Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation
Simple Summary
Abstract
1. Introduction
1.1. Highlighting the Persistent Challenge of Pancreatic Cancer
1.2. The Promise of IHC in the Era of Advanced Molecular Technologies
- •
- Delineate the current landscape of predictive IHC biomarkers in PDAC, highlighting successes and challenges.
- •
- Analyze the ethical implications of using IHC to guide treatment decisions, focusing on the principles of autonomy, beneficence, non-maleficence, and justice.
- •
- Assess how IHC-based approaches align with clinical needs and patient welfare.
2. Methods
3. Results
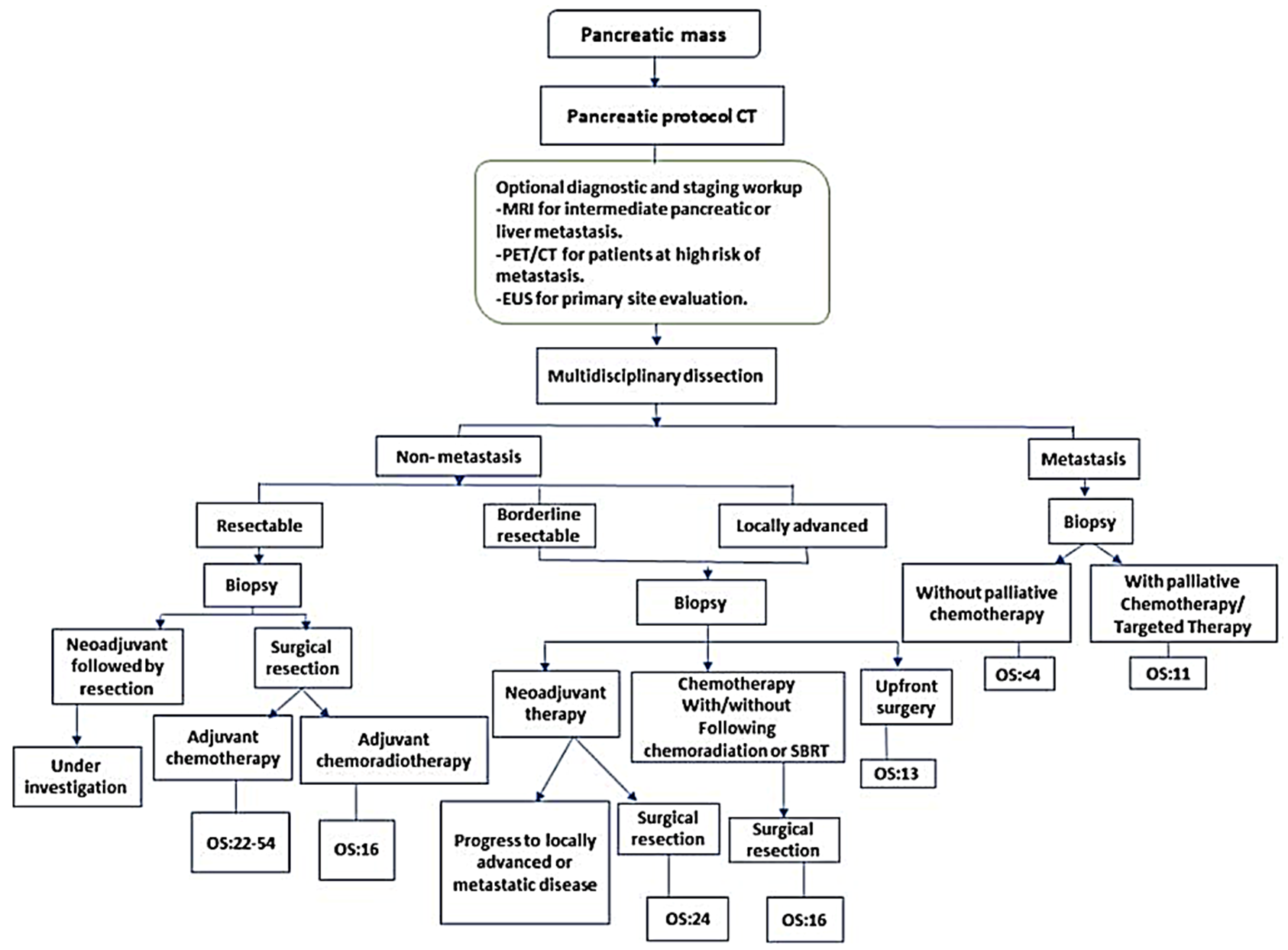
3.1. The Utility and Clinical Imperative of IHC Biomarkers in PDAC
3.2. The Utility Dilemma: Balancing Cost, Actionability, and Patient Benefit
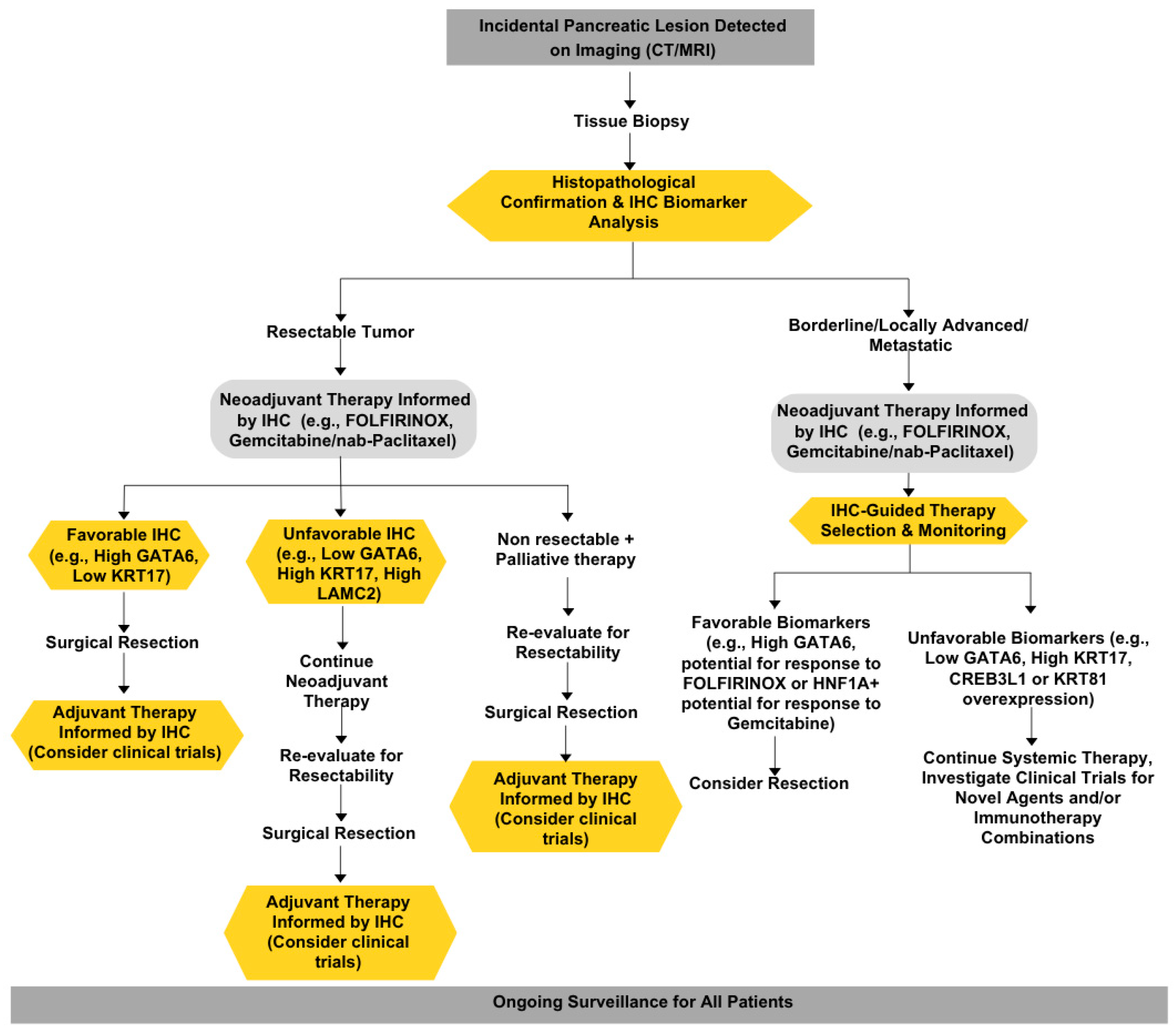
3.3. IHC: A Value-Based Solution to the Utility Dilemma
3.4. Ethical Principles Guiding the Implementation of IHC Predictive Biomarkers
3.5. Autonomy
3.6. Beneficence
3.7. Non-Maleficence
3.8. Justice
4. Discussion
5. Ethical Considerations: Utility, Equity, and Transparency
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schepis, T.; De Lucia, S.S.; Pellegrino, A.; Del Gaudio, A.; Maresca, R.; Coppola, G.; Chiappetta, M.F.; Gasbarrini, A.; Franceschi, F.; Candelli, M.; et al. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers 2023, 15, 3423. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Q.; Castaneda, C.; Cook, S. Targeted Therapies in Pancreatic Cancer: A New Era of Precision Medicine. Biomedicines 2024, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.L.; Canto, M.I.; Dbouk, M.; Hruban, R.H.; Katona, B.W.; Chak, A.; Brand, R.E.; Syngal, S.; Farrell, J.; Kastrinos, F.; et al. Pancreatic Cancer Surveillance and Survival of High-Risk Individuals. JAMA Oncol. 2024, 10, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.; Arnold, D.; Brunner, T.; Cardone, A.; Carrato, A.; Coll-Ortega, C.; De Luze, S.; Garel, P.; Goossens, M.E.; Grilli, R.; et al. Bratislava Statement: Consensus recommendations for improving pancreatic cancer care. ESMO Open 2020, 5, e001051. [Google Scholar] [CrossRef]
- Bugazia, D.; Al-Najjar, E.; Esmail, A.; Abdelrahim, S.; Abboud, K.; Abdelrahim, A.; Umoru, G.; Rayyan, H.A.; Abudayyeh, A.; Al Moustafa, A.E.; et al. Pancreatic ductal adenocarcinoma: The latest on diagnosis, molecular profiling, and systemic treatments. Front. Oncol. 2024, 14, 1386699. [Google Scholar] [CrossRef]
- Garajova, I.; Peroni, M.; Gelsomino, F.; Leonardi, F. A Simple Overview of Pancreatic Cancer Treatment for Clinical Oncologists. Curr. Oncol. 2023, 30, 9587–9601. [Google Scholar] [CrossRef]
- Tirpe, A.; Streianu, C.; Isachesku, E.; Simon, I.; Berindan-Neagoe, I. The road to overcome pancreatic cancer: Where are we? Heliyon 2024, 10, e38196. [Google Scholar] [CrossRef]
- de la Fouchardiere, C.; Adham, M.; Marion-Audibert, A.M.; Duclos, A.; Darcha, C.; Berthelet, O.; Hervieu, V.; Artru, P.; Labrosse, H.; Fayet, Y.; et al. Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database. Cancers 2021, 13, 3515. [Google Scholar] [CrossRef]
- Hilmi, M.; Delecourt, F.; Raffenne, J.; Bourega, T.; Dusetti, N.; Iovanna, J.; Blum, Y.; Richard, M.; Neuzillet, C.; Couvelard, A.; et al. Redefining phenotypic intratumor heterogeneity of pancreatic ductal adenocarcinoma: A bottom-up approach. J. Pathol. 2025, 265, 448–461. [Google Scholar] [CrossRef]
- Yu, B.; Shao, S.; Ma, W. Frontiers in pancreatic cancer on biomarkers, microenvironment, and immunotherapy. Cancer Lett. 2025, 610, 217350. [Google Scholar] [CrossRef]
- Robertson, F.P.; Cameron, A.; Spiers, H.V.M.; Joseph, N.; Taylor, E.; Ratnayake, B.; Jamieson, N.B.; Pandanaboyana, S. Evidence for molecular subtyping in pancreatic ductal adenocarcinoma: A systematic review. HPB 2024, 26, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Coka, L.A.; Roa-Pena, L.; Babu, S.; Horowitz, M.; Petricoin, E.F., III; Matrisian, L.M.; Blais, E.M.; Marchenko, N.; Allard, F.D.; Akalin, A.; et al. Keratin 17 is a prognostic and predictive biomarker in pancreatic ductal adenocarcinoma. Am. J. Clin. Pathol. 2024, 162, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Juiz, N.; Elkaoutari, A.; Bigonnet, M.; Gayet, O.; Roques, J.; Nicolle, R.; Iovanna, J.; Dusetti, N. Basal-like and classical cells coexist in pancreatic cancer revealed by single-cell analysis on biopsy-derived pancreatic cancer organoids from the classical subtype. FASEB J. 2020, 34, 12214–12228. [Google Scholar] [CrossRef] [PubMed]
- Kokumai, T.; Omori, Y.; Ishida, M.; Ohtsuka, H.; Mizuma, M.; Nakagawa, K.; Maeda, C.; Ono, Y.; Mizukami, Y.; Miura, S.; et al. GATA6 and CK5 Stratify the Survival of Patients with Pancreatic Cancer Undergoing Neoadjuvant Chemotherapy. Mod. Pathol. 2023, 36, 100102. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Raghavan, S.; Winter, P.S.; Navia, A.W.; Williams, H.L.; DenAdel, A.; Lowder, K.E.; Galvez-Reyes, J.; Kalekar, R.L.; Mulugeta, N.; Kapner, K.S.; et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 2021, 184, 6119–6137.e26. [Google Scholar] [CrossRef]
- Hayashi, H.; Higashi, T.; Miyata, T.; Yamashita, Y.I.; Baba, H. Recent advances in precision medicine for pancreatic ductal adenocarcinoma. Ann. Gastroenterol. Surg. 2021, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ahmadvand, P.; Farahani, H.; Farnell, D.; Darbandsari, A.; Topham, J.; Karasinska, J.; Nelson, J.; Naso, J.; Jones, S.J.M.; Renouf, D.; et al. A Deep Learning Approach for the Identification of the Molecular Subtypes of Pancreatic Ductal Adenocarcinoma Based on Whole Slide Pathology Images. Am. J. Pathol. 2024, 194, 2302–2312. [Google Scholar] [CrossRef]
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; Andre, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef]
- Giannis, D.; Moris, D.; Barbas, A.S. Diagnostic, Predictive and Prognostic Molecular Biomarkers in Pancreatic Cancer: An Overview for Clinicians. Cancers 2021, 13, 1071. [Google Scholar] [CrossRef]
- Lyu, J.; Jiang, M.; Zhu, Z.; Wu, H.; Kang, H.; Hao, X.; Cheng, S.; Guo, H.; Shen, X.; Wu, T.; et al. Identification of biomarkers and potential therapeutic targets for pancreatic cancer by proteomic analysis in two prospective cohorts. Cell Genom. 2024, 4, 100561. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Goerke, L.; Anderson, A.; Wilsdon, T. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health 2024, 27, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Z.; Cheng, T.; Hu, Y.; Zhong, F.; Ren, S.; Wang, S. Single-cell transcriptomics analysis reveals dynamic changes and prognostic signature in tumor microenvironment of PDAC. Sci. Rep. 2025, 15, 4025. [Google Scholar]
- Pompella, L.; Tirino, G.; Pappalardo, A.; Caterino, M.; Ventriglia, A.; Nacca, V.; Orditura, M.; Ciardiello, F.; De Vita, F. Pancreatic Cancer Molecular Classifications: From Bulk Genomics to Single Cell Analysis. Int. J. Mol. Sci. 2020, 21, 2814. [Google Scholar] [CrossRef]
- Duan, K.; Jang, G.H.; Grant, R.C.; Wilson, J.M.; Notta, F.; O’Kane, G.M.; Knox, J.J.; Gallinger, S.; Fischer, S. The value of GATA6 immunohistochemistry and computer-assisted diagnosis to predict clinical outcome in advanced pancreatic cancer. Sci. Rep. 2021, 11, 14951. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Hayashi, A.; Toki, M.; Kitahama, K.; Ho, Y.J.; Kato, K.; Yamada, T.; Kawamoto, S.; Kambayashi, K.; Ochiai, K.; et al. Combination immunohistochemistry for CK5/6, p63, GATA6, and HNF4a predicts clinical outcome in treatment-naive pancreatic ductal adenocarcinoma. Sci. Rep. 2024, 14, 15598. [Google Scholar] [CrossRef]
- Rao, J.; Sinn, M.; Pelzer, U.; Riess, H.; Oettle, H.; Demir, I.E.; Friess, H.; Jager, C.; Steiger, K.; Muckenhuber, A. KRT81 and HNF1A expression in pancreatic ductal adenocarcinoma: Investigation of predictive and prognostic value of immunohistochemistry-based subtyping. J. Pathol. Clin. Res. 2024, 10, e12377. [Google Scholar] [CrossRef]
- Xu, H.; Xue, S.; Sun, Y.; Ma, J.; Li, S.; Wang, Y.; Mao, T.; Ge, W.; Yue, M.; Shentu, D.; et al. CREB3L1 facilitates pancreatic tumor progression and reprograms intratumoral tumor-associated macrophages to shape an immunotherapy-resistance microenvironment. J. Immunother. Cancer 2025, 13, e010029. [Google Scholar] [CrossRef]
- van Eijck, C.W.; Real, F.X.; Malats, N.; Vadgama, D.; van den Bosch, T.P.; Doukas, M.; van Eijck, C.H.; Mustafa, D.A. GATA6 identifies an im-mune-enriched phenotype linked to favorable outcomes in patients with pancreatic cancer undergoing upfront surgery. Cell Rep. Med. 2024, 5, 101557. [Google Scholar] [CrossRef] [PubMed]
- Muckenhuber, A.; Berger, A.K.; Schlitter, A.M.; Steiger, K.; Konukiewitz, B.; Trumpp, A.; Eils, R.; Werner, J.; Friess, H.; Esposito, I.; Klöppel, G. Pancreatic Ductal Adenocar-cinoma Subtyping Using the Biomarkers Hepatocyte Nuclear Factor-1A and Cy-tokeratin-81 Correlates with Outcome and Treatment Response. Clin. Cancer Res. 2018, 24, 351–359. [Google Scholar] [CrossRef]
- Lansbergen, M.F.; Dings, M.P.G.; Manoukian, P.; Farina, A.; Waasdorp, C.; Hooijer, G.K.J.; Verheij, J.; Koster, J.; Zwijnenburg, D.A.; Wilmink, J.W.; et al. Transcriptome-based classification to predict FOLFIRINOX response in a real-world metastatic pancreatic cancer cohort. Transl. Res. 2024, 273, 137–147. [Google Scholar] [CrossRef]
- Okada, Y.; Nishiwada, S.; Yamamura, K.; Sho, M.; Baba, H.; Takayama, T.; Goel, A. Identification of laminin γ2 as a prognostic and predictive biomarker for determining response to gemcitabine-based therapy in pancreatic ductal adenocarcinoma. Eur. J. Cancer 2021, 146, 125–134. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Rae, S.; Bisset, K.; Upstill-Goddard, R.; Gemenetzis, G.; Johns, A.L.; Dickson, E.J.; Mittal, A.; Gill, A.J.; Duthie, F.; et al. The Impact of Molecular Subtyping on Pathological Staging of Pancreatic Cancer. Ann. Surg. 2023, 277, e396–e405. [Google Scholar] [CrossRef]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hu, K.; Bailey, P.; Springfeld, C.; Roth, S.; Kurilov, R.; Brors, B.; Gress, T.; Buchholz, M.; An, J.; et al. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 743908. [Google Scholar] [CrossRef] [PubMed]
- Christofyllakis, K.; Bittenbring, J.T.; Thurner, L.; Ahlgrimm, M.; Stilgenbauer, S.; Bewarder, M.; Kaddu-Mulindwa, D. Cost-effectiveness of precision cancer medicine-current challenges in the use of next generation sequencing for comprehensive tumour genomic profiling and the role of clinical utility frameworks (Review). Mol. Clin. Oncol. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Roa-Pena, L.; Leiton, C.V.; Babu, S.; Pan, C.H.; Vanner, E.A.; Akalin, A.; Bandovic, J.; Moffitt, R.A.; Shroyer, K.R.; Escobar-Hoyos, L.F. Keratin 17 identifies the most lethal molecular subtype of pancreatic cancer. Sci. Rep. 2019, 9, 11239. [Google Scholar] [CrossRef]
- Roa-Pena, L.; Babu, S.; Leiton, C.V.; Wu, M.; Taboada, S.; Akalin, A.; Buscaglia, J.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 testing in pancreatic cancer needle aspiration biopsies predicts survival. Cancer Cytopathol. 2021, 129, 865–873. [Google Scholar] [CrossRef]
- Rashid, N.U.; Peng, X.L.; Jin, C.; Moffitt, R.A.; Volmar, K.E.; Belt, B.A.; Panni, R.Z.; Nywening, T.M.; Herrera, S.G.; Moore, K.J.; et al. Purity Independent Subtyping of Tumors (PurIST), A Clinically Robust, Single-sample Classifier for Tumor Subtyping in Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 82–92. [Google Scholar] [CrossRef]
- Mosele, M.F.; Westphalen, C.B.; Stenzinger, A.; Barlesi, F.; Bayle, A.; Bieche, I.; Bonastre, J.; Castro, E.; Dienstmann, R.; Kramer, A.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2024, 35, 588–606. [Google Scholar] [CrossRef]
- Racu, M.L.; Schiavo, A.A.; Van Campenhout, C.; De Neve, N.; Masuy, T.; Maris, C.; Decaestecker, C.; Remmelink, M.; Salmon, I.; D’Haene, N. Validation of a targeted next-generation sequencing panel for pancreatic ductal adenocarcinomas. Exp. Mol. Pathol. 2024, 139, 104920. [Google Scholar] [CrossRef]
- Torres, C.; Grippo, P.J. Pancreatic cancer subtypes: A roadmap for precision medicine. Ann. Med. 2018, 50, 277–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [PubMed]
- Frank, T.S.; Sun, X.; Zhang, Y.; Yang, J.; Fisher, W.E.; Gingras, M.C.; Li, M. Genomic profiling guides the choice of molecular targeted therapy of pancreatic cancer. Cancer Lett. 2015, 363, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M.; Katerji, H.; Zhang, H.; Hicks, D.G. Biomarker and multigene assay testing in ER positive, HER-2 negative breast carcinomas: An international guidelines-based approach. Hum. Pathol. Rep. 2021, 26, 300574. [Google Scholar] [CrossRef]
- Zaha, D.C. Significance of immunohistochemistry in breast cancer. World J. Clin. Oncol. 2014, 5, 382–392. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef]
- Sholl, L.M. Biomarkers of response to checkpoint inhibitors beyond PD-L1 in lung cancer. Mod. Pathol. 2022, 35 (Suppl. S1), 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, J.H. PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future. J. Pathol. Transl. Med. 2019, 53, 199–206. [Google Scholar] [CrossRef]
- Horgan, D.; Hamdi, Y.; Lal, J.A.; Nyawira, T.; Meyer, S.; Kondji, D.; Francisco, N.M.; De Guzman, R.; Paul, A.; Bernard, B.; et al. Framework for Adoption of Next-Generation Sequencing (NGS) Globally in the Oncology Area. Healthcare 2023, 11, 431. [Google Scholar] [CrossRef]
- Herbst, B.; Zheng, L. Precision medicine in pancreatic cancer: Treating every patient as an exception. Lancet Gastroenterol. Hepatol. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Ubel, P.A.; Scherr, K.A.; Fagerlin, A. Autonomy: What’s Shared Decision Making Have to Do with It? Am. J. Bioeth. 2018, 18, W11–W12. [Google Scholar] [CrossRef]
- Varkey, B. Principles of Clinical Ethics and Their Application to Practice. Med. Princ. Pract. 2021, 30, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Schildmann, J.; Ritter, P.; Salloch, S.; Uhl, W.; Vollmann, J. ‘One also needs a bit of trust in the doctor …’: A qualitative interview study with pancreatic cancer patients about their perceptions and views on information and treatment decision-making. Ann. Oncol. 2013, 24, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Mattison, R.; Bercovitz, R.; Lottenberg, R.; Rezende, S.M.; Silverstein, R.; Terrell, D.; Kunkle, R.; Smith, D.; Bollard, C.; et al. Identifying experts for clinical practice guidelines: Perspectives from the ASH Guideline Oversight Subcommittee. Blood Adv. 2023, 7, 4323–4326. [Google Scholar] [CrossRef]
- Entwistle, V.A.; Carter, S.M.; Cribb, A.; McCaffery, K. Supporting patient autonomy: The importance of clinician-patient relationships. J. Gen. Intern. Med. 2010, 25, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Marakala, V.; Madathil, L.P.; George, T.; D’Souza, R.F.; Palatty, P.L. Ethical and moral principles for oncology healthcare workers: A brief report from a Bioethics consortium emphasizing on need for education. J. Educ. Health Promot. 2024, 13, 145. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C.; Malleo, G.; Paiella, S.; Bassi, C.; Milella, M.; Dreyer, S.B.; Froeling, F.E.M.; Chang, D.K.; Biankin, A.V.; et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann. Oncol. 2021, 32, 183–196. [Google Scholar] [CrossRef]
- Marron, J.M.; Charlot, M.; Gaddy, J.; Rosenberg, A.R. The Ethical Imperative of Equity in Oncology: Lessons Learned From 2020 and a Path Forward. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e13–e19. [Google Scholar] [CrossRef]
- Zakout, G.A. Practicing equitable principles in cancer clinical research: Has the EU got it right? J. Cancer Policy 2023, 37, 100435. [Google Scholar] [CrossRef]
- Kirkwood, M.K.; Schenkel, C.; Hinshaw, D.C.; Bruinooge, S.S.; Waterhouse, D.M.; Peppercorn, J.M.; Subbiah, I.M.; Levit, L.A. State of Geographic Access to Cancer Treatment Trials in the United States: Are Studies Located Where Patients Live? JCO Oncol. Pract. 2025, 21, 427–437. [Google Scholar] [CrossRef]
- Villamar, D.M.P.; Blasé, N. The Promise of Cancer Health Justice: How Stakeholders and the Community Can Build a Sustained and Equitable System of Cancer Care Through the Lens of Colorectal Cancer Interventions. Cancer J. 2023, 29, 338–342. [Google Scholar] [CrossRef]
- Zalis, M.; Viana Veloso, G.G.; Aguiar, P.N., Jr.; Gimenes, N.; Reis, M.X.; Matsas, S.; Ferreira, C.G. Next-generation sequencing impact on cancer care: Applications, challenges, and future directions. Front. Genet. 2024, 15, 1420190. [Google Scholar] [CrossRef] [PubMed]
- Qadir, R.; Umair, M.B.; Tariq, U.B.; Ahmad, A.; Kiran, W.; Shahid, M.H. Unraveling Pancreatic Cancer: Epidemiology, Risk Factors, and Global Trends. Cureus 2024, 16, e72816. [Google Scholar] [CrossRef]
- Vidal, L.; Dlamini, Z.; Qian, S.; Rishi, P.; Karmo, M.; Joglekar, N.; Abedin, S.; Previs, R.A.; Orbegoso, C.; Joshi, C.; et al. Equitable inclusion of diverse populations in oncology clinical trials: Deterrents and drivers. ESMO Open 2024, 9, 103373. [Google Scholar] [CrossRef]
- Singh, R.R.; Goldberg, J.; Varghese, A.M.; Yu, K.H.; Park, W.; O’Reilly, E.M. Genomic profiling in pancreatic ductal adenocarcinoma and a pathway towards therapy individualization: A scoping review. Cancer Treat. Rev. 2019, 75, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Holderfield, M.; Lee, B.J.; Jiang, J.; Tomlinson, A.; Seamon, K.J.; Mira, A.; Patrucco, E.; Goodhart, G.; Dilly, J.; Gindin, Y.; et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Nature 2024, 629, 919–926. [Google Scholar] [CrossRef]
- Wasko, U.N.; Jiang, J.; Dalton, T.C.; Curiel-Garcia, A.; Edwards, A.C.; Wang, Y.; Lee, B.; Orlen, M.; Tian, S.; Stalnecker, C.A.; et al. Tumour-selective activity of RAS-GTP inhibition in pancreatic cancer. Nature 2024, 629, 927–936. [Google Scholar] [CrossRef]
- Dilly, J.; Hoffman, M.T.; Abbassi, L.; Li, Z.; Paradiso, F.; Parent, B.D.; Hennessey, C.J.; Jordan, A.C.; Morgado, M.; Dasgupta, S.; et al. Mechanisms of Resistance to Oncogenic KRAS Inhibition in Pancreatic Cancer. Cancer Discov. 2024, 14, 2135–2161. [Google Scholar] [CrossRef]

| Journal Name | Journal Indexed | Impact Factor | Journal Peer Review Process | Conflict of Interest | Author | Title | Study ID | Country in Which the Study Conducted | Biomarker(s) | Methods | Aim of Study | Study Design | Total Number of Participants | Comparison | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Journal for ImmunoTherapy of Cancer | Yes | 10.3 | Single anonymized | None | Xu, 2025 [28] | CREB3L1 facilitates pancreatic tumor progression and reprograms intratumoral tumor-associated macrophages to shape an immunotherapy-resistance microenvironment | https://doi.org/10.1136/jitc-2024-010029 | China | CREB3L1 | IHC | Explore CREB3L1’s role in PDAC and its correlation with clinical features | Cohort study | 94 | Responders (ORR) vs. non- ORR; IHC-based CREB3L1 expression | Lower CREB3L1 expression found in those responding well to immunotherapy | CREB3L1 expression predicts immunotherapy response and outcome in PDAC |
| Cell Reports Medicine | Yes | 11.7 | Single anonymized | None | Van Eijck, 2024 [29] | GATA6 identifies an immune-enriched phenotype linked to favorable outcomes in patients with pancreatic cancer undergoing upfront surgery | https://doi.org/10.1016/j.xcrm.2024.101557 | Other: the Netherlands | GATA6 | Transcriptomic profiling/IHC | Investigate prognostic value of GATA6 IHC in treatment-naive vs. gemcitabine-based nCRT-treated PDAC patients | Cohort study | 88 | GATA6 expression, immune cell infiltration, and survival in treatment-naive vs. chemotherapy-treated patients | Strongly treatment-naive GATA6 tumors exhibit enhanced immunological features and immunostimulatory mechanisms | GATA6 expression correlates with distinct immune landscapes and better outcomes, underscoring its prognostic significance |
| The Journal of Pathology: Clinical Research | Yes | 3.4 | Single anonymized | None | Rao, 2024 [27] | KRT81 and HNF1A expression in pancreatic ductal adenocarcinoma: investigation of predictive and prognostic value of immunohistochemistry-based subtyping | https://doi.org/10.1002/2056-4538.12377 | Other: Germany | KRT81 and HNF1A | IHC | Validate revised subtyping in a controlled phase III trial (CONKO-005) assessing erlotinib + gemcitabine vs. gemcitabine | Cohort study | 269 | Gemcitabine–erlotinib combination vs. gemcitabine only | HNF1A-positive subtype benefited more from gemcitabine–erlotinib compared with KRT81-positive or double-negative subtypes | KRT81/HNF1A IHC serves as a partial surrogate for RNA-defined PDAC subtypes and informs potential treatment choices |
| Clinical Cancer Research | Yes | 10.4 | double-blind review | A. Stenzinger reports receiving speaker’s bureau honoraria from AstraZeneca, Illumina, Novartis, and Thermo Fisher Scientific and is a consultant/advisory board member for AstraZeneca, Bristol-Myers Squibb, Illumina, Novartis, and Thermo Fisher Scientific. M.R. Sprick is listed as a co-inventor on a pending patent application on the use of KRT81 and HNF1A for the stratification of PDAC patients. This is owned by HI-STEM gGmbH. No potential conflicts of interest were disclosed by the other authors. | Muckenhuber, 2018 [30] | Pancreatic Ductal Adenocarcinoma Subtyping Using the Biomarkers Hepatocyte Nuclear Factor-1A and Cytokeratin-81 Correlates with Outcome and Treatment Response | https://doi.org/10.1158/1078-0432.CCR-17-2180 | Other: Germany | HNF1A and KRT81 | IHC | Validate HNF1A/KRT81 subtypes in resectable and unresectable PDAC, and assess their predictive value for chemotherapy | Cohort study | 204 | Survival and treatment response (FOLFIRINOX vs. gemcitabine) by HNF1A/KRT81 IHC subtypes | KRT81-positive patients derived less benefit from FOLFIRINOX; HNF1A-positive patients had a significantly better initial response to FOLFIRINOX compared with gemcitabine | An IHC-based HNF1A/KRT81 classification reliably identifies PDAC subtypes, aiding pre-therapeutic stratification and personalized therapy |
| Translational Research | Yes | 6.4 | Single anonymized | MFB has received research funding from Celgene, Frame Therapeutics, and Lead Pharma, and has acted as a consultant to Servier and Olympus. HWML Consultant or advisory role: BMS, Daiichy, Dragonfly, Eli Lilly, MSD, Nordic Pharma, Servier. Research funding and/or medication supply: Bayer, BMS, Celgene, Janssen, Incyte, Eli Lilly, MSD, Nordic Pharma, Philips, Roche, Servier. Speaker role: Astellas, Daiichy, Novartis. JPM has acted as a consultant to AbbVie. JWW Consultant or advisory role: MSD, Servier, Astra Zeneca, Research funding: MSD, Nordic, Servier. Speaker role: MSD, Servier. None of these parties were involved in the design of this study or drafting of the manuscript. All other authors declare no conflicts of interest. | Lansbergen, 2024 [31] | Transcriptome-based classification to predict FOLFIRINOX response in a real-world metastatic pancreatic cancer cohort | https://doi.org/10.1016/j.trsl.2024.08.002 | Other: the Netherlands | GATA6 and keratin-17 (KRT17) | IHC | Assess predictive value of molecular subtypes for FOLFIRINOX response in advanced PDAC | Cohort study | 86 | IHC-based stratification into good vs. poor responders to FOLFIRINOX | GATA6 H-score reliably predicts response to FOLFIRINOX | GATA6 (±KRT17) IHC can be integrated into diagnostic routines to guide chemotherapy choice and inform patient prognosis |
| Scientific Reports | Yes | 3.8 | Single anonymized | The authors declare no competing interests. | Duan, 2021 [25] | The value of GATA6 immunohistochemistry and computer-assisted diagnosis to predict clinical outcome in advanced pancreatic cancer | https://doi.org/10.1038/s41598-021-94544-3 | Canada | GATA6 | IHC | Evaluate GATA6 IHC as a single biomarker for advanced PDAC and examine computer-assisted analysis | Cohort study | 110 | Response to chemotherapy according to GATA6 IHC | Among mFFX-treated patients, disease progression was 39% in GATA6-low vs. 12% in GATA6-high PDAC | GATA6 IHC can serve as a single predictive biomarker for mFFX response in advanced PDAC; warrants prospective validation |
| American Journal of Clinical Pathology | Yes | 5.4 | double-blind review | K.R.S. and L.F.E.-H. are consultants for KDx Diagnostics. E.M.B. is an employee of Perthera and owns stocks in the company. He also has consulted for Theralink Technologies and received compensation as chair of the Science Advisory Board and owns stock in the company. The additional authors have nothing to disclose. | Delgado-Coka, 2024 [12] | Keratin 17 is a prognostic and predictive biomarker in pancreatic ductal adenocarcinoma | https://doi.org/10.1093/AJCP/AQAE038 | United States | Keratin 17 | IHC | Define K17 IHC thresholds for chemotherapy response to optimize therapeutic interventions in PDAC | Cohort study | 305 | K17 expression in the context of survival after adjuvant chemotherapy | High K17 marks resistance to gemcitabine-based therapies and predicts better response to 5-FU-based regimens | K17 IHC testing serves as a rapid predictive marker to guide the best chemotherapy choice based on tumor expression profiles |
| Scientific Reports | Yes | 3.8 | Single anonymized | CI-D receives research support from BMS. All other authors (TS, AH, MT, K-Ki, YJH, K-Ka, TY, SK, Ko-K, KO, KG, NO, JM, YS, TH, JS) declare that they have no conflicts of interest in relation to this work. | Shibayama, 2024 [26] | Combination immunohistochemistry for CK5/6, p63, GATA6, and HNF4a predicts clinical outcome in treatment-naive pancreatic ductal adenocarcinoma | https://doi.org/10.1038/s41598-024-65900-w | Other: Japan | CK5/6, p63, GATA6, and HNF4a | Immunohistochemistry (IHC) on endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB) samples | Determine surrogate biomarkers (K5/6, p63, GATA6, HNF4a) for molecular signatures in advanced PDAC | Cohort study | 190 | IHC-based subtypes (Classical, Transitional, Basal-like) | Basal-like pattern correlated with squamous differentiation and worst survival; (Transitional/Basal vs. Classical) = poor prognosis | IHC expression patterns identify Basal-like PDAC, aiding in prognostic assessment and therapeutic decision-making. |
| Cancers | Yes | 5.2 | Single anonymized | Authors declare no conflict of interest | Okada, 2021 [32] | Identification of LAMC2 as a prognostic and predictive biomarker for determining response to gemcitabine-based therapy in pancreatic ductal adenocarcinoma | https://doi.org/10.1016/j.ejca.2020.12.031 | Japan | LAMC2 (Laminin γ2) | IHC on EUS-FNAB samples. | Identify and validate biomarkers for gemcitabine-based therapy response in PDAC | Retrospective analysis of datasets and clinical cohorts | 423 | LAMC2 expression compared with overall survival, relapse-free survival, and gemcitabine response | High LAMC2 correlates with poor prognosis and reduced response to gemcitabine | LAMC2 serves as a prognostic and predictive biomarker for gemcitabine-based therapies in PDAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Coka, L.A.; Roa-Peña, L.; Flescher, A.; Escobar-Hoyos, L.F.; Shroyer, K.R. Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation. Cancers 2025, 17, 1899. https://doi.org/10.3390/cancers17121899
Delgado-Coka LA, Roa-Peña L, Flescher A, Escobar-Hoyos LF, Shroyer KR. Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation. Cancers. 2025; 17(12):1899. https://doi.org/10.3390/cancers17121899
Chicago/Turabian StyleDelgado-Coka, Lyanne A., Lucia Roa-Peña, Andrew Flescher, Luisa F. Escobar-Hoyos, and Kenneth R. Shroyer. 2025. "Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation" Cancers 17, no. 12: 1899. https://doi.org/10.3390/cancers17121899
APA StyleDelgado-Coka, L. A., Roa-Peña, L., Flescher, A., Escobar-Hoyos, L. F., & Shroyer, K. R. (2025). Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation. Cancers, 17(12), 1899. https://doi.org/10.3390/cancers17121899





