Propofol Total Intravenous Anesthesia for Pediatric Proton Radiotherapy and Its Effect on Patient Outcomes
Simple Summary
Abstract
1. Introduction
2. Methods
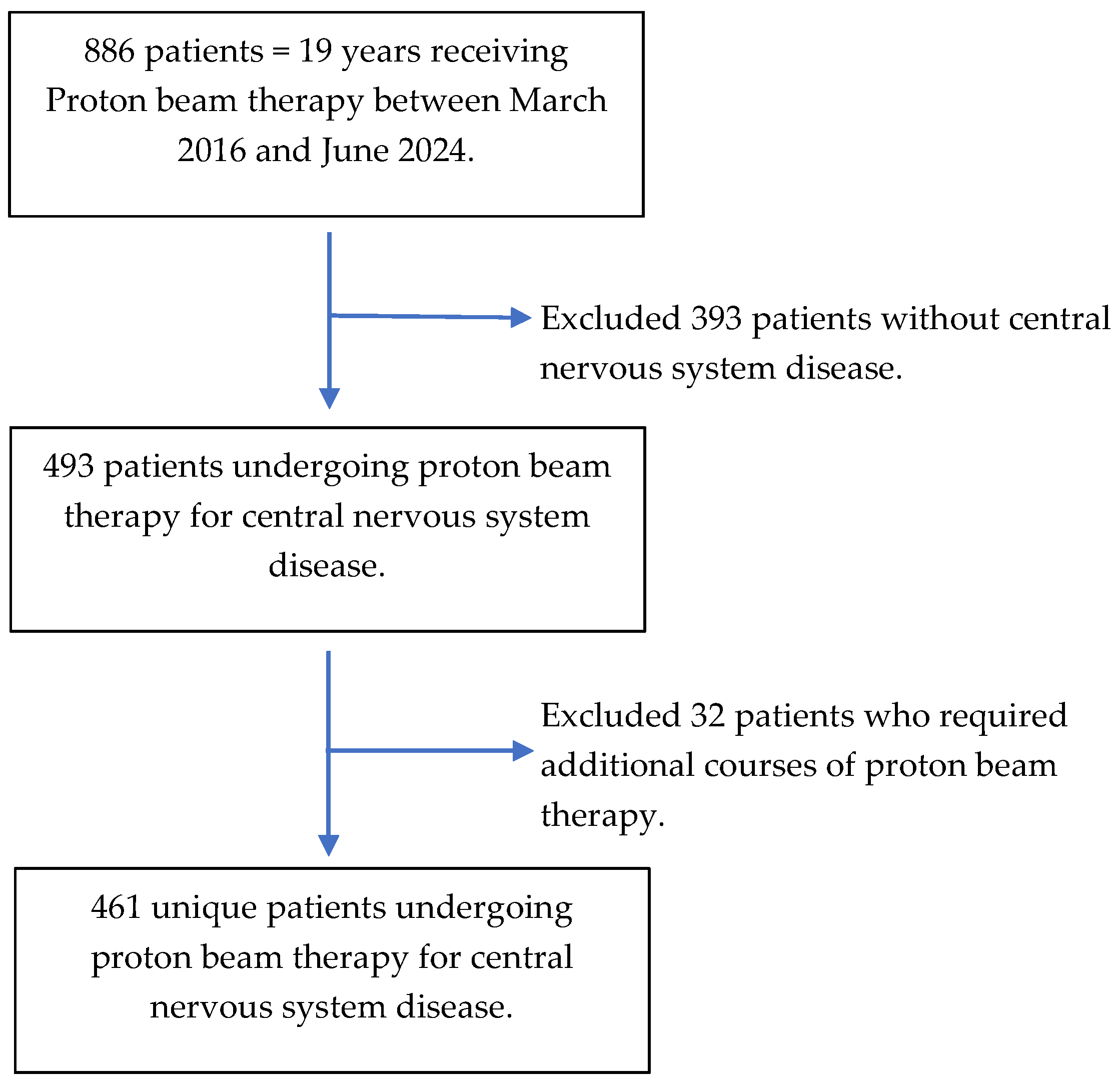
2.1. Study Population
2.2. Study Covariates
2.3. Anesthetic Management and Exposure Variable
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Oncological Outcome
3.3. Thirty-Day Unplanned Admissions or Emergency Room Visits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debel, W.; Ramadhan, A.; Vanpeteghem, C.; Forsyth, R.G. Does the Choice of Anaesthesia Affect Cancer? A Molecular Crosstalk between Theory and Practice. Cancers 2022, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Gan, T.J. Total intravenous anesthesia versus inhalation anesthesia: How do outcomes compare? Curr. Opin. Anaesthesiol. 2023, 36, 399–406. [Google Scholar] [CrossRef]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, G.H.; Wang, B.N.; Sun, L.; Zheng, H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-β and prognosis after breast cancer surgery: A prospective, randomized and controlled study. BMC Anesth. 2018, 18, 131. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, N.J.; Zhang, H.; Zhu, Y.M. Effects of combined general-epidural anesthesia and total intravenous anesthesia on cellular immunity and prognosis in patients with non-small cell lung cancer: A comparative study. Mol. Med. Rep. 2017, 16, 4445–4454. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, J.; Yeo, H.; Kim, K.; Rhu, J.; Choi, G.S.; Kim, J.; Joh, J.W.; Kim, K.; Kim, M.J.; et al. Recurrence-free survival after hepatectomy using propofol-based total intravenous anaesthesia and sevoflurane-based inhalational anaesthesia: A randomised controlled study. Anaesthesia 2025, 80, 366–377. [Google Scholar] [CrossRef]
- Cao, S.J.; Zhang, Y.; Zhang, Y.X.; Zhao, W.; Pan, L.H.; Sun, X.D.; Jia, Z.; Ouyang, W.; Ye, Q.S.; Zhang, F.X.; et al. Long-term survival in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: Follow-up of a multicentre randomised trial. Br. J. Anaesth. 2023, 131, 266–275. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Enlund, A.; Lundberg, J.; Wärnberg, F.; Wang, D.X.; Ekman, A.; Ahlstrand, R.; Flisberg, P.; Hedlund, L.; et al. Impact of general anaesthesia on breast cancer survival: A 5-year follow up of a pragmatic, randomised, controlled trial, the CAN-study, comparing propofol and sevoflurane. EClinicalMedicine 2023, 60, 102037. [Google Scholar] [CrossRef]
- Jansen, L.; Dubois, B.F.H.; Hollmann, M.W. The Effect of Propofol versus Inhalation Anesthetics on Survival after Oncological Surgery. J. Clin. Med. 2022, 11, 6741. [Google Scholar] [CrossRef]
- Xie, S.; Li, L.; Meng, F.; Wang, H. Regional anesthesia might reduce recurrence and metastasis rates in adult patients with cancers after surgery: A meta-analysis. BMC Anesth. 2024, 24, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Qin, C.; Zhang, C.; Du, Y.; Xu, T. Effect of regional versus general anesthesia on recurrence of non-muscle invasive bladder cancer: A systematic review and meta-analysis of eight retrospective cohort studies. BMC Anesth. 2023, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyemang, P.; Tsai, J.Y.; Kapoor, R.; Van Meter, A.; Tan, G.M.; Peters, S.; Opitz, L.; Pedrotti, D.; DeSoto, H.S.; Zavala, A.M. Survey of Anesthesia, Sedation, and Non-sedation Practices for Children Undergoing Repetitive Cranial or Craniospinal Radiotherapy. Cureus 2022, 14, e24075. [Google Scholar] [CrossRef]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: Mediating mechanisms and prophylactic measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef]
- Xu, J.; Xu, W.; Zhu, J. Propofol suppresses proliferation and invasion of glioma cells by upregulating microRNA-218 expression. Mol. Med. Rep. 2015, 12, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Zhang, J.; Li, J.; Shu, Y. Propofol Suppresses Glioma Tumorigenesis by Regulating circ_0047688/miR-516b-5p/IFI30 Axis. Biochem. Genet. 2023, 61, 151–169. [Google Scholar] [CrossRef]
- Cen, S.; Yang, G.; Bao, H.; Yu, Z.; Liang, L. Impact of propofol versus sevoflurane anesthesia on molecular subtypes and immune checkpoints of glioma during surgery. Health Sci. Rep. 2023, 6, e1366. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zeng, M.; Ji, N.; Hao, S.; Zhou, Y.; Gao, Z.; Gu, H.; Zhang, L.; Ma, D.; Peng, Y.; et al. Impact of Anesthesia on Long-term Outcomes in Patients With Supratentorial High-grade Glioma Undergoing Tumor Resection: A Retrospective Cohort Study. J. Neurosurg. Anesth. 2020, 32, 227–233. [Google Scholar] [CrossRef]
- Grau, S.J.; Löhr, M.; Taurisano, V.; Trautner, H.; Timmer, M.; Schwab, S.G.; Hampl, J.; Annecke, T. The choice of anaesthesia for glioblastoma surgery does not impact the time to recurrence. Sci. Rep. 2020, 10, 5556. [Google Scholar] [CrossRef]
- Chow, R.; Hasan, S.; Choi, J.I.; Fox, J.; Chhabra, A.M.; Marshall, D.C.; Bakst, R.L.; Simone, C.B., 2nd. Effect of treatment interruptions on overall survival in patients with triple-negative breast cancer. J. Natl. Cancer Inst. 2023, 115, 1029–1035. [Google Scholar] [CrossRef]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef]
- Waddle, M.R.; Chen, R.C.; Arastu, N.H.; Green, R.L.; Jackson, M.; Qaqish, B.F.; Camporeale, J.; Collichio, F.A.; Marks, L.B. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract. Radiat. Oncol. 2015, 5, e245–e253. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, W.; Hamada, T.; Suzuki, J.; Matsuoka, Y.; Omori-Miyake, M.; Kuwahara, M.; Matsumoto, A.; Nomura, S.; Konishi, A.; Yorozuya, T.; et al. Suppressive effect of the anesthetic propofol on the T cell function and T cell-dependent immune responses. Sci. Rep. 2024, 14, 19337. [Google Scholar] [CrossRef] [PubMed]
- Visvabharathy, L.; Xayarath, B.; Weinberg, G.; Shilling, R.A.; Freitag, N.E. Propofol Increases Host Susceptibility to Microbial Infection by Reducing Subpopulations of Mature Immune Effector Cells at Sites of Infection. PLoS ONE 2015, 10, e0138043. [Google Scholar] [CrossRef]
- Yi, S.; Tao, X.; Wang, Y.; Cao, Q.; Zhou, Z.; Wang, S. Effects of propofol on macrophage activation and function in diseases. Front. Pharmacol. 2022, 13, 964771. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, N.; Valls, N.; González, R. Techniques and Complications of Anesthesia in Pediatric Radiotherapy: A Retrospective Cohort Study. J. Pediatr. Hematol. Oncol. 2023, 45, 377–382. [Google Scholar] [CrossRef]
- McFadyen, J.G.; Pelly, N.; Orr, R.J. Sedation and anesthesia for the pediatric patient undergoing radiation therapy. Curr. Opin. Anaesthesiol. 2011, 24, 433–438. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 461) | No Anesthesia (n = 267) | Propofol Anesthesia (n = 194) | p-Value |
|---|---|---|---|---|
| Age, years, median | 9 | 13 | 4 | <0.001 |
| (interquartile range) | (5–13) | (10–16) | (2–6) | |
| Gender, No. (%) | 0.975 | |||
| Female | 200 (43.4) | 116 (43.4) | 84 (43.3) | |
| Male | 261 (56.6) | 151 (56.6) | 110 (56.7) | |
| Ethnicity/Race of patients with available data a, No. (%) | 0.520 | |||
| American Indian or Alaska Native | 2 (0.5) | 0 (0) | 2 (1.1) | |
| Asian | 31 (7.3) | 19 (7.7) | 12 (6.9) | |
| Black or African American | 46 (10.9) | 29 (11.7) | 17 (9.7) | |
| Hispanic or Latino | 182 (43) | 103 (41.5) | 79 (45.1) | |
| White | 162 (38.3) | 97 (39.1) | 65 (37.1) | |
| Prior Chemotherapy, No. (%) | <0.001 | |||
| No | 379 (82.2) | 249 (93.3) | 130 (67) | |
| Yes | 82 (17.8) | 18 (6.7) | 64 (33) | |
| Concurrent Chemotherapy, No. (%) | 0.006 | |||
| No | 397 (86.1) | 240 (89.9) | 157 (80.9) | |
| Yes | 64 (13.9) | 27 (10.1) | 37 (19.1) | |
| Number of Chemotherapy Agents, No. (%) | <0.001 | |||
| None | 346 (75.1) | 230 (86.1) | 116 (59.8) | |
| One | 31 (6.7) | 11 (4.1) | 20 (10.3) | |
| Two | 34 (7.4) | 14 (5.2) | 20 (10.3) | |
| Three or more | 50 (10.8) | 12 (4.5) | 38 (19.6) | |
| Days between Cancer-Related Surgery and Start of PBT, mean (SD) | 241 (569) | 281 (663) | 186 (406) | 0.898 |
| Proton Radiotherapy Field, No. (%) | <0.001 | |||
| Brain | 234 (50.8) | 156 (58.4) | 78 (40.2) | |
| Craniospinal | 207 (44.9) | 99 (37.1) | 108 (55.7) | |
| Spine | 20 (4.3) | 12 (4.5) | 8 (4.1) | |
| PBT Interrupted, No. (%) | 0.006 | |||
| No | 232 (50.3) | 149 (55.8) | 83 (42.8) | |
| Yes | 229 (49.7) | 118 (44.2) | 111 (57.2) | |
| Days of Interruption, No. (%) | 0.019 | |||
| 1 | 93 (20.2) | 50 (18.7) | 43 (22.2) | |
| ≥2 | 136 (29.5) | 68 (25.5) | 68 (35.1) | |
| Reasons for Interruption, No. (%) | ||||
| Medical | 123 (26.7) | 66 (24.7) | 57 (29.4) | 0.264 |
| Equipment Failure and Non-medical | 84 (18.2) | 34 (12.7) | 50 (25.8) | <0.001 |
| Unknown | 62 (13.4) | 31 (11.6) | 31 (16) | 0.175 |
| 30-day Admission and/or ER Visit, No. (%) | <0.001 | |||
| No | 434 (94.1) | 266 (99.6) | 168 (86.6) | |
| Yes | 27 (5.9) | 1 (0.4) | 26 (13.4) |
| Variable | Patients | Events | OS Rate at 1 Year | OS Rate at 2 Years | OS Rate at 3 Years | p-Value * |
|---|---|---|---|---|---|---|
| (95%CI) | (95%CI) | (95%CI) | ||||
| All patients | 461 | 39 | 0.95 (0.93, 0.97) | 0.89 (0.86, 0.93) | 0.89 (0.85, 0.93) | |
| Age, years: | 0.838 | |||||
| 0: <13 | 326 | 27 | 0.95 (0.92, 0.98) | 0.9 (0.86, 0.94) | 0.89 (0.85, 0.94) | |
| 1: ≥13 | 135 | 12 | 0.96 (0.92, 1) | 0.88 (0.81, 0.95) | 0.88 (0.81, 0.95) | |
| Gender: | 0.923 | |||||
| Female | 200 | 16 | 0.94 (0.9, 0.98) | 0.89 (0.83, 0.95) | 0.89 (0.83, 0.95) | |
| Male | 261 | 23 | 0.96 (0.93, 0.99) | 0.9 (0.85, 0.95) | 0.89 (0.84, 0.94) | |
| Race/Ethnicity: | 0.702 | |||||
| Asian | 31 | 2 | 1 (1, 1) | 0.94 (0.84, 1) | 0.94 (0.84, 1) | |
| Black/African American | 46 | 7 | 0.91 (0.82, 1) | 0.85 (0.73, 0.98) | 0.85 (0.73, 0.98) | |
| Hispanic or Latino | 182 | 17 | 0.94 (0.9, 0.98) | 0.88 (0.82, 0.94) | 0.86 (0.8, 0.93) | |
| Other | 2 | 0 | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | |
| White | 162 | 11 | 0.96 (0.92, 1) | 0.92 (0.86, 0.97) | 0.92 (0.86, 0.97) | |
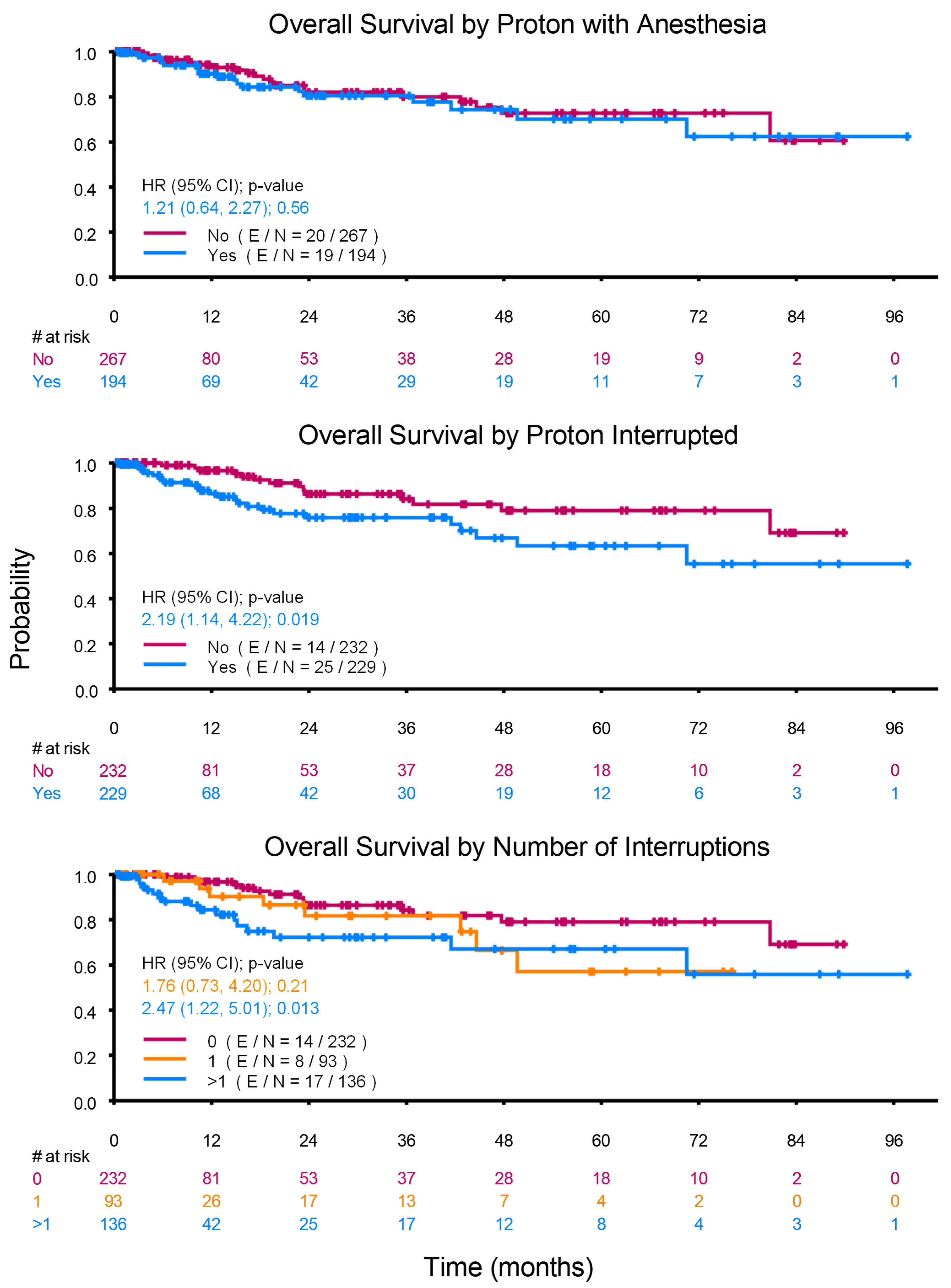
| PBT with Anesthesia: | 0.558 | |||||
| None | 267 | 20 | 0.96 (0.93, 0.99) | 0.9 (0.86, 0.95) | 0.89 (0.84, 0.94) | |
| Propofol Anesthesia | 194 | 19 | 0.94 (0.9, 0.98) | 0.88 (0.83, 0.94) | 0.88 (0.83, 0.94) | |
| Prior Chemotherapy: | 0.152 | |||||
| No | 379 | 28 | 0.96 (0.93, 0.98) | 0.91 (0.87, 0.95) | 0.9 (0.86, 0.94) | |
| Yes | 82 | 11 | 0.92 (0.86, 0.99) | 0.84 (0.75, 0.95) | 0.84 (0.75, 0.95) | |
| Concurrent Chemotherapy: | 0.003 | |||||
| No | 397 | 27 | 0.96 (0.94, 0.99) | 0.92 (0.88, 0.95) | 0.91 (0.87, 0.95) | |
| Yes | 64 | 12 | 0.88 (0.8, 0.98) | 0.79 (0.68, 0.92) | 0.79 (0.68, 0.92) | |
| PBT Interrupted: | 0.019 | |||||
| No | 232 | 14 | 0.98 (0.96, 1) | 0.93 (0.89, 0.97) | 0.92 (0.87, 0.97) | |
| Yes | 229 | 25 | 0.92 (0.88, 0.96) | 0.86 (0.8, 0.92) | 0.86 (0.8, 0.92) | |
| Number of Interruptions: | 0.033 | |||||
| 0 days | 232 | 14 | 0.98 (0.96, 1) | 0.93 (0.89, 0.97) | 0.92 (0.87, 0.97) | |
| 1 day | 93 | 8 | 0.95 (0.9, 1) | 0.91 (0.83, 0.99) | 0.91 (0.83, 0.99) | |
| ≥2 days | 136 | 17 | 0.80 (0.84, 0.96) | 0.83 (0.75, 0.91) | 0.83 (0.75, 0.91) | |
| Variable | 30-Day Unplanned Admission and/ or Emergency Room Visit (Before Propensity Score Matching) | 30-Day Unplanned Admission and/or Emergency Room Visit (After Propensity Score Matching) | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 461) | No (n = 434) | Yes (n = 27) | p-Value | All (n = 296) | No (n = 278) | Yes (n = 18) | p-Value | |
| Age, years (median, IQR) | 9 (5, 13) | 9 (5, 13) | 4 (2, 8) | <0.001 | 8.0 (4, 12) | 8.0 (4.0, 13.0) | 6.0 (4.0, 8.0) | 0.099 |
| Gender, n (%) | 0.278 | |||||||
| Female | 200 (43.4) | 191 (95.5) | 9 (4.5) | |||||
| Male | 261 (56.6) | 243 (93.1) | 18 (6.9) | |||||
| Ethnicity/Race, n (%) | 2 (0.5) | 2 (100) | 0 (0) | 0.725 | ||||
| AIAN | 31 (7.3) | 29 (93.5) | 2 (6.5) | |||||
| Asian | 46 (10.9) | 44 (95.7) | 2 (4.3) | |||||
| Black/AA | ||||||||
| Hispanic or Latino | 182 (43) | 169 (92.9) | 13 (7.1) | |||||
| White | 162 (38.3) | 155 (95.7) | 7 (4.3) | |||||
| PBT with Propofol Anesthesia, n (%) | <0.001 | <0.001 | ||||||
| No | 267 (57.9) | 266 (99.6) | 1 (0.4) | 148 (50.0) | 147 (99.3) | 1 (0.7) | ||
| Yes | 194 (42.1) | 168 (86.6) | 26 (13.4) | 148 (50.0) | 131 (88.5) | 17 (11.5) | ||
| Previous Chemotherapy, n (%) | 0.029 | 0.1427 | ||||||
| No | 379 (82.2) | 361 (95.3) | 18 (4.7) | 260 (87.8) | 242 (93.1) | 18 (6.9) | ||
| Yes | 82 (17.8) | 73 (89) | 9 (11) | 36 (12.2) | 36 (100.0) | 0 (0.0) | ||
| Concurrent Chemotherapy | 0.403 | |||||||
| No | 397 (86.1) | 375 (94.5) | 22 (5.5) | |||||
| Yes | 64 (13.9) | 59 (92.2) | 5 (7.8) | |||||
| Number of Chemotherapy Agents, n (%) | 0.101 | |||||||
| None | 346 (75.1) | 330 (95.4) | 16 (4.6) | |||||
| One | 31 (6.7) | 27 (87.1) | 4 (12.9) | |||||
| Two | 34 (7.4) | 32 (94.1) | 2 (5.9) | |||||
| Three or more | 50 (10.8) | 45 (90) | 5 (10) | |||||
| Days between Cancer-Related Surgery and Start of PBT, mean (SD) | 241 (569) | 245 (578) | 164(402) | 0.834 | ||||
| Proton Radiotherapy Field, No. (%) | 0.182 | 0.1302 | ||||||
| Brain | 234 (50.8) | 222 (94.9) | 12 (5.1) | 122 (41.2) | 114 (93.4) | 8 (6.6) | ||
| Craniospinal | 207 (44.9) | 195 (94.2) | 12 (5.8) | 164 (55.4) | 156 (95.1) | 8 (4.9) | ||
| Spine | 20 (4.3) | 17 (85.0) | 3 (15) | 10 (3.4) | 8 (80.0) | 2 (20.0) | ||
| PBT Interrupted, n (%) | 0.027 | |||||||
| No | 232 (50.3) | 224 (96.6) | 8 (3.4) | |||||
| Yes | 229 (49.7) | 210 (91.7) | 19 (8.3) | |||||
| Days of Interruption, n (%) | 0.013 | |||||||
| 1 | 93 (20.2) | 89 (95.7) | 4 (4.3) | |||||
| ≥2 | 136 (29.5) | 121 (89) | 15 (11) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owusu-Agyemang, P.; Mani, J.; Idowu, T.; Zavala, A.; Tsai, J.; Kapoor, R.; Idowu, O.; Galdamez Melara, J.; Muraleedharan, P.; Francis, C.; et al. Propofol Total Intravenous Anesthesia for Pediatric Proton Radiotherapy and Its Effect on Patient Outcomes. Cancers 2025, 17, 1904. https://doi.org/10.3390/cancers17121904
Owusu-Agyemang P, Mani J, Idowu T, Zavala A, Tsai J, Kapoor R, Idowu O, Galdamez Melara J, Muraleedharan P, Francis C, et al. Propofol Total Intravenous Anesthesia for Pediatric Proton Radiotherapy and Its Effect on Patient Outcomes. Cancers. 2025; 17(12):1904. https://doi.org/10.3390/cancers17121904
Chicago/Turabian StyleOwusu-Agyemang, Pascal, Julie Mani, Techecia Idowu, Acsa Zavala, January Tsai, Ravish Kapoor, Olakunle Idowu, Jose Galdamez Melara, Pallavi Muraleedharan, Clara Francis, and et al. 2025. "Propofol Total Intravenous Anesthesia for Pediatric Proton Radiotherapy and Its Effect on Patient Outcomes" Cancers 17, no. 12: 1904. https://doi.org/10.3390/cancers17121904
APA StyleOwusu-Agyemang, P., Mani, J., Idowu, T., Zavala, A., Tsai, J., Kapoor, R., Idowu, O., Galdamez Melara, J., Muraleedharan, P., Francis, C., Feng, L., & Cata, J. (2025). Propofol Total Intravenous Anesthesia for Pediatric Proton Radiotherapy and Its Effect on Patient Outcomes. Cancers, 17(12), 1904. https://doi.org/10.3390/cancers17121904






