, M.; Asukai, K.; Yanagimoto, Y.; Takahashi, Y.; Tomokuni, A.; Yamamoto, K.; Akita, H.; et al. Prognostic Significance of Sarcopenia and Systemic Inflammatory Response in Patients with Esophageal Cancer. Anticancer. Res. 2019, 39, 449–458. [Google Scholar] [CrossRef]
- Yabusaki, H.; Kodera, Y.; Fukushima, N.; Hiki, N.; Kinami, S.; Yoshida, M.; Aoyagi, K.; Ota, S.; Hata, H.; Noro, H.; et al. Comparison of Postoperative Quality of Life among Three Different Reconstruction Methods After Proximal Gastrectomy: Insights From the PGSAS Study. World J. Surg. 2020, 44, 3433–3440. [Google Scholar] [CrossRef]
- Kunisaki, C.; Yoshida, K.; Yoshida, M.; Matsumoto, S.; Arigami, T.; Sugiyama, Y.; Seto, Y.; Akiyama, Y.; Oshio, A.; Nakada, K. Effects of Proximal Gastrectomy and Various Clinical Factors on Postoperative Quality of Life for Upper-third Gastric Cancer Assessed using the Postgastrectomy Syndrome Assessment Scale-45 (PGSAS-45): A PGSAS NEXT Study. Ann. Surg. Oncol. 2022, 29, 3899–3908. [Google Scholar] [CrossRef]
- Jezerskyte, E.; van Berge Henegouwen, M.I.; van Laarhoven, H.W.M.; van Kleef, J.J.; Eshuis, W.J.; Heisterkamp, J.; Hartgrink, H.H.; Rosman, C.; van Hillegersberg, R.; Hulshof, M.; et al. Postoperative Complications and Long-Term Quality of Life After Multimodality Treatment for Esophageal Cancer: An Analysis of the Prospective Observational Cohort Study of Esophageal-Gastric Cancer Patients (POCOP). Ann. Surg. Oncol. 2021, 28, 7259–7276. [Google Scholar] [CrossRef]
- Iwata, Y.; Ito, S.; Misawa, K.; Ito, Y.; Komori, K.; Abe, T.; Shimizu, Y.; Tajika, M.; Niwa, Y.; Yoshida, K.; et al. Incidence and treatment of metachronous gastric cancer after proximal gastrectomy. Surg. Today 2018, 48, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kuroda, S.; Choda, Y.; Otsuka, S.; Ueyama, S.; Tanaka, N.; Hato, S.; Kimura, T.; Muraoka, A.; Tanakaya, K.; et al. Incidence of Metachronous Remnant Gastric Cancer after Proximal Gastrectomy with the Double-flap Technique (rD-FLAP-rGC Study): A Multicenter, Retrospective Study. Ann. Surg. Oncol. 2023, 30, 2307–2316. [Google Scholar] [CrossRef]
- Ishizu, K.; Hayashi, T.; Ogawa, R.; Nishino, M.; Sakon, R.; Wada, T.; Otsuki, S.; Yamagata, Y.; Katai, H.; Matsui, Y.; et al. Characteristics of Metachronous Remnant Gastric Cancer After Proximal Gastrectomy: A Retrospective Analysis. J. Gastric Cancer 2024, 24, 280–290. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, M.; Peng, W.; Liu, T.; Zhang, J.; Sheng, M.; Ren, R.; Chen, Q.; Gong, W.; Wu, Y. Incidence and risk of remnant gastric cancer after gastrectomy for gastric cancer: A population-based study from the SEER database. BMC Gastroenterol. 2024, 24, 35. [Google Scholar] [CrossRef]
- Li, Z.W.; Qiu, Y.Y.; Liu, F.; Liu, X.R.; Zhang, W.; Peng, D. The Effect of Surgical Approach on Clinical Outcomes in 535 Patients with Remnant Gastric Cancer. J. Laparoendosc. Adv. Surg. Tech. A 2023, 33, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, N.; Nomura, E.; Niki, M.; Shinohara, H.; Nishiguchi, K.; Okuzawa, M.; Toyoda, M.; Morita, S. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer 2002, 5, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Riva, P.; Da Roit, A.; Basato, S.; Marano, S.; Castoro, C. Gastric tube cancer after esophagectomy for cancer: A systematic review. Dis. Esophagus 2019, 32, doz049. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Morita, M.; Ikebe, M.; Nakashima, Y.; Yamamoto, M.; Matsubara, H.; Kakeji, Y.; Doki, Y.; Toh, Y. Clinicopathological features and prognosis of gastric tube cancer after esophagectomy for esophageal cancer: A nationwide study in Japan. Esophagus 2022, 19, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Noma, K.; Maeda, N.; Ninomiya, T.; Tanabe, S.; Kikuchi, S.; Kuroda, S.; Nishizaki, M.; Kagawa, S.; Kawahara, Y.; et al. Clinical characteristics and management of gastric tube cancer after esophagectomy. Esophagus 2018, 15, 180–189. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ueno, M.; Okamoto, K.; Shimoyama, H.; Ohkura, Y.; Haruta, S.; Udagawa, H. Successful Robot-Assisted Surgery for Advanced Metachronous Cancer in a Gastric Conduit after Esophagectomy: A Case Report. Ann. Thorac. Cardiovasc. Surg. 2024, 30, cr.23–00202. [Google Scholar] [CrossRef]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Young Rha, S.; Moon Bae, J.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Hoon Noh, S.; et al. International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef]
- Min, S.H.; Won, Y.; Lee, K.; Youn, S.I.; Kim, G.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Laparoscopic gastrectomy and metastasectomy for stage IV gastric cancer. Surg. Endosc. 2021, 35, 1879–1887. [Google Scholar] [CrossRef]
- Hisamori, S.; Okabe, H.; Tsunoda, S.; Nishigori, T.; Ganeko, R.; Fukui, Y.; Okamura, R.; Maekawa, H.; Sakai, Y.; Obama, K. Long-Term Outcomes of Laparoscopic Radical Gastrectomy for Highly Advanced Gastric Cancer: Final Report of a Prospective Phase II Trial (KUGC04). Ann. Surg. Oncol. 2021, 28, 8962–8972. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Tokunaga, M.; Sato, Y.; Nakagawa, M.; Aburatani, T.; Matsuyama, T.; Nakajima, Y.; Kinugasa, Y. Perioperative chemotherapy for locally advanced gastric cancer in Japan: Current and future perspectives. Surg. Today 2020, 50, 30–37. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [PubMed]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar]
- Rha, S.Y.; Oh, D.Y.; Yanez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Tokunaga, M.; Yamasaki, Y.; Kayasuga, H.; Nishihara, T.; Tadano, J.; Kawashima, K.; Haruki, S.; Kinugasa, Y. Evaluating the benefit of contact-force feedback in robotic surgery using the Saroa surgical system: A preclinical study. Asian J. Endosc. Surg. 2024, 17, e13395. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, S.; Ganguly, K.; Mandal, D. Advancements in robotic surgery: Innovations, challenges and future prospects. J. Robot Surg. 2024, 18, 28. [Google Scholar] [CrossRef]
- Ebihara, Y.; Hirano, S.; Kurashima, Y.; Takano, H.; Okamura, K.; Murakami, S.; Shichinohe, T.; Morohashi, H.; Oki, E.; Hakamada, K.; et al. Tele-robotic distal gastrectomy with lymph node dissection on a cadaver. Asian J. Endosc. Surg. 2024, 17, e13246. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hakamada, K.; Morohashi, H.; Akasaka, H.; Ebihara, Y.; Oki, E.; Hirano, S.; Mori, M. Reappraisal of telesurgery in the era of high-speed, high-bandwidth, secure communications: Evaluation of surgical performance in local and remote environments. Ann. Gastroenterol. Surg. 2023, 7, 167–174. [Google Scholar] [CrossRef]
- Sun, L.F.; Liu, K.; Su, X.S.; Wei, X.; Chen, X.L.; Zhang, W.H.; Chen, X.Z.; Yang, K.; Zhou, Z.G.; Hu, J.K. Robot-Assisted versus Laparoscopic-Assisted Gastrectomy among Gastric Cancer Patients: A Retrospective Short-Term Analysis from a Single Institution in China. Gastroenterol. Res. Pract. 2019, 2019, 9059176. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, W.J.; Hyung, W.J.; Kim, H.I.; Han, S.U.; Kim, Y.W.; Ryu, K.W.; Park, S. Comprehensive Learning Curve of Robotic Surgery: Discovery From a Multicenter Prospective Trial of Robotic Gastrectomy. Ann. Surg. 2021, 273, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Yan, L.; Feng, Q.; Yan, S.; Ji-Peng, L.; Qing-Chuan, Z.; Bo, T.; Rui-Zi, G.; Zhi-Guo, S.; Xia, L.; Qing, F.; et al. Learning curve of robotic distal and total gastrectomy. Br. J. Surg. 2021, 108, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Suda, K.; Kadoya, S.; Ishida, Y.; Nakauchi, M.; Nakamura, K.; Akimoto, S.; Tanaka, T.; Kikuchi, K.; Inaba, K.; et al. The safe performance of robotic gastrectomy by second-generation surgeons meeting the operating surgeon’s criteria in the Japan Society for Endoscopic Surgery guidelines. Asian J. Endosc. Surg. 2022, 15, 70–81. [Google Scholar] [CrossRef]
- Sakai, Y.; Morishita, T. The financial impact of robotic surgery on hospital gross profits in Japan compared to laparoscopic surgery. Asian J. Endosc. Surg. 2025, 18, e13410. [Google Scholar] [CrossRef]
| Author | Year | Design | Procedure | No. of Cases | Conversion to Open Surgery | Thoracic Operative Time (min) | Blood Loss (mL) | No. of Retrieved LNs | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Osugi. [50] | 2003 | Retrospective | MIE | 77 | NR | 227 | 284 | 33.9 | Pneumonia 15.6% RLNP 14.3% |
| OE | 72 | NA | 186 | 310 | 32.8 | Pneumonia 19.4% RLNP 12.5% | |||
| Biere [51] | 2012 | RCT | MIE | 59 | 14% | 329 | 200 | 20 | Pneumonia 9% RLNP 2% |
| OE | 56 | NA | 299 | 475 | 21 | Pneumonia 29% RLNP 14% | |||
| Weksler [75] | 2012 | Retrospective | RAMIE | 569 | 6.7% | NR | NR | 16.0 | NR |
| PSM | MIE | 569 | 12.0% | NR | NR | 16.0 | NR | ||
| NDB | OE | 569 | NA | NR | NR | 13.0 | NR | ||
| Van der Sluis [56] | 2017 | RCT | RAMIE | 54 | 2% | 170 | 120 | 27 | Pneumonia 28% RLNP 9% |
| De Groot [57] | 2020 | OE | 55 | NA | 134 | 200 | 25 | Pneumonia 55% RLNP 11% | |
| Sarkaria [76] | 2019 | Prospective | RAMIE | 64 | NR | 384 | 250 | 25 | Pneumonia 14.1% RLNP 3.1% |
| OE | 106 | NA | 326 | 350 | 22 | Pneumonia 34% RLNP 0% | |||
| Zhang [77] | 2019 | Retrospective | RAMIE | 76 | 2.6% | 303.5 | 200 | 19.7 | Pneumonia 6.6% RLNP 6.6% |
| MIE | 108 | 0 | 277.2 | 200 | 20.3 | Pneumonia 9.3% RLNP 6.5% | |||
| Yun [78] | 2020 | Retrospective | RAMIE | 130 | 2.3% | 275.6 | 110.8 | 39.1 | Pneumonia 3.8% RLNP 25.4% |
| PSM | OE | 241 | NA | 240.0 | 93.8 | 38.3 | Pneumonia 10.8% RLNP 19.9% | ||
| Tagkalos [3] | 2020 | Prospective | RAMIE | 50 | NA | 223 | 331 | 27 | Pneumonia 18% |
| MIE | 50 | NA | 202 | 350 | 23 | Pneumonia 12% | |||
| Gong [80] | 2020 | Retrospective | RAMIE | 91 | NR | 318.0 | 215 | 22.8 (Upper mediastinal LN 6.2) | Pneumonia 9.9% RLNP 22.0% |
| MIE | 144 | NR | 321.1 | 200 | 23.1 (Upper mediastinal LN 5.6) | Pneumonia 10.4% RLNP 23.6% | |||
| OE | 74 | NA | 299.4 | 290 | 24.1 (Upper mediastinal LN 4.3) | Pneumonia 13.0% RLNP 15.6% | |||
| Yang [79] | 2022 | RCT | RAMIE | 181 | 7 | 203.8 | 200 | 15 | Pneumonia 9.9% RLNP 32.6% |
| MIE | 177 | 6 | 244.9 | 200 | 14 | Pneumonia 11.9% RLNP 27.1% | |||
| Fujita [81] | 2022 | Retrospective | RAMIE | 50 | 0 | Total 448.1 | Total 111.6 | NR | Pneumonia 8.0% RLNP 8.0% |
| PSM | MIE | 50 | 0 | Total 383.6 | Total 153.5 | NR | Pneumonia 12.0% RLNP 34.0% |
| Author | Year | Stage Design | Procedure | No. of Cases | Conversion to Open Surgery (%) | Mean Operative Time (min) | Mean Blood Loss (mL) | Retrieved Lymph Node | Postoperative Hospital Stay (Days) | Overall Morbidity (%) | 5 yr RFS | 5 yr OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Katai [37] Katai [38] JCOG0912 | 2017 2020 | I RCT | LDG | 457 | 3.5 | 278 | 38 | 39 | 11.3 | 3.3 (C–D III≦) | 95.1% | NR |
| ODG | 455 | NA | 194 | 115 | 39 | 24.9 | 3.7 (C–D III≦) | 94.0% | NR | |||
| Kim [40] Kim [41] KLASS-01 | 2016 2019 | I RCT | LDG | 644 | 0.9 | 184.1 | 190.6 | 40.5 | 7.1 | 13.0 | 97.1% | 94.2% |
| ODG | 612 | NA | 139.4 | 110.8 | 43.7 | 7.9 | 19.9 | 97.2% | 93.3% | |||
| Yoshida [42] | 2017 | I NDB PSM | LDG | 14,386 | 47.1 | 287 | 50 | NR | 12 | SSSI 1.0% PF 1.0% | NR | NR |
| ODG | 14,386 | NA | 209 | 185 | NR | 15 | SSSI 1.9% PF 0.8% | NR | NR | |||
| Hu [43] Huang [44] CLASS-01 | 2016 2022 | I-IV RCT | LDG | 519 | 6.4 | 217.3 | 105.5 | 36.1 | 10.8 | 15.2 | 3 yr 76.6% | 72.6% |
| ODG | 520 | NA | 186.0 | 117.3 | 36.9 | 11.3 | 12.9 | 3 yr 77.8% | 76.3% | |||
| Lee [45] Son [46] KLASS-02 | 2019 2022 | IB-III RCT | LDG | 460 | 3.7 | 225.7 | 138.3 | 46.6 | 8.1 | 22.0 | 79.5% | 88.9% |
| ODG | 458 | NA | 162.3 | 222.0 | 46.9 | 9.1 | 24.5 | 81.1% | 88.7% | |||
| Etoh [47] JLSSG0901 | 2023 | IB-III RCT | LDG | 227 | 1.2 | 205 | 30 | 43 | NR | 11.5 | 75.7% | 81.7% |
| ODG | 233 | NA | 291 | 141 | 43 | NR | 10.7 | 73.9% | 79.8% | |||
| Kinoshita [48] LOC-A Study | 2019 | II-III PSM | LG | 305 | 1.3 | 365 | 140 | 43 | 12 | 20.1 | Recurrence rate 29.8% | 54.2% |
| OG | 305 | NA | 228 | 396 | 34 | 12 | 18.7 | Recurrence rate 30.8% | 53.0% | |||
| Yoshida [42] | 2017 | IIA-IV NDB PSM | LDG | 3738 | 47.1 | 296 | 50 | NR | 13 | NS | NR | NR |
| ODG | 3738 | NA | 222 | 240 | NR | 15 | NR | NR |
| Author | Year | Design | Procedure | No. of Cases | Stage ≥ II | TG or PG (%) | Conversion to Open Surgery | Operative Time (min) | Blood Loss (mL) | Retrieved Lymph Node | Postoperative Hospital Stay (Days) | Overall Morbidity (%) | RFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang [59], China | 2016 | RCT | RG | 151 | 76 | 37 | 1.9% | 243 | 94 | 30.1 | 5.6 | 10.3 | NR | NR |
| OG | 145 | 79 | 31 | NA | 192 | 153 | 29.1 | 6.7 | 9.3 | NR | NR | |||
| Pan [60], China | 2017 | RCT | RG | 102 | 78.0 | 64.7 | 0 | 153 | 41 | 36.1 | 3.8 | 5.0 | NR | NR |
| LG | 61 | 89.0 | 73.8 | 0 | 152 | 84 | 30.0 | 5.4 | 19.7 | NR | NR | |||
| Lu J [61], China | 2021 | RCT | RG | 141 | NR | 0 | NR | 201 | 41 | 17.6 | 7.9 | 9.2 | NR | NR |
| LG | 142 | NR | 0 | NR | 182 | 56 | 15.8 | 8.2 | 17.6 | NR | NR | |||
| Ojima [7], Japan | 2021 | RCT | RG | 117 | 41.9 | 40.7 | 3.4 | 297 | 25 | 35 | 12 | 8.8 | NR | NR |
| LG | 119 | 40.3 | 31.6 | 1.7 | 245 | 25 | 30 | 13 | 19.7 | NR | NR | |||
| Kim [62], South Korea | 2016 | Prospective | RG | 185 | 18.9 | 16.2 | 1.1 | 221 | 50 | 34 | 6 | 11.9 | NR | NR |
| LG | 185 | 10.2 | 16.2 | 0.5 | 178 | 55 | 32 | 6 | 10.3 | NR | NR | |||
| Uyama [54], Japan | 2019 | Prospective, | RG | 326 | 12 | 22 | 0.3 | 313 | 20 | 38.5 | 9 | 41.1 | NR | NR |
| Okabe [63], Japan | 2019 | Prospective, | RG | 115 | 40.9 | 37.4 | 1.7 | 372 | 15 | 46 | 12 | 9.6 | NR | NR |
| Tokunaga [64], Japan | 2016 | Prospective | RG | 120 | 1 | 12 | 2.5 | 349 | 19 | NR | 9 | 14.2 | NR | NR |
| Parisi [65], Italy | 2017 | Retrospective, PSM | RG | 151 | 44 | 26 | 4.6% | 365 | 118 | 27.8 | 8.9 | 17.9 | NR | NR |
| LG | 151 | 44 | 32 | 5.3% | 220 | 96 | 24.6 | 9.1 | 11.9 | NR | NR | |||
| OG | 302 | 53 | 32 | NA | 199 | 127 | 25.8 | 12.7 | 19.5 | NR | NR | |||
| Wang [66], China | 2019 | Retrospective, PSM | RG | 253 | 76 | 43 | NR | 242 | 149 | NR | 10.2 | 18.8 | NR | NR |
| LG | 253 | 76 | 44 | NR | 238 | 144 | NR | 11.6 | 24.5 | NR | NR | |||
| Ryan [67], USA | 2020 | Retrospective | RG | 631 | 66 | 28 | NR | NR | NR | 19.6 | 10.2 | NR | NR | MST 56.2 mo. |
| LG | 1262 | 66 | 28 | NR | NR | NR | 17.4 | 11.6 | NR | NR | MST 49.2 mo. | |||
| Shibasaki [68], Japan | 2020 | Retrospective, PSM | RG | 354 | 38 | 30 | 0 | 360 | 37 | 37 | 12 | 3.7 (C–D III ≦) | NR | NR |
| LG | 354 | 37 | 29 | 0.1 | 347 | 28 | 36 | 13 | 7.6 (C–D III ≦) | NR | NR | |||
| Hikage [53], Japan | 2021 | Retrospective, PSM | RG | 342 | 4.7 | 16 | 2.0 | 321 | 15 | 42.0 | 8 | 13.2 | 5 yr 95.2 | 5 yr 96.4 |
| LG | 342 | 7.0 | 15 | 2.5 | 282 | 14 | 40.5 | 9 | 18.4 | 5 yr 93.4 | 5 yr 94.8 | |||
| Suda [6], Japan | 2022 | Retrospective, NCD, PSM | RG | 2671 | NA | 14.5 | 0.3 | 354 | 20 | NR | 10 | 4.9(C–D III ≦) | NR | NR |
| LG | 2671 | NA | 14.5 | 0.5 | 268 | 15 | NR | 11 | 3.9 (C–D III ≦) | NR | NR | |||
| Shimoike [69], Japan | 2022 | Retrospective | RG | 336 | 33 | 24 | 0 | 370 | 0 | NR | 10 | 14.9 (C–D II ≦) | NR | NR |
| Omori [70], Japan | 2022 | Retrospective, PSM | RG | 210 | 48 | 32 | NR | 208 | 13 | NR | 7 | 1.0 | NR | NR |
| LG | 210 | 48 | 35 | NR | 231 | 42 | NR | 8 | 4.8 | NR | NR | |||
| Tian [71], China | 2022 | Retrospective, PSM | RG | 463 | 65 | 20 | NR | 205 | 74 | 32.2 | 7.3 | 2.7 | 3 yr 77.0 | 3 yr 81.2 |
| LG | 877 | 68 | 21 | NR | 185 | 78 | 30.8 | 7.6 | 3.2 | 3 yr 77.0 | 3 yr 80.3 | |||
| Gao [72], China | 2022 | Retrospective, PSM | RG | 410 | 88 | 0 | 0.6 | 205 | 139 | 31.4 | 9.0 | 13.7 | 3 yr 72.9 | 3 yr 75.5 |
| LG | 410 | 87 | 0 | 1.4 | 185 | 167 | 29.4 | 9.1 | 16.6 | 3 yr 71.4 | 3 yr 73.1 | |||
| Li [73], China | 2023 | Retrospective, PSM | RG | 1776 | 64.6 | 30.7 | 1.2 | 249 | 127 | 32.5 | 9.2 | 12.6 | 5 yr 79.8 | 5 yr 80.8 |
| LG | 1776 | 65.0 | 30.7 | 1.6 | 220 | 143 | 30.7 | 9.3 | 15.2 | 5 yr 78.5 | 5 yr 79.5 | |||
| Lu [74], China | 2024 | Retrospective, PSM | RG | 1034 | 61.9 | 34.3 | 0.4 | 223 | 98 | 30.8 | 9.4 | 12.2 | 3 yr 77.4 | 3 yr 79.7 |
| LG | 1034 | 61.6 | 33.1 | 1.5 | 210 | 118 | 30.8 | 9.9 | 12.1 | 3 yr 76.7 | 3 yr 78.4 |
| Author | Year | Procedure | No. of Cases | Stage (Early/Advanced) | Conversion to Open Surgery | Mean Operative Time (min) | Mean Blood Loss (mL) | Retrieved Lymph Node | Postoperative Hospital Stay (Days) | Complications | RFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qian F [15] | 2010 | Lap | 15 | 0/15 | 6.7 | 205 | 110 | 18 | 13 | 6.7 | NR | NR |
| Kim [16] | 2014 | Lap | 17 | 13/4 | 0 | 197.2 | NR | 12.9 | 11.1 | 23.5 | NR | NR |
| Open | 50 | NR | NA | 149.3 | NR | NR | 13.8 | 30.0 | NR | NR | ||
| Nagai [17] | 2014 | Lap | 12 | 10/2 | 0 | 362.3 | 68.5 | 23.7 | 11.3 | 0 | NR | 3 yr 77.8 |
| Open | 6/4 | NA | 270.5 | 746.3 | 15.7 | 24.9 | 20 | NR | 3 yr 100 5 yr 72.9 | |||
| Son [18] | 2015 | Lap | 11/6 | 47.1 | 234.4 | 227.6 | 18.8 | 9.3 | 35.2 | NR | 5 yr 66.7 | |
| Open | 4/13 | NA | 170.0 | 184.1 | 22.3 | 9.3 | 29.4 | NR | 5 yr 60.3 | |||
| Tsunoda [19] | 2016 | Lap | 10 | 9/1 | 0 | 325 | 55 | 22 | 13 | 10 | NR | NR |
| Otsuka [20] | 2019 | Lap | 7 | 6/1 | 0 | 364 | 70 | 22 | 13.4 | 28.6 | NR | NR |
| Open | 20 | 12/8 | NA | 309 | 1066 | 12 | 16 | 50 | NR | NR | ||
| Booka [21] | 2019 | Lap | 8 | 2/6 | 25.0 | 307.5 | 135.5 | 8.8 | 10.6 | 37.5 | NR | NR |
| Open | 23 | 8/15 | NA | 295.8 | 568.3 | 6 | 21.3 | 26.1 | NR | NR | ||
| Kaihara [22] | 2019 | Lap | 6 | 2/4 | 16.7 | 310.5 | 50 | 7 | 9 | 50.0 (C–D II ≦) | NR | 5 yr 80.0 |
| Open | 5/10 | NA | 263 | 465 | 3 | 9 | 33.3 (C–D II ≦) | NR | 5 yr 60.6 | |||
| Ota [23] | 2020 | LG | 2/5 | 0 | 397 | 70 | 21 | 30 | 13.3 | NR | 3 yr 100 | |
| OG | 15 | 11/4 | NA | 271 | 245 | 8 | 23 | 20.0 | NR | 3 yr 86.7 | ||
| Kitadani [24] | 2020 | LG | 23 | 18/5 | 13.0 | 302 | 115 | 8 | 11 | 21.7 | 5 yr 87 | 5 yr 62 |
| OG | 15 | 5/10 | NA | 281 | 290 | 12 | 14 | 40.0 | 5 yr 78 | 5 yr 77 | ||
| Albossani [25] | 2020 | LG | 30 | 24/6 | 13.3 | 225 | 166 | 16 | 9.5 | 37.0 | NR | NR |
| RG | 25 | 18/7 | 0 | 292 | 202 | 18 | 8.9 | 40.0 | NR | NR | ||
| Li [26] | 2021 | LG | 41 | 14/27 | 19.5 | 297.9 | 288.8 | 13.6 | 9 | 22.0 | 3 yr 57.5 | 3 yr 60.0 |
| RG | 29 | 10/19 | 17.2 | 272 | 229.2 | 13.6 | 9 | 27.6 | 3 yr 65.5 | 3 yr 69.0 | ||
| Wu [27] | 2022 | LG | 16/20 | NR | 243.1 | 188.3 | 14 | 7.6 | 8.3 | NR | 3 yr 75.6 | |
| OG | 17/31 | NA | 215.7 | 305.8 | 10.7 | 11.2 | 20.8 | NR | 3 yr 73.3 | |||
| Aoyama [28] | 2023 | LG | 327 | 52/79 | NR | 344 | Less | 14 | 13 | 28.4 | 3 yr 71.9 | 3 yr 77.9 |
| OG | 195 | 75/114 | NA | 273 | 10 | 16 | 47.7 | 3 yr 62.2 | 3 yr 76.2 | |||
| Umeki [29] | 2023 | LG | 46 | 34/12 | 0 | 311.5 | 35.5 | 13.5 | 16.5 | 8.7 | 3 yr 72.3 | 3 yr 80.2 |
| Zhong [30] | 2024 | LG | 46 | 12/34 | 4.3 | 163.9 | 59.7 | 19.2 | 11.9 | 28.0 | 3 yr 61.6 | 3 yr 56.3 |
| OG | 160 | 20/140 | NA | 225.7 | 220.4 | 15.6 | 18.7 | 35.0 | 3 yr 60.8 | 3 yr 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, K.; Miyata, T.; Nagayama, T.; Sannomiya, Y.; Hashimoto, A.; Nishiki, H.; Kaida, D.; Fujita, H.; Kinami, S.; Takamura, H. Current Status and Future Applications of Robotic Surgery in Upper Gastrointestinal Surgery: A Narrative Review. Cancers 2025, 17, 1933. https://doi.org/10.3390/cancers17121933
Okamoto K, Miyata T, Nagayama T, Sannomiya Y, Hashimoto A, Nishiki H, Kaida D, Fujita H, Kinami S, Takamura H. Current Status and Future Applications of Robotic Surgery in Upper Gastrointestinal Surgery: A Narrative Review. Cancers. 2025; 17(12):1933. https://doi.org/10.3390/cancers17121933
Chicago/Turabian StyleOkamoto, Koichi, Takashi Miyata, Taigo Nagayama, Yuta Sannomiya, Akifumi Hashimoto, Hisashi Nishiki, Daisuke Kaida, Hideto Fujita, Shinichi Kinami, and Hiroyuki Takamura. 2025. "Current Status and Future Applications of Robotic Surgery in Upper Gastrointestinal Surgery: A Narrative Review" Cancers 17, no. 12: 1933. https://doi.org/10.3390/cancers17121933
APA StyleOkamoto, K., Miyata, T., Nagayama, T., Sannomiya, Y., Hashimoto, A., Nishiki, H., Kaida, D., Fujita, H., Kinami, S., & Takamura, H. (2025). Current Status and Future Applications of Robotic Surgery in Upper Gastrointestinal Surgery: A Narrative Review. Cancers, 17(12), 1933. https://doi.org/10.3390/cancers17121933








