DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges
Simple Summary
Abstract
1. Introduction
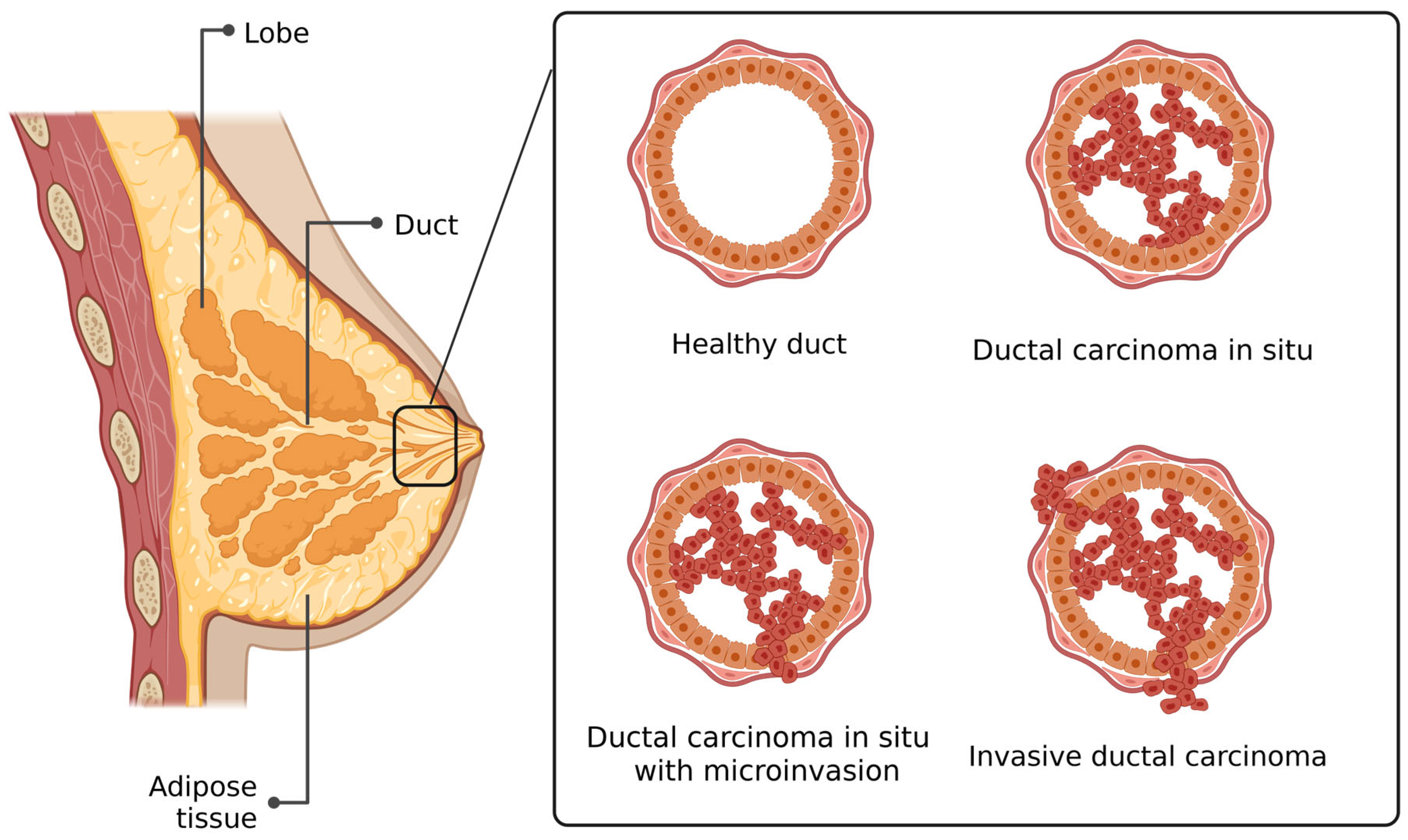
2. Histology of DCIS
3. DCIS with Microinvasion
4. Molecular Characteristics of DCIS
4.1. HER2
4.2. Germline Mutations
4.3. Somatic Mutations
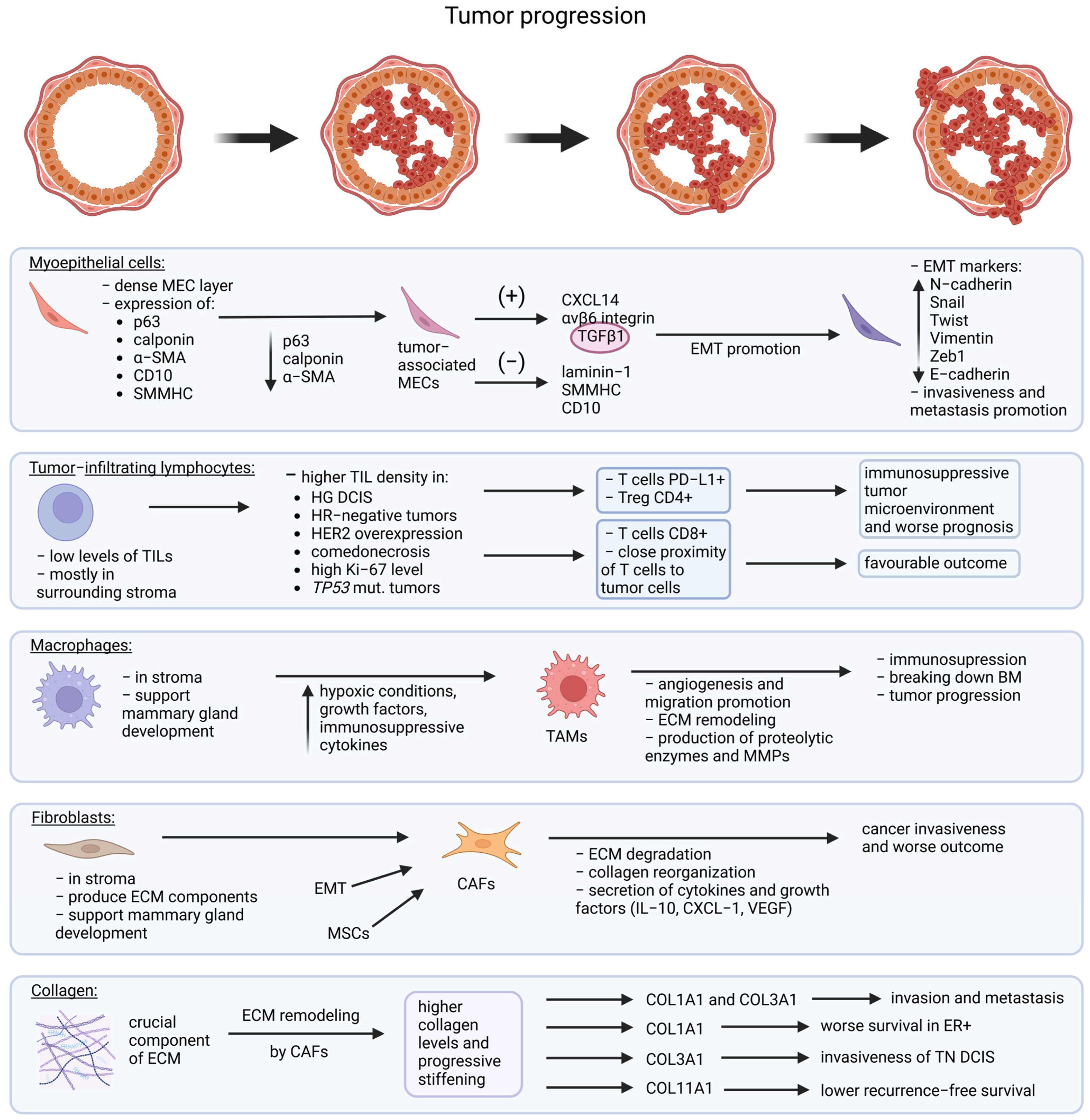
5. Myoepithelial Markers
6. Tumor Microenvironment
6.1. Tumor-Infiltrating Lymphocytes
6.2. Fibroblasts
6.3. Collagen
6.4. Macrophages
7. Epigenetics
8. Current Approach
9. Future Perspectives
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BM | Basement membrane |
| CAFs | Cancer-associated fibroblasts |
| CK14 | Cytokeratin 14 |
| COL1A1 | Collagen like type I |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein 4 |
| CXCL14 | Chemokine (C-X-C motif) ligand 14 |
| DCIS | Ductal carcinoma in situ |
| DCIS-Mi | DCIS with microinvasion |
| DCs | Dendritic cells |
| DFS | Disease-free survival |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| EZH2 | Enhancer of zeste homolog 2 |
| HDAC | Histone deacetylases |
| HER2 | Human epidermal growth factor 2 |
| HR | Hormone receptor |
| IDC | Invasive ductal carcinoma |
| IL-6 | Interleukin-6 |
| lncRNA | Long noncoding RNA |
| LSD1 | Lysine-specific demethylase 1 |
| MECs | Myoepithelial cells |
| miRNA | MicroRNA |
| MMP3 | Matrix metalloproteinase-3 |
| MSCs | Mesenchymal stem cells |
| NK | Natural killer |
| PD-1 | Programmed cell death receptor 1 |
| PD-L1 | Programmed death-ligand 1 |
| PR | Progesterone receptor |
| PRC2 | Polycomb repressive complex 2 |
| SMMHC | Smooth muscle myosin heavy chain |
| TAMs | Tumor-associated macrophages |
| TCF7 | T-cell factor 7 |
| TGFβ1 | Transforming growth factor beta 1 |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TILs | Tumor-infiltrating lymphocytes |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| Tregs | Regulatory T cells |
| VEGF | Vascular endothelial growth factor |
| α-SMA | α-Smooth muscle actin |
References
- Glover, J.A.; Bannon, F.J.; Hughes, C.M.; Cantwell, M.M.; Comber, H.; Gavin, A.; Deady, S.; Murray, L.J. Increased Diagnosis and Detection Rates of Carcinoma in Situ of the Breast. Breast Cancer Res. Treat. 2012, 133, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K. Epidemiology of Ductal Carcinoma In Situ. JNCI Monogr. 2010, 2010, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.R.; Agahozo, M.C.; Koppert, L.B.; van Deurzen, C.H.M. A Retrospective Alternative for Active Surveillance Trials for Ductal Carcinoma in Situ of the Breast. Int. J. Cancer 2020, 146, 1189–1197. [Google Scholar] [CrossRef]
- Mathelin, C.; Lodi, M.; Alghamdi, K.; Arboleda-Osorio, B.; Avisar, E.; Anyanwu, S.; Boubnider, M.; Costa, M.M.; Elder, E.; Elonge, T.; et al. The Senologic International Society Survey on Ductal Carcinoma In Situ: Present and Future. Eur. J. Breast Health 2022, 18, 205–221. [Google Scholar] [CrossRef]
- Grimm, L.J.; Rahbar, H.; Abdelmalak, M.; Hall, A.H.; Ryser, M.D. Ductal Carcinoma in Situ: State-of-the-Art Review. Radiology 2022, 302, 246–255. [Google Scholar] [CrossRef]
- Ma, T.; Semsarian, C.R.; Barratt, A.; Parker, L.; Pathmanathan, N.; Nickel, B.; Bell, K.J.L. Should Low-Risk DCIS Lose the Cancer Label? An Evidence Review. Breast Cancer Res. Treat. 2023, 199, 415–433. [Google Scholar] [CrossRef]
- Narod, S.A.; Sopik, V. Countercurrents: DCIS or Cancer? Why All the Confusion? Curr. Oncol. 2022, 29, 4936–4940. [Google Scholar] [CrossRef]
- Brackstone, M.; Durocher-Allen, L.; Koch, C.A.; Califaretti, N.; Eisen, A.; Knowles, S.; Plexman, T.; Salim, A. A Systematic Review on the Management of Ductal Carcinoma in Situ of the Breast. Surg. Oncol. 2025, 60, 102223. [Google Scholar] [CrossRef]
- Riggi, J.; Galant, C.; Vernaeve, H.; Vassilieff, M.; Berlière, M.; Van Bockstal, M.R. Risk Perception of Patients with Ductal Carcinoma in Situ (DCIS) of the Breast and Their Healthcare Practitioners: The Importance of Histopathological Terminology, and the Gaps in Our Knowledge. Histol. Histopathol. 2025, 40, 297–306. [Google Scholar] [CrossRef]
- Segnan, N.; Minozzi, S.; Armaroli, P.; Cinquini, M.; Bellisario, C.; González-Lorenzo, M.; Gianola, S.; Ponti, A. Epidemiologic Evidence of Slow Growing, Nonprogressive or Regressive Breast Cancer: A Systematic Review. Int. J. Cancer 2016, 139, 554–573. [Google Scholar] [CrossRef]
- Ryser, M.D.; Weaver, D.L.; Zhao, F.; Worni, M.; Grimm, L.J.; Gulati, R.; Etzioni, R.; Hyslop, T.; Lee, S.J.; Hwang, E.S. Cancer Outcomes in DCIS Patients Without Locoregional Treatment. JNCI J. Natl. Cancer Inst. 2019, 111, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Hilton, B.; Clements, K.; Provenzano, E.; Cheung, S.; Wallis, M.G.; Sawyer, E.; Thomas, J.S.; Hanby, A.M.; Pinder, S.E.; et al. Pathological Features of 11,337 Patients with Primary Ductal Carcinoma in Situ (DCIS) and Subsequent Events: Results from the UK Sloane Project. Br. J. Cancer 2021, 124, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Dinh, P.; Pathmanathan, N.; Graham, J.D. Ductal Carcinoma in Situ: Molecular Changes Accompanying Disease Progression. J. Mammary Gland Biol. Neoplasia 2022, 27, 101–131. [Google Scholar] [CrossRef]
- Hophan, S.L.; Odnokoz, O.; Liu, H.; Luo, Y.; Khan, S.; Gradishar, W.; Zhou, Z.; Badve, S.; Torres, M.A.; Wan, Y. Ductal Carcinoma In Situ of Breast: From Molecular Etiology to Therapeutic Management. Endocrinology 2022, 163, bqac027. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shamliyan, T.; Virnig, B.A.; Kane, R. Tumor Characteristics as Predictors of Local Recurrence after Treatment of Ductal Carcinoma in Situ: A Meta-Analysis. Breast Cancer Res. Treat. 2011, 127, 1–14. [Google Scholar] [CrossRef]
- Cheung, S.; Booth, M.E.; Kearins, O.; Dodwell, D. Risk of Subsequent Invasive Breast Cancer after a Diagnosis of Ductal Carcinoma in Situ (DCIS). Breast 2014, 23, 807–811. [Google Scholar] [CrossRef]
- van Luijt, P.A.; Heijnsdijk, E.A.M.; Fracheboud, J.; Overbeek, L.I.H.; Broeders, M.J.M.; Wesseling, J.; den Heeten, G.J.; de Koning, H.J. The Distribution of Ductal Carcinoma in Situ (DCIS) Grade in 4232 Women and Its Impact on Overdiagnosis in Breast Cancer Screening. Breast Cancer Res. 2016, 18, 47. [Google Scholar] [CrossRef]
- Bane, A. Ductal Carcinoma In Situ: What the Pathologist Needs to Know and Why. Int. J. Breast Cancer 2013, 2013, 914053. [Google Scholar] [CrossRef]
- van Seijen, M.; Jóźwiak, K.; Pinder, S.E.; Hall, A.; Krishnamurthy, S.; Thomas, J.S.; Collins, L.C.; Bijron, J.; Bart, J.; Cohen, D.; et al. Variability in Grading of Ductal Carcinoma in Situ among an International Group of Pathologists. J. Pathol. Clin. Res. 2021, 7, 233–242. [Google Scholar] [CrossRef]
- Silverstein, M.J. The University of Southern California/Van Nuys Prognostic Index for Ductal Carcinoma in Situ of the Breast. Am. J. Surg. 2003, 186, 337–343. [Google Scholar] [CrossRef]
- Di Saverio, S.; Catena, F.; Santini, D.; Ansaloni, L.; Fogacci, T.; Mignani, S.; Leone, A.; Gazzotti, F.; Gagliardi, S.; De Cataldis, A.; et al. 259 Patients with DCIS of the Breast Applying USC/Van Nuys Prognostic Index: A Retrospective Review with Long Term Follow Up. Breast Cancer Res. Treat. 2008, 109, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, T.; Li, F.; Shi, J.; Zhang, L. Clinic-Pathological Features of Breast Ductal Carcinoma in Situ with Micro-Invasion. Cancer Investig. 2020, 38, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, J.; Wang, W.; Fei, X.; Zong, Y.; Chen, X.; Huang, O.; He, J.; Chen, W.; Li, Y.; et al. Biologic Behavior and Long-Term Outcomes of Breast Ductal Carcinoma in Situ with Microinvasion. Oncotarget 2016, 7, 64182–64190. [Google Scholar] [CrossRef]
- de Mascarel, I.; MacGrogan, G.; Mathoulin-Pélissier, S.; Soubeyran, I.; Picot, V.; Coindre, J.-M. Breast Ductal Carcinoma in Situ with Microinvasion. Cancer 2002, 94, 2134–2142. [Google Scholar] [CrossRef]
- Liu, B.-T.; Ding, J.-N.; Wang, J.-L.; Li, Z.-S.; Ding, Y.-L.; Ma, R. Differences in Pathologic Characteristics between Ductal Carcinoma in Situ (DCIS), DCIS with Microinvasion and DCIS with Invasive Ductal Carcinoma. Int. J. Clin. Exp. Pathol. 2020, 13, 1066–1072. [Google Scholar]
- Rakovitch, E.; Sutradhar, R.; Lalani, N.; Nofech-Mozes, S.; Gu, S.; Goldberg, M.; Hanna, W.; Fong, C.; Paszat, L. Multiple Foci of Microinvasion Is Associated with an Increased Risk of Invasive Local Recurrence in Women with Ductal Carcinoma in Situ Treated with Breast-Conserving Surgery. Breast Cancer Res. Treat. 2019, 178, 169–176. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.J.; Chung, Y.R.; Kang, E.; Kim, E.-K.; Kim, S.H.; Kim, Y.J.; Kim, J.H.; Kim, I.A.; Park, S.Y. Microinvasive Carcinoma versus Ductal Carcinoma In Situ: A Comparison of Clinicopathological Features and Clinical Outcomes. J. Breast Cancer 2018, 21, 197–205. [Google Scholar] [CrossRef]
- Pu, T.; Zhong, X.; Deng, L.; Li, S.; Qiu, Y.; Liu, Y.; Zheng, H.; Ye, F.; Bu, H. Long Term Prognosis of Ductal Carcinoma in Situ with Microinvasion: A Retrospective Cohort Study. Int. J. Clin. Exp. Pathol. 2018, 11, 2665–2674. [Google Scholar]
- Yu, K.-D.; Wu, L.-M.; Liu, G.-Y.; Wu, J.; Di, G.-H.; Shen, Z.-Z.; Shao, Z.-M. Different Distribution of Breast Cancer Subtypes in Breast Ductal Carcinoma in Situ (DCIS), DCIS with Microinvasion, and DCIS with Invasion Component. Ann. Surg. Oncol. 2011, 18, 1342–1348. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Zhou, W.; Jirström, K.; Amini, R.-M.; Fjällskog, M.-L.; Sollie, T.; Lindman, H.; Sørlie, T.; Blomqvist, C.; Wärnberg, F. Molecular Subtypes in Ductal Carcinoma in Situ of the Breast and Their Relation to Prognosis: A Population-Based Cohort Study. BMC Cancer 2013, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Pape-Zambito, D.; Jiang, Z.; Wu, H.; Devarajan, K.; Slater, C.M.; Cai, K.Q.; Patchefsky, A.; Daly, M.B.; Chen, X. Identifying a Highly-Aggressive DCIS Subgroup by Studying Intra-Individual DCIS Heterogeneity among Invasive Breast Cancer Patients. PLoS ONE 2014, 9, e100488. [Google Scholar] [CrossRef] [PubMed]
- Shee, K.; Muller, K.E.; Marotti, J.; Miller, T.W.; Wells, W.A.; Tsongalis, G.J. Ductal Carcinoma in Situ Biomarkers in a Precision Medicine Era. Am. J. Pathol. 2019, 189, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.R.; Wesseling, J.; Lips, E.H.; Smidt, M.; Galant, C.; van Deurzen, C.H.M. Systematic Assessment of HER2 Status in Ductal Carcinoma in Situ of the Breast: A Perspective on the Potential Clinical Relevance. Breast Cancer Res. 2024, 26, 125. [Google Scholar] [CrossRef]
- Van Bockstal, M.; Libbrecht, L.; Floris, G.; Lambein, K.; Pinder, S. Stromal Inflammation, Necrosis and HER2 Overexpression in Ductal Carcinoma in Situ of the Breast: Another Causality Dilemma? Ann. Oncol. 2017, 28, 2317. [Google Scholar] [CrossRef]
- Schettini, F.; Buono, G.; Cardalesi, C.; Desideri, I.; De Placido, S.; Del Mastro, L. Hormone Receptor/Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Where We Are Now and Where We Are Going. Cancer Treat. Rev. 2016, 46, 20–26. [Google Scholar] [CrossRef]
- Mardekian, S.K.; Bombonati, A.; Palazzo, J.P. Ductal Carcinoma in Situ of the Breast: The Importance of Morphologic and Molecular Interactions. Hum. Pathol. 2016, 49, 114–123. [Google Scholar] [CrossRef]
- Tanei, T.; Seno, S.; Sota, Y.; Hatano, T.; Kitahara, Y.; Abe, K.; Masunaga, N.; Tsukabe, M.; Yoshinami, T.; Miyake, T.; et al. High HER2 Intratumoral Heterogeneity Is a Predictive Factor for Poor Prognosis in Early-Stage and Locally Advanced HER2-Positive Breast Cancer. Cancers 2024, 16, 1062. [Google Scholar] [CrossRef]
- Akrida, I.; Mulita, F. The Clinical Significance of HER2 Expression in DCIS. Med. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]
- Holmes, P.; Lloyd, J.; Chervoneva, I.; Pequinot, E.; Cornfield, D.B.; Schwartz, G.F.; Allen, K.G.; Palazzo, J.P. Prognostic Markers and Long-Term Outcomes in Ductal Carcinoma in Situ of the Breast Treated with Excision Alone. Cancer 2011, 117, 3650–3657. [Google Scholar] [CrossRef]
- Ciniselli, C.M.; Verderio, P.; Baili, P.; Sant, M.; Pizzamiglio, S.; Duroni, V.; de Braud, F.G.; Folli, S.; Scaperrotta, G.; De Santis, M.C.; et al. Clinical and Biological Significance of HER2-Low in Ductal Carcinoma In Situ of the Breast. Clin. Breast Cancer 2025, 25, e79–e85.e1. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Couch, R.E.; Karam, R.; Hu, C.; Boddicker, N.; Polley, E.C.; Na, J.; Ambrosone, C.B.; Yao, S.; Trentham-Dietz, A.; et al. Pathogenic Variants in Cancer Susceptibility Genes Predispose to Ductal Carcinoma In Situ of the Breast. Clin. Cancer Res. 2025, 31, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Petridis, C.; Arora, I.; Shah, V.; Megalios, A.; Moss, C.; Mera, A.; Clifford, A.; Gillett, C.; Pinder, S.E.; Tomlinson, I.; et al. Frequency of Pathogenic Germline Variants in BRCA1, BRCA2, PALB2, CHEK2 and TP53 in Ductal Carcinoma in Situ Diagnosed in Women under the Age of 50 Years. Breast Cancer Res. 2019, 21, 58. [Google Scholar] [CrossRef]
- Feszak, S.; Feszak, I.J.; Kluźniak, W.; Wokołorczyk, D.; Stempa, K.; Gliniewicz, K.; Uciński, J.; Huzarski, T.; Dębniak, T.; Gronwald, J.; et al. BRCA1 and BRCA2 Mutations in Polish Women with Ductal Carcinoma In Situ. Cancers 2025, 17, 613. [Google Scholar] [CrossRef]
- Hernandez, L.; Wilkerson, P.M.; Lambros, M.B.; Campion-Flora, A.; Rodrigues, D.N.; Gauthier, A.; Cabral, C.; Pawar, V.; Mackay, A.; A’Hern, R.; et al. Genomic and Mutational Profiling of Ductal Carcinomas in Situ and Matched Adjacent Invasive Breast Cancers Reveals Intra-Tumour Genetic Heterogeneity and Clonal Selection. J. Pathol. 2012, 227, 42–52. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jung, S.-H.; Kim, M.S.; Baek, I.-P.; Lee, S.H.; Kim, T.-M.; Chung, Y.-J.; Lee, S.H. Genomic Differences between Pure Ductal Carcinoma in Situ and Synchronous Ductal Carcinoma in Situ with Invasive Breast Cancer. Oncotarget 2015, 6, 7597–7607. [Google Scholar] [CrossRef]
- Bergholtz, H.; Kumar, S.; Wärnberg, F.; Lüders, T.; Kristensen, V.; Sørlie, T. Comparable Cancer-Relevant Mutation Profiles in Synchronous Ductal Carcinoma in Situ and Invasive Breast Cancer. Cancer Rep. 2020, 3, e1248. [Google Scholar] [CrossRef]
- Pang, J.-M.B.; Savas, P.; Fellowes, A.P.; Mir Arnau, G.; Kader, T.; Vedururu, R.; Hewitt, C.; Takano, E.A.; Byrne, D.J.; Choong, D.Y.; et al. Breast Ductal Carcinoma in Situ Carry Mutational Driver Events Representative of Invasive Breast Cancer. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc 2017, 30, 952–963. [Google Scholar] [CrossRef]
- Miron, A.; Varadi, M.; Carrasco, D.; Li, H.; Luongo, L.; Kim, H.J.; Park, S.Y.; Cho, E.Y.; Lewis, G.; Kehoe, S.; et al. PIK3CA Mutations in In Situ and Invasive Breast Carcinomas. Cancer Res. 2010, 70, 5674–5678. [Google Scholar] [CrossRef]
- Agahozo, M.C.; Sieuwerts, A.M.; Doebar, S.C.; Verhoef, E.I.; Beaufort, C.M.; Ruigrok-Ritstier, K.; de Weerd, V.; Sleddens, H.F.B.M.; Dinjens, W.N.M.; Martens, J.W.M.; et al. PIK3CA Mutations in Ductal Carcinoma in Situ and Adjacent Invasive Breast Cancer. Endocr. Relat. Cancer 2019, 26, 471–482. [Google Scholar] [CrossRef]
- Morrissey, R.L.; Thompson, A.M.; Lozano, G. Is Loss of P53 a Driver of Ductal Carcinoma in Situ Progression? Br. J. Cancer 2022, 127, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Doebar, S.C.; Krol, N.M.; van Marion, R.; Brouwer, R.W.W.; van Ijcken, W.F.J.; Martens, J.M.; Dinjens, W.N.M.; van Deurzen, C.H.M. Progression of Ductal Carcinoma in Situ to Invasive Breast Cancer: Comparative Genomic Sequencing. Virchows Arch. 2019, 474, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Pareja, F.; Brown, D.N.; Lee, J.Y.; Da Cruz Paula, A.; Selenica, P.; Bi, R.; Geyer, F.C.; Gazzo, A.; da Silva, E.M.; Vahdatinia, M.; et al. Whole-Exome Sequencing Analysis of the Progression from Non–Low-Grade Ductal Carcinoma In Situ to Invasive Ductal Carcinoma. Clin. Cancer Res. 2020, 26, 3682–3693. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.R. Role of Myoepithelial Cells in Breast Tumor Progression. Front. Biosci. 2010, 15, 226. [Google Scholar] [CrossRef]
- Moumen, M.; Chiche, A.; Cagnet, S.; Petit, V.; Raymond, K.; Faraldo, M.M.; Deugnier, M.-A.; Glukhova, M.A. The Mammary Myoepithelial Cell. Int. J. Dev. Biol. 2011, 55, 763–771. [Google Scholar] [CrossRef]
- Zou, Z.; Anisowicz, A.; Hendrix, M.J.C.; Thor, A.; Neveu, M.; Sheng, S.; Rafidi, K.; Seftor, E.; Sager, R. Maspin, a Serpin with Tumor-Suppressing Activity in Human Mammary Epithelial Cells. Science 1994, 263, 526–529. [Google Scholar] [CrossRef]
- McLachlan, E.; Shao, Q.; Wang, H.; Langlois, S.; Laird, D.W. Connexins Act as Tumor Suppressors in Three-Dimensional Mammary Cell Organoids by Regulating Differentiation and Angiogenesis. Cancer Res. 2006, 66, 9886–9894. [Google Scholar] [CrossRef]
- Rohilla, M.; Bal, A.; Singh, G.; Joshi, K. Phenotypic and Functional Characterization of Ductal Carcinoma In Situ–Associated Myoepithelial Cells. Clin. Breast Cancer 2015, 15, 335–342. [Google Scholar] [CrossRef]
- Hilson, J.B.; Schnitt, S.J.; Collins, L.C. Phenotypic Alterations in Ductal Carcinoma In Situ-Associated Myoepithelial Cells: Biologic and Diagnostic Implications. Am. J. Surg. Pathol. 2009, 33, 227. [Google Scholar] [CrossRef]
- Barbareschi, M.; Pecciarini, L.; Cangi, M.G.; Macrì, E.; Rizzo, A.; Viale, G.; Doglioni, C. P63, a P53 Homologue, Is a Selective Nuclear Marker of Myoepithelial Cells of the Human Breast. Am. J. Surg. Pathol. 2001, 25, 1054–1060. [Google Scholar] [CrossRef]
- Taniguchi, S. Suppression of Cancer Phenotypes through a Multifunctional Actin-Binding Protein, Calponin, That Attacks Cancer Cells and Simultaneously Protects the Host from Invasion. Cancer Sci. 2005, 96, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, F.N.; Cirqueira, C.S.; Bacchi, C.E.; Carvalho, F.M. Morphologic, Molecular and Microenvironment Factors Associated with Stromal Invasion in Breast Ductal Carcinoma in Situ: Role of Myoepithelial Cells. Breast Dis. 2015, 35, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Bachelard-Cascales, E.; Chapellier, M.; Delay, E.; Pochon, G.; Voeltzel, T.; Puisieux, A.; Caron de Fromentel, C.; Maguer-Satta, V. The CD10 Enzyme Is a Key Player to Identify and Regulate Human Mammary Stem Cells. Stem Cells 2010, 28, 1081–1088. [Google Scholar] [CrossRef]
- Arce, L.; Yokoyama, N.N.; Waterman, M.L. Diversity of LEF/TCF Action in Development and Disease. Oncogene 2006, 25, 7492–7504. [Google Scholar] [CrossRef]
- Roose, J.; Huls, G.; van Beest, M.; Moerer, P.; van der Horn, K.; Goldschmeding, R.; Logtenberg, T.; Clevers, H. Synergy Between Tumor Suppressor APC and the β-Catenin-Tcf4 Target Tcf1. Science 1999, 285, 1923–1926. [Google Scholar] [CrossRef]
- Russell, T.D.; Jindal, S.; Agunbiade, S.; Gao, D.; Troxell, M.; Borges, V.F.; Schedin, P. Myoepithelial Cell Differentiation Markers in Ductal Carcinoma in Situ Progression. Am. J. Pathol. 2015, 185, 3076–3089. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Liu, X.; Yang, Y.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Liu, J.; Meng, Q.; et al. TGF-Β1 Stimulates Epithelial–Mesenchymal Transition and Cancer-Associated Myoepithelial Cell during the Progression from in Situ to Invasive Breast Cancer. Cancer Cell Int. 2019, 19, 343. [Google Scholar] [CrossRef]
- Allen, M.D.; Thomas, G.J.; Clark, S.; Dawoud, M.M.; Vallath, S.; Payne, S.J.; Gomm, J.J.; Dreger, S.A.; Dickinson, S.; Edwards, D.R.; et al. Altered Microenvironment Promotes Progression of Preinvasive Breast Cancer: Myoepithelial Expression of Avβ6 Integrin in DCIS Identifies High-Risk Patients and Predicts Recurrence. Clin. Cancer Res. 2014, 20, 344–357. [Google Scholar] [CrossRef]
- Stephenson, J.M.; Banerjee, S.; Saxena, N.K.; Cherian, R.; Banerjee, S.K. Neuropilin-1 Is Differentially Expressed in Myoepithelial Cells and Vascular Smooth Muscle Cells in Preneoplastic and Neoplastic Human Breast: A Possible Marker for the Progression of Breast Cancer. Int. J. Cancer 2002, 101, 409–414. [Google Scholar] [CrossRef]
- Lo, P.-K.; Zhang, Y.; Yao, Y.; Wolfson, B.; Yu, J.; Han, S.-Y.; Duru, N.; Zhou, Q. Tumor-Associated Myoepithelial Cells Promote the Invasive Progression of Ductal Carcinoma in Situ through Activation of TGFβ Signaling. J. Biol. Chem. 2017, 292, 11466–11484. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Su, Y.; Fassl, A.; Hinohara, K.; Qiu, X.; Harper, N.W.; Huh, S.J.; Bloushtain-Qimron, N.; Jovanović, B.; Ekram, M.; et al. Perturbed Myoepithelial Cell Differentiation in BRCA Mutation Carriers and in Ductal Carcinoma in Situ. Nat. Commun. 2019, 10, 4182. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Marshall, J.F.; Jones, J.L. Avβ6 Expression in Myoepithelial Cells: A Novel Marker for Predicting DCIS Progression with Therapeutic Potential. Cancer Res. 2014, 74, 5942–5947. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Ertel, A.; Davicioni, E.; Kline, J.; Schwartz, G.F.; Witkiewicz, A.K. Progression of Ductal Carcinoma in Situ to Invasive Breast Cancer Is Associated with Gene Expression Programs of EMT and Myoepithelia. Breast Cancer Res. Treat. 2012, 133, 1009–1024. [Google Scholar] [CrossRef]
- Lu, J.; Guo, H.; Treekitkarnmongkol, W.; Li, P.; Zhang, J.; Shi, B.; Ling, C.; Zhou, X.; Chen, T.; Chiao, P.J.; et al. 14-3-3ζ Cooperates with ErbB2 to Promote Ductal Carcinoma In Situ Progression to Invasive Breast Cancer by Inducing Epithelial-Mesenchymal Transition. Cancer Cell 2009, 16, 195–207. [Google Scholar] [CrossRef]
- Hulahan, T.S.; Angel, P.M. From Ductal Carcinoma in Situ to Invasive Breast Cancer: The Prognostic Value of the Extracellular Microenvironment. J. Exp. Clin. Cancer Res. 2024, 43, 329. [Google Scholar] [CrossRef]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef]
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic Significance of Tumour Infiltrating B Lymphocytes in Breast Ductal Carcinoma in Situ. Histopathology 2017, 71, 258–268. [Google Scholar] [CrossRef]
- Agahozo, M.C.; Van Bockstal, M.R.; Groenendijk, F.H.; Van Den Bosch, T.P.P.; Westenend, P.J.; Van Deurzen, C.H.M. Ductal Carcinoma in Situ of the Breast: Immune Cell Composition According to Subtype. Mod. Pathol. 2020, 33, 196–205. [Google Scholar] [CrossRef]
- Campbell, M.J.; Baehner, F.; O’Meara, T.; Ojukwu, E.; Han, B.; Mukhtar, R.; Tandon, V.; Endicott, M.; Zhu, Z.; Wong, J.; et al. Characterizing the Immune Microenvironment in High-Risk Ductal Carcinoma in Situ of the Breast. Breast Cancer Res. Treat. 2017, 161, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Thike, A.A.; Chen, X.; Koh, V.C.Y.; Binte Md Nasir, N.D.; Yeong, J.P.S.; Bay, B.H.; Tan, P.H. Higher Densities of Tumour-Infiltrating Lymphocytes and CD4+ T Cells Predict Recurrence and Progression of Ductal Carcinoma in Situ of the Breast. Histopathology 2020, 76, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Almekinders, M.M.; Bismeijer, T.; Kumar, T.; Yang, F.; Thijssen, B.; van der Linden, R.; van Rooijen, C.; Vonk, S.; Sun, B.; Parra Cuentas, E.R.; et al. Comprehensive Multiplexed Immune Profiling of the Ductal Carcinoma in Situ Immune Microenvironment Regarding Subsequent Ipsilateral Invasive Breast Cancer Risk. Br. J. Cancer 2022, 127, 1201–1213. [Google Scholar] [CrossRef]
- Borowsky, A.; Glencer, A.; Ramalingam, K.; Schindler, N.; Mori, H.; Ghule, P.; Lee, K.; Nachmanson, D.; Officer, A.; Harismendy, O.; et al. Tumor Microenvironmental Determinants of High-Risk DCIS Progression. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Hendry, S.; Pang, J.-M.B.; Byrne, D.J.; Lakhani, S.R.; Cummings, M.C.; Campbell, I.G.; Mann, G.B.; Gorringe, K.L.; Fox, S.B. Relationship of the Breast Ductal Carcinoma In Situ Immune Microenvironment with Clinicopathological and Genetic Features. Clin. Cancer Res. 2017, 23, 5210–5217. [Google Scholar] [CrossRef]
- Kim, M.; Chung, Y.R.; Kim, H.J.; Woo, J.W.; Ahn, S.; Park, S.Y. Immune Microenvironment in Ductal Carcinoma in Situ: A Comparison with Invasive Carcinoma of the Breast. Breast Cancer Res. 2020, 22, 32. [Google Scholar] [CrossRef]
- Toss, M.S.; Abidi, A.; Lesche, D.; Joseph, C.; Mahale, S.; Saunders, H.; Kader, T.; Miligy, I.M.; Green, A.R.; Gorringe, K.L.; et al. The Prognostic Significance of Immune Microenvironment in Breast Ductal Carcinoma in Situ. Br. J. Cancer 2020, 122, 1496–1506. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Pasetto, C.V.; Aguiar, F.N.; Peixoto, M.B.; Dória, M.T.; Mota, B.S.; Maesaka, J.Y.; Filassi, J.R.; Baracat, E.C.; Gonçalves, R. Evaluation of Tumor Infiltrating Lymphocytes as a Prognostic Biomarker in Patients with Ductal Carcinoma in Situ of the Breast. Breast Cancer Res. Treat. 2024, 208, 9–18. [Google Scholar] [CrossRef]
- Darvishian, F.; Ozerdem, U.; Adams, S.; Chun, J.; Pirraglia, E.; Kaplowitz, E.; Guth, A.; Axelrod, D.; Shapiro, R.; Price, A.; et al. Tumor-Infiltrating Lymphocytes in a Contemporary Cohort of Women with Ductal Carcinoma In Situ (DCIS). Ann. Surg. Oncol. 2019, 26, 3337–3343. [Google Scholar] [CrossRef] [PubMed]
- Rask, G.; Wadsten, C.; Acs, B.; Hartman, J.; Fredriksson, I.; Garmo, H.; Wärnberg, F.; Sund, M. Immune Cell Infiltrate in Ductal Carcinoma in Situ and the Risk of Dying from Breast Cancer: Case–Control Study. Br. J. Surg. 2024, 111, znae037. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Makker, A.; Negi, M.P.S.; Goel, M.M. CD3+, CD4+ & CD8+ Tumour Infiltrating Lymphocytes (TILs) Are Predictors of Favourable Survival Outcome in Infiltrating Ductal Carcinoma of Breast. Indian J. Med. Res. 2014, 140, 361–369. [Google Scholar]
- Chung, Y.R.; Kim, H.J.; Jang, M.H.; Park, S.Y. Prognostic Value of Tumor Infiltrating Lymphocyte Subsets in Breast Cancer Depends on Hormone Receptor Status. Breast Cancer Res. Treat. 2017, 161, 409–420. [Google Scholar] [CrossRef]
- Risom, T.; Glass, D.R.; Averbukh, I.; Liu, C.C.; Baranski, A.; Kagel, A.; McCaffrey, E.F.; Greenwald, N.F.; Rivero-Gutiérrez, B.; Strand, S.H.; et al. Transition to Invasive Breast Cancer Is Associated with Progressive Changes in the Structure and Composition of Tumor Stroma. Cell 2022, 185, 299–310.e18. [Google Scholar] [CrossRef]
- Morita, M.; Yamaguchi, R.; Tanaka, M.; Tse, G.M.; Yamaguchi, M.; Kanomata, N.; Naito, Y.; Akiba, J.; Hattori, S.; Minami, S.; et al. CD8+ Tumor-infiltrating Lymphocytes Contribute to Spontaneous “Healing” in HER2-positive Ductal Carcinoma in Situ. Cancer Med. 2016, 5, 1607–1618. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural Killer Cell Therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Sun, Y.; Rodgers Furones, A.; Gultekin, O.; Khare, S.; Neo, S.Y.; Shi, W.; Moyano-Galceran, L.; Lam, K.-P.; Dasgupta, R.; Fuxe, J.; et al. Adaptive NK Cells Exhibit Tumor-Specific Immune Memory and Cytotoxicity in Ovarian Cancer. Cancer Immunol. Res. 2025. [Google Scholar] [CrossRef]
- Hassouneh, Z.; Noel, O.D.V.; Ji, N.; Kim, M.E.; Bowman, N.; Svatek, R.S.; Mace, E.; Mukherjee, N. CD56 on Intratumoral NK Cells: Orchestrating NK Cell-Mediated Anti-Tumor Effects in Bladder Cancer. Neoplasia N. Y. N 2025, 66, 101187. [Google Scholar] [CrossRef]
- Guevara Lopez, M.L.; Gebo, A.; Parodi, M.; Persano, S.; Maus-Conn, J.; Mingari, M.C.; Loiacono, F.; Orecchia, P.; Sivori, S.; Cantoni, C.; et al. CD56bright Cytokine-Induced Memory-like NK Cells and NK-Cell Engagers Synergize against Non-Small Cell Lung Cancer Cancer-Stem Cells. J. Immunother. Cancer 2025, 13, e010205. [Google Scholar] [CrossRef]
- Ascierto, M.L.; Idowu, M.O.; Zhao, Y.; Khalak, H.; Payne, K.K.; Wang, X.-Y.; Dumur, C.I.; Bedognetti, D.; Tomei, S.; Ascierto, P.A.; et al. Molecular Signatures Mostly Associated with NK Cells Are Predictive of Relapse Free Survival in Breast Cancer Patients. J. Transl. Med. 2013, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Levi, I.; Amsalem, H.; Nissan, A.; Darash-Yahana, M.; Peretz, T.; Mandelboim, O.; Rachmilewitz, J. Characterization of Tumor Infiltrating Natural Killer Cell Subset. Oncotarget 2015, 6, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.-L.; Voron, T.; Tartour, E.; Taieb, J. Chapter Seven—Immunomodulatory Activity of VEGF in Cancer. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 330, pp. 295–342. [Google Scholar]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune Tumor Microenvironment in Breast Cancer and the Participation of Estrogen and Its Receptors in Cancer Physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human Breast Cancer Cells Enhance Self Tolerance by Promoting Evasion from NK Cell Antitumor Immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Romero-Olmedo, A.J.; Schulz, A.R.; Huber, M.; Brehm, C.U.; Chang, H.-D.; Chiarolla, C.M.; Bopp, T.; Skevaki, C.; Berberich-Siebelt, F.; Radbruch, A.; et al. Deep Phenotypical Characterization of Human CD3+CD56+ T Cells by Mass Cytometry. Eur. J. Immunol. 2021, 51, 672–681. [Google Scholar] [CrossRef]
- Almeida, J.-S.; Casanova, J.M.; Santos-Rosa, M.; Tarazona, R.; Solana, R.; Rodrigues-Santos, P. Natural Killer T-like Cells: Immunobiology and Role in Disease. Int. J. Mol. Sci. 2023, 24, 2743. [Google Scholar] [CrossRef]
- Allaoui, R.; Hagerling, C.; Desmond, E.; Warfvinge, C.-F.; Jirström, K.; Leandersson, K. Infiltration of γδ T Cells, IL-17+ T Cells and FoxP3+ T Cells in Human Breast Cancer. Cancer Biomark. 2017, 20, 395–409. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Song, W.; Lu, C.; Chen, Z.; He, L.; Wang, X.; Wang, Y.; Shi, C.; Liu, Z.; et al. Integrating Multi-Omics Data Reveals the Antitumor Role and Clinical Benefits of Gamma-Delta T Cells in Triple-Negative Breast Cancer. BMC Cancer 2025, 25, 623. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary Gland Development: Cell Fate Specification, Stem Cells and the Microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef]
- Bu, L.; Baba, H.; Yoshida, N.; Miyake, K.; Yasuda, T.; Uchihara, T.; Tan, P.; Ishimoto, T. Biological Heterogeneity and Versatility of Cancer-Associated Fibroblasts in the Tumor Microenvironment. Oncogene 2019, 38, 4887–4901. [Google Scholar] [CrossRef] [PubMed]
- Krzysiek-Maczka, G.; Targosz, A.; Szczyrk, U.; Strzalka, M.; Brzozowski, T.; Ptak-Belowska, A. Involvement of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in the Mechanism of Helicobacter Pylori-Induced Fibroblasts Activation. J. Physiol. Pharmacol. 2019, 70, 727–736. [Google Scholar] [CrossRef]
- Raz, Y.; Cohen, N.; Shani, O.; Bell, R.E.; Novitskiy, S.V.; Abramovitz, L.; Levy, C.; Milyavsky, M.; Leider-Trejo, L.; Moses, H.L.; et al. Bone Marrow–Derived Fibroblasts Are a Functionally Distinct Stromal Cell Population in Breast Cancer. J. Exp. Med. 2018, 215, 3075–3093. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Myers, M.; Fang, W.B.; Zinda, B.; Smart, C.; Lambert, D.; Zou, A.; Fan, F.; Cheng, N. CXCL1 Derived from Mammary Fibroblasts Promotes Progression of Mammary Lesions to Invasive Carcinoma through CXCR2 Dependent Mechanisms. J. Mammary Gland Biol. Neoplasia 2018, 23, 249–267. [Google Scholar] [CrossRef]
- Osuala, K.O.; Sameni, M.; Shah, S.; Aggarwal, N.; Simonait, M.L.; Franco, O.E.; Hong, Y.; Hayward, S.W.; Behbod, F.; Mattingly, R.R.; et al. Il-6 Signaling between Ductal Carcinoma in Situ Cells and Carcinoma-Associated Fibroblasts Mediates Tumor Cell Growth and Migration. BMC Cancer 2015, 15, 584. [Google Scholar] [CrossRef]
- Sung, K.E.; Yang, N.; Pehlke, C.; Keely, P.J.; Eliceiri, K.W.; Friedl, A.; Beebe, D.J. Transition to Invasion in Breast Cancer: A Microfluidic in Vitro Model Enables Examination of Spatial and Temporal Effects. Integr. Biol. 2011, 3, 439–450. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-Led Collective Invasion of Carcinoma Cells with Differing Roles for RhoGTPases in Leading and Following Cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Giussani, M.; Landoni, E.; Merlino, G.; Turdo, F.; Veneroni, S.; Paolini, B.; Cappelletti, V.; Miceli, R.; Orlandi, R.; Triulzi, T.; et al. Extracellular Matrix Proteins as Diagnostic Markers of Breast Carcinoma. J. Cell. Physiol. 2018, 233, 6280–6290. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human Breast Cancer Invasion and Aggression Correlates with ECM Stiffening and Immune Cell Infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.-X.; Wu, H.-T.; Li, X.-L.; Wen, X.-F.; Du, C.-W.; Zhang, G.-J. Collagen 1A1 (COL1A1) Promotes Metastasis of Breast Cancer and Is a Potential Therapeutic Target. Discov. Med. 2018, 25, 211–223. [Google Scholar] [PubMed]
- Salimian, N.; Peymani, M.; Ghaedi, K.; Hashemi, M.; Rahimi, E. Collagen 1A1 (COL1A1) and Collagen11A1(COL11A1) as Diagnostic Biomarkers in Breast, Colorectal and Gastric Cancers. Gene 2024, 892, 147867. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lin, L.; Li, X.; Wen, R.; Zhang, X. Silencing of COL3A1 Represses Proliferation, Migration, Invasion, and Immune Escape of Triple Negative Breast Cancer Cells via down-Regulating PD-L1 Expression. Cell Biol. Int. 2022, 46, 1959–1969. [Google Scholar] [CrossRef]
- Toss, M.S.; Miligy, I.M.; Gorringe, K.L.; Aleskandarany, M.A.; Alkawaz, A.; Mittal, K.; Aneja, R.; Ellis, I.O.; Green, A.R.; Rakha, E.A. Collagen (XI) Alpha-1 Chain Is an Independent Prognostic Factor in Breast Ductal Carcinoma in Situ. Mod. Pathol. 2019, 32, 1460–1472. [Google Scholar] [CrossRef]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-Associated Macrophages: Unwitting Accomplices in Breast Cancer Malignancy. NPJ Breast Cancer 2016, 2, 15025. [Google Scholar] [CrossRef]
- Pollard, J.W. Trophic Macrophages in Development and Disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The Role of Myeloid Cells in the Promotion of Tumour Angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Li, M.O. TGF-β: Guardian of T Cell Function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Ojalvo, L.S.; King, W.; Cox, D.; Pollard, J.W. High-Density Gene Expression Analysis of Tumor-Associated Macrophages from Mouse Mammary Tumors. Am. J. Pathol. 2009, 174, 1048–1064. [Google Scholar] [CrossRef]
- Gopinathan, G.; Diekwisch, T.G.H. Epigenetics and Early Development. J. Dev. Biol. 2022, 10, 26. [Google Scholar] [CrossRef]
- DeVaux, R.S.; Herschkowitz, J.I. Beyond DNA: The Role of Epigenetics in the Premalignant Progression of Breast Cancer. J. Mammary Gland Biol. Neoplasia 2018, 23, 223–235. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwon, H.J.; Lee, H.E.; Ryu, H.S.; Kim, S.-W.; Kim, J.H.; Kim, I.A.; Jung, N.; Cho, N.-Y.; Kang, G.H. Promoter CpG Island Hypermethylation during Breast Cancer Progression. Virchows Arch. 2011, 458, 73–84. [Google Scholar] [CrossRef]
- Verschuur-Maes, A.H.J.; de Bruin, P.C.; van Diest, P.J. Epigenetic Progression of Columnar Cell Lesions of the Breast to Invasive Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 705–715. [Google Scholar] [CrossRef]
- van Hoesel, A.Q.; Sato, Y.; Elashoff, D.A.; Turner, R.R.; Giuliano, A.E.; Shamonki, J.M.; Kuppen, P.J.K.; van de Velde, C.J.H.; Hoon, D.S.B. Assessment of DNA Methylation Status in Early Stages of Breast Cancer Development. Br. J. Cancer 2013, 108, 2033–2038. [Google Scholar] [CrossRef]
- Lehmann, U.; Länger, F.; Feist, H.; Glöckner, S.; Hasemeier, B.; Kreipe, H. Quantitative Assessment of Promoter Hypermethylation during Breast Cancer Development. Am. J. Pathol. 2002, 160, 605–612. [Google Scholar] [CrossRef]
- Tommasi, S.; Karm, D.L.; Wu, X.; Yen, Y.; Pfeifer, G.P. Methylation of Homeobox Genes Is a Frequent and Early Epigenetic Event in Breast Cancer. Breast Cancer Res. 2009, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.C.; Koestler, D.C.; Fleischer, T.; Chen, P.; Jenson, E.G.; Marotti, J.D.; Onega, T.; Kristensen, V.N.; Christensen, B.C. DNA Methylation in Ductal Carcinoma in Situ Related with Future Development of Invasive Breast Cancer. Clin. Epigenetics 2015, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lin, J.-R.; Zhang, Q.; O’Brien, K.; Montagna, C.; Zhang, Z.D. Epigenetic Alterations to Polycomb Targets Precede Malignant Transition in a Mouse Model of Breast Cancer. Sci. Rep. 2018, 8, 5535. [Google Scholar] [CrossRef]
- Fleischer, T.; Frigessi, A.; Johnson, K.C.; Edvardsen, H.; Touleimat, N.; Klajic, J.; Riis, M.L.; Haakensen, V.D.; Wärnberg, F.; Naume, B.; et al. Genome-Wide DNA Methylation Profiles in Progression to in Situ and Invasive Carcinoma of the Breast with Impact on Gene Transcription and Prognosis. Genome Biol. 2014, 15, 435. [Google Scholar] [CrossRef]
- Tessarz, P.; Kouzarides, T. Histone Core Modifications Regulating Nucleosome Structure and Dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef]
- Ding, L.; Erdmann, C.; Chinnaiyan, A.M.; Merajver, S.D.; Kleer, C.G. Identification of EZH2 as a Molecular Marker for a Precancerous State in Morphologically Normal Breast Tissues. Cancer Res. 2006, 66, 4095–4099. [Google Scholar] [CrossRef]
- Kleer, C.G.; Cao, Q.; Varambally, S.; Shen, R.; Ota, I.; Tomlins, S.A.; Ghosh, D.; Sewalt, R.G.A.B.; Otte, A.P.; Hayes, D.F.; et al. EZH2 Is a Marker of Aggressive Breast Cancer and Promotes Neoplastic Transformation of Breast Epithelial Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11606–11611. [Google Scholar] [CrossRef]
- Serce, N.; Gnatzy, A.; Steiner, S.; Lorenzen, H.; Kirfel, J.; Buettner, R. Elevated Expression of LSD1 (Lysine-Specific Demethylase 1) during Tumour Progression from Pre-Invasive to Invasive Ductal Carcinoma of the Breast. BMC Clin. Pathol. 2012, 12, 13. [Google Scholar] [CrossRef]
- Suzuki, J.; Chen, Y.-Y.; Scott, G.K.; DeVries, S.; Chin, K.; Benz, C.C.; Waldman, F.M.; Hwang, E.S. Protein Acetylation and Histone Deacetylase Expression Associated with Malignant Breast Cancer Progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 3163–3171. [Google Scholar] [CrossRef]
- Dabi, Y.; Bendifallah, S.; Suisse, S.; Haury, J.; Touboul, C.; Puchar, A.; Favier, A.; Daraï, E. Overview of Non-Coding RNAs in Breast Cancers. Transl. Oncol. 2022, 25, 101512. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour Invasion and Metastasis Initiated by microRNA-10b in Breast Cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Blenkiron, C.; Goldstein, L.D.; Thorne, N.P.; Spiteri, I.; Chin, S.-F.; Dunning, M.J.; Barbosa-Morais, N.L.; Teschendorff, A.E.; Green, A.R.; Ellis, I.O.; et al. MicroRNA Expression Profiling of Human Breast Cancer Identifies New Markers of Tumor Subtype. Genome Biol. 2007, 8, R214. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bracken, C.P.; Bert, A.G.; Goodall, G.J. MicroRNAs as Regulators of Epithelial-Mesenchymal Transition. Cell Cycle 2008, 7, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, C.; Zhang, F. MicroRNA-206 Suppresses Growth and Metastasis of Breast Cancer Stem Cells via Blocking EVI-1-Mediated CALR Expression. PLoS ONE 2022, 17, e0274919. [Google Scholar] [CrossRef]
- Bhan, A.; Mandal, S.S. LncRNA HOTAIR: A Master Regulator of Chromatin Dynamics and Cancer. Biochim. Biophys. Acta BBA-Rev. Cancer 2015, 1856, 151–164. [Google Scholar] [CrossRef]
- Iacoangeli, A.; Lin, Y.; Morley, E.J.; Muslimov, I.A.; Bianchi, R.; Reilly, J.; Weedon, J.; Diallo, R.; Böcker, W.; Tiedge, H. BC200 RNA in Invasive and Preinvasive Breast Cancer. Carcinogenesis 2004, 25, 2125–2133. [Google Scholar] [CrossRef]
- van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal Carcinoma in Situ: To Treat or Not to Treat, That Is the Question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef]
- Elshof, L.E.; Tryfonidis, K.; Slaets, L.; Van Leeuwen-Stok, A.E.; Skinner, V.P.; Dif, N.; Pijnappel, R.M.; Bijker, N.; Rutgers, E.J.T.; Wesseling, J. Feasibility of a Prospective, Randomised, Open-Label, International Multicentre, Phase III, Non-Inferiority Trial to Assess the Safety of Active Surveillance for Low Risk Ductal Carcinoma in Situ—The LORD Study. Eur. J. Cancer 2015, 51, 1497–1510. [Google Scholar] [CrossRef]
- Francis, A.; Thomas, J.; Fallowfield, L.; Wallis, M.; Bartlett, J.M.S.; Brookes, C.; Roberts, T.; Pirrie, S.; Gaunt, C.; Young, J.; et al. Addressing Overtreatment of Screen Detected DCIS; the LORIS Trial. Eur. J. Cancer 2015, 51, 2296–2303. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Frank, E.; Pinto, D.; Basila, D.; Collyar, D.; Bennett, A.; Kaplan, C.; Rosenberg, S.; et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) Trial: A Phase III Randomised Controlled Clinical Trial for Low-Risk Ductal Carcinoma in Situ (DCIS). BMJ Open 2019, 9, e026797. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Ryser, M.D.; Weiss, A.; Wolf, A.; Norris, K.; Witten, M.; Grimm, L.; Schnitt, S.; et al. Active Monitoring With or Without Endocrine Therapy for Low-Risk Ductal Carcinoma In Situ: The COMET Randomized Clinical Trial. JAMA 2025, 333, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.A. COMETgazing—Interesting Insights, Lessons for Clinical Practice and a Call for More Precision Using the biomarkerSCOPE. Oncotarget 2025, 16, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.; Rodin, D.; Sutradhar, R.; Nofech-Mozes, S.; Trebinjac, S.; Paszat, L.F.; Rakovitch, E. Can Molecular Biomarkers Help Reduce the Overtreatment of DCIS? Curr. Oncol. 2023, 30, 5795–5806. [Google Scholar] [CrossRef]
- Cilento, M.A.; Sweeney, C.J.; Butler, L.M. Spatial Transcriptomics in Cancer Research and Potential Clinical Impact: A Narrative Review. J. Cancer Res. Clin. Oncol. 2024, 150, 296. [Google Scholar] [CrossRef]
- Page, D.B.; Broeckx, G.; Jahangir, C.A.; Verbandt, S.; Gupta, R.R.; Thagaard, J.; Khiroya, R.; Kos, Z.; Abduljabbar, K.; Acosta Haab, G.; et al. Spatial Analyses of Immune Cell Infiltration in Cancer: Current Methods and Future Directions: A Report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. J. Pathol. 2023, 260, 514–532. [Google Scholar] [CrossRef]
- Donisi, C.; Pretta, A.; Pusceddu, V.; Ziranu, P.; Lai, E.; Puzzoni, M.; Mariani, S.; Massa, E.; Madeddu, C.; Scartozzi, M. Immunotherapy and Cancer: The Multi-Omics Perspective. Int. J. Mol. Sci. 2024, 25, 3563. [Google Scholar] [CrossRef]
- Ji, A.L.; Rubin, A.J.; Thrane, K.; Jiang, S.; Reynolds, D.L.; Meyers, R.M.; Guo, M.G.; George, B.M.; Mollbrink, A.; Bergenstråhle, J.; et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell 2020, 182, 497–514.e22. [Google Scholar] [CrossRef]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S.; et al. Spatial Transcriptomics Reveals Distinct and Conserved Tumor Core and Edge Architectures That Predict Survival and Targeted Therapy Response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Wang, K.; Zhou, L.; Jiang, M.; Gao, Y.; Yang, R.; Yan, S.; Zhang, W.; Lu, B.; et al. Spatial Transcriptomics Analysis of Esophageal Squamous Precancerous Lesions and Their Progression to Esophageal Cancer. Nat. Commun. 2023, 14, 4779. [Google Scholar] [CrossRef]
- Ravi, V.M.; Will, P.; Kueckelhaus, J.; Sun, N.; Joseph, K.; Salié, H.; Vollmer, L.; Kuliesiute, U.; von Ehr, J.; Benotmane, J.K.; et al. Spatially Resolved Multi-Omics Deciphers Bidirectional Tumor-Host Interdependence in Glioblastoma. Cancer Cell 2022, 40, 639–655.e13. [Google Scholar] [CrossRef]
- Yousuf, S.; Qiu, M.; Voith Von Voithenberg, L.; Hulkkonen, J.; Macinkovic, I.; Schulz, A.R.; Hartmann, D.; Mueller, F.; Mijatovic, M.; Ibberson, D.; et al. Spatially Resolved Multi-Omics Single-Cell Analyses Inform Mechanisms of Immune Dysfunction in Pancreatic Cancer. Gastroenterology 2023, 165, 891–908.e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Long, J.; Li, L.; Wu, Z.-X.; Da, T.-T.; Wang, X.-Q.; Huang, C.; Jiang, Y.-H.; Yao, X.-Q.; Ma, H.-Q.; et al. Single-Cell and Spatial Transcriptome Analysis Reveals the Cellular Heterogeneity of Liver Metastatic Colorectal Cancer. Sci. Adv. 2023, 9, eadf5464. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-D.; Sun, Z.; Zhu, Z.-B.; Liu, X.; Chen, J.-Z.; Hao, L.; Zhu, J.-F.; Pang, K.; Wu, D.; Dong, Y.; et al. Integrated Single-Cell and Spatial Transcriptomic Profiling Reveals Higher Intratumour Heterogeneity and Epithelial–Fibroblast Interactions in Recurrent Bladder Cancer. Clin. Transl. Med. 2023, 13, e1338. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, P.; Wang, B.G.; Murdock, T.; Cope, L.; Hsu, F.-C.; Wang, T.-L.; Shih, I.-M. Spatial Transcriptomic Analysis of Ovarian Cancer Precursors Reveals Reactivation of IGFBP2 during Pathogenesis. Cancer Res. 2022, 82, 4528–4541. [Google Scholar] [CrossRef]
- Bassiouni, R.; Idowu, M.O.; Gibbs, L.D.; Robila, V.; Grizzard, P.J.; Webb, M.G.; Song, J.; Noriega, A.; Craig, D.W.; Carpten, J.D. Spatial Transcriptomic Analysis of a Diverse Patient Cohort Reveals a Conserved Architecture in Triple-Negative Breast Cancer. Cancer Res. 2023, 83, 34–48. [Google Scholar] [CrossRef]
- Coutant, A.; Cockenpot, V.; Muller, L.; Degletagne, C.; Pommier, R.; Tonon, L.; Ardin, M.; Michallet, M.-C.; Caux, C.; Laurent, M.; et al. Spatial Transcriptomics Reveal Pitfalls and Opportunities for the Detection of Rare High-Plasticity Breast Cancer Subtypes. Lab. Investig. J. Tech. Methods Pathol. 2023, 103, 100258. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Ge, J.-Y.; Chen, Y.-F.; Liu, T.; Chen, L.; Liu, C.-C.; Ma, D.; Chen, Y.-Y.; Cai, Y.-W.; Xu, Y.-Y.; et al. Combined Single-Cell and Spatial Transcriptomics Reveal the Metabolic Evolvement of Breast Cancer during Early Dissemination. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2205395. [Google Scholar] [CrossRef]
- Wang, X.; Venet, D.; Lifrange, F.; Larsimont, D.; Rediti, M.; Stenbeck, L.; Dupont, F.; Rouas, G.; Garcia, A.J.; Craciun, L.; et al. Spatial Transcriptomics Reveals Substantial Heterogeneity in Triple-Negative Breast Cancer with Potential Clinical Implications. Nat. Commun. 2024, 15, 10232. [Google Scholar] [CrossRef]
- Sobhani, F.; Muralidhar, S.; Hamidinekoo, A.; Hall, A.H.; King, L.M.; Marks, J.R.; Maley, C.; Horlings, H.M.; Hwang, E.S.; Yuan, Y. Spatial Interplay of Tissue Hypoxia and T-Cell Regulation in Ductal Carcinoma in Situ. NPJ Breast Cancer 2022, 8, 105. [Google Scholar] [CrossRef]
- Hulahan, T.S.; Spruill, L.; Wallace, E.N.; Park, Y.; West, R.B.; Marks, J.R.; Hwang, E.S.; Drake, R.R.; Angel, P.M. Extracellular Microenvironment Alterations in Ductal Carcinoma In Situ and Invasive Breast Cancer Pathologies by Multiplexed Spatial Proteomics. Int. J. Mol. Sci. 2024, 25, 6748. [Google Scholar] [CrossRef]
- Badve, S.S.; Cho, S.; Gökmen-Polar, Y.; Sui, Y.; Chadwick, C.; McDonough, E.; Sood, A.; Taylor, M.; Zavodszky, M.; Tan, P.H.; et al. Multi-Protein Spatial Signatures in Ductal Carcinoma in Situ (DCIS) of Breast. Br. J. Cancer 2021, 124, 1150–1159. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajzendanc, K. DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges. Cancers 2025, 17, 1925. https://doi.org/10.3390/cancers17121925
Prajzendanc K. DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges. Cancers. 2025; 17(12):1925. https://doi.org/10.3390/cancers17121925
Chicago/Turabian StylePrajzendanc, Karolina. 2025. "DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges" Cancers 17, no. 12: 1925. https://doi.org/10.3390/cancers17121925
APA StylePrajzendanc, K. (2025). DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges. Cancers, 17(12), 1925. https://doi.org/10.3390/cancers17121925





