Perineural Invasion in Breast Cancer: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Historical Perspectives on Perineural Invasion in Breast Cancer
3. Prevalence and Clinicopathological Correlates of Perineural Invasion in Breast Pathology
3.1. Perineural Invasion in Pre-Malignant and Benign Breast Lesions
| References | Total Cases Studied | Lesion Associated with PNI | Benign Breast Disease | DCIS | PNI Cases | PNI Prevalence (%) | Follow Up | Level of Evidence * |
|---|---|---|---|---|---|---|---|---|
| Ackerman, 1957 [29] | 1 | Benign breast disease | 1 | - | 1 | - | - | 5 |
| Taylor and Norris, 1967 [30] | 1000 breast biopsies coded as sclerosing adenosis | Sclerosing adenosis | 20 | - | 20 | 2.00% | Follow-up for 17 of 20 patients was available; all were well (median, 7 years) | 2 |
| Davies, 1973 [37] | 316 mastopathies (excluding carcinomas) | Benign breast disease | 4 | - | 4 | 1.30% | Patients were well after 8, 13, 28, and 38 months of follow-up | 2 |
| Gould et al., 1975 [38] | 2 | Extensive adenosis and papillomatosis | 2 | - | 2 | - | - | 4 |
| Tsang and Chan, 1992 [42] | 1 | DCIS (cribriform type) | - | 1 | 1 | - | - | 4 |
| Cerilli and Fechner, 2000 [40] | 1 | Intraductal papillomas with superimposed hyperplasia | 1 | - | 1 | - | Patient was well after 5 months of follow-up | 4 |
| Gobbi et al., 2001 [31] | 10,000 breast biopsies (excluding carcinoma) | DCIS (cribriform and comedo types) and benign breast disease | 11 | 3 | 14 | 0.14% | - | 2 |
| Doyle et al., 2007 [39] | 125 | Radial scars | 4 | - | 4 | 3.20% | - | 2 |
| Fellegara and Kuhn, 2007 [2] | 1 | Florid adenosis | 1 | - | 1 | - | - | 4 |
| Chan and Chen, 2009 [41] | 1 | Fibrocystic changes with ductal hyperplasia and stromal sclerosis | 1 | - | 1 | - | Patient was well after 31 months of follow-up | 4 |
| Elfituri and Emmadi, 2019 [10] | 1 | DCIS (cribriform, papillary and micropapillary types) | - | 1 | 1 | - | Patient was well after 20 months of follow-up | 4 |
| Total | - | - | 45 | 5 | 50 | 0.14–2.00% | - | - |
3.2. Perineural Invasion in Breast Cancer
3.3. Perineural Invasion Across Breast Cancer Subtypes
| Reference | Cohort Size | PNI Cases | PNI Prevalence (%) | Clinicopathological Correlates of PNI | Prognostic Significance | Level of Evidence # |
|---|---|---|---|---|---|---|
| Roses et al., 1982 [52] | 122 patients with breast cancer (pT1; T1N0M0) | 17/122 | 13.93% | Not assessed | PNI was not a significant predictor of LRR | 2 |
| Mate et al., 1986 [53] | 180 patients with clinical stage I or II operable invasive mammary carcinoma treated by radiotherapy following local tumor excision | 12/151 | 7.95% | Not assessed | PNI was not a significant predictor of LRR, although neither did LVI or tumor grade | 2 |
| McCready et al., 1996 [54] * | 293 patients with primary invasive breast carcinoma s/p lumpectomy and negative margins | Not assessed | Not quantified | Not assessed | LVPI was a significant predictor of LRR | 2 |
| McCready et al., 2000 [55] * | 244 patients with breast carcinoma s/p lumpectomy alone without adjuvant radiotherapy or adjuvant systemic therapy | LVPI in 82/229 | LVPI prevalence 35.82% | Not assessed | LVPI was a significant predictor of LRR | 2 |
| Duraker et al., 2006 [49] | 377 patients with invasive mammary carcinoma | 97/377 | 25.73% |
| PNI was not prognostic for disease-free survival | 2 |
| Karak et al., 2010 [48] | 1136 patients with invasive mammary carcinoma | 13/1136 | 1.14% |
| Patients with PNI can expect a meaningful survival at 5 years with appropriately aggressive adjuvant therapy (only 1 of the 13 patients died after mean follow-up of 5.9 years) | 2 |
| Koca et al., 2013 [56] | 218 patients with pN3a breast cancer | 20/218 | 9.17% | Not assessed | PNI was significantly associated with DFS (HR 2.519) | 2 |
| Narayan et al., 2021 [16] | 8864 patients with invasive mammary carcinoma | 1384/8864 | 15.61% | Not assessed |
| 2 |
| Hosoya et al., 2023 [51] | 191 patients with invasive mammary carcinoma s/p surgical resection | 27/191 | 14.14% |
| PNI had a significant adverse effect on DMFS and DSS | 2 |
| Barb et al., 2023 [57] | 229 cases (Romania, FFPE analysis) | Not assessed | Not quantified |
|
| 2 |
4. Pathophysiology and Mechanisms of Perineural Invasion
4.1. Tumor Microenvironment
4.2. Tertiary Lymphoid Structures
4.3. Molecular Pathways
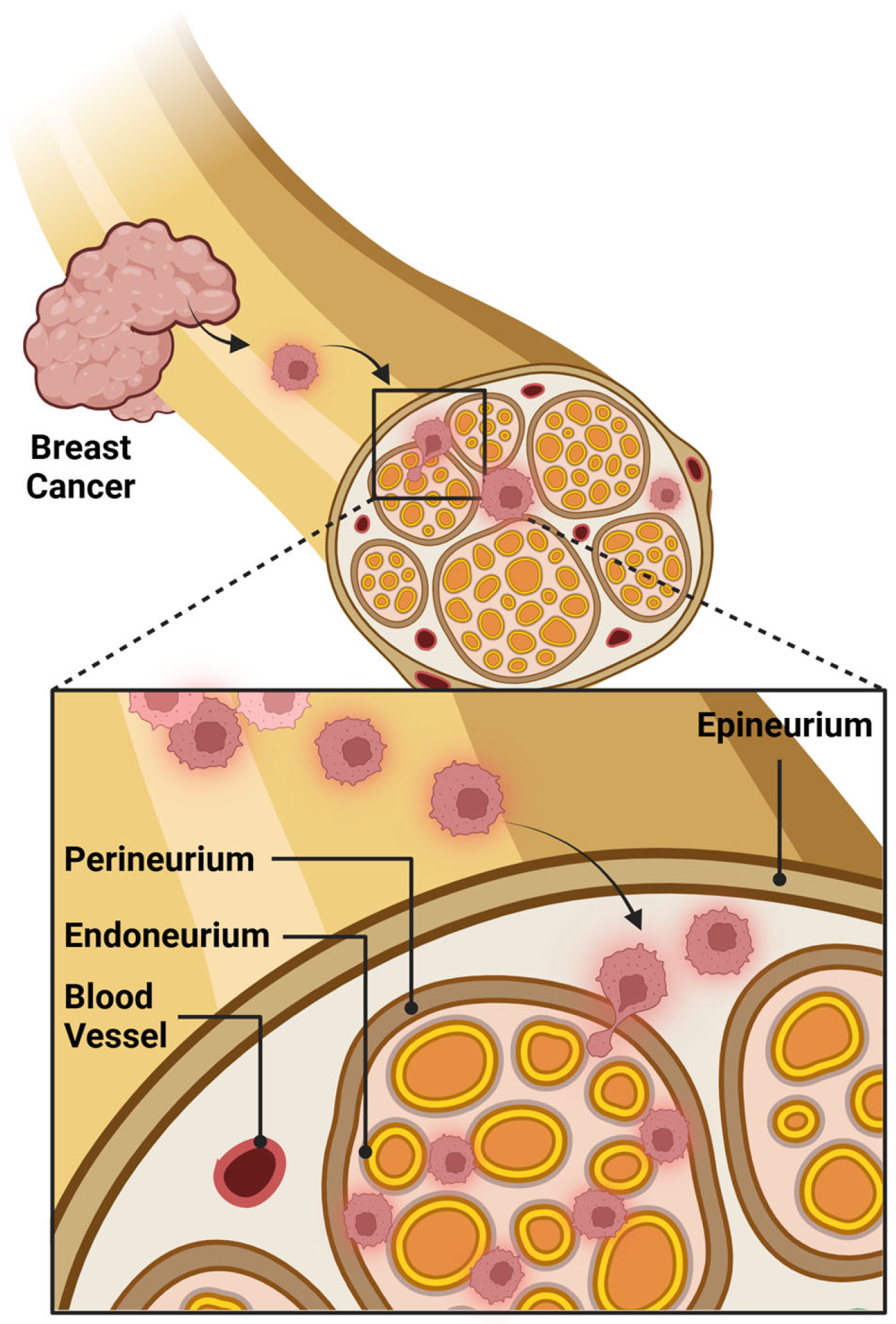
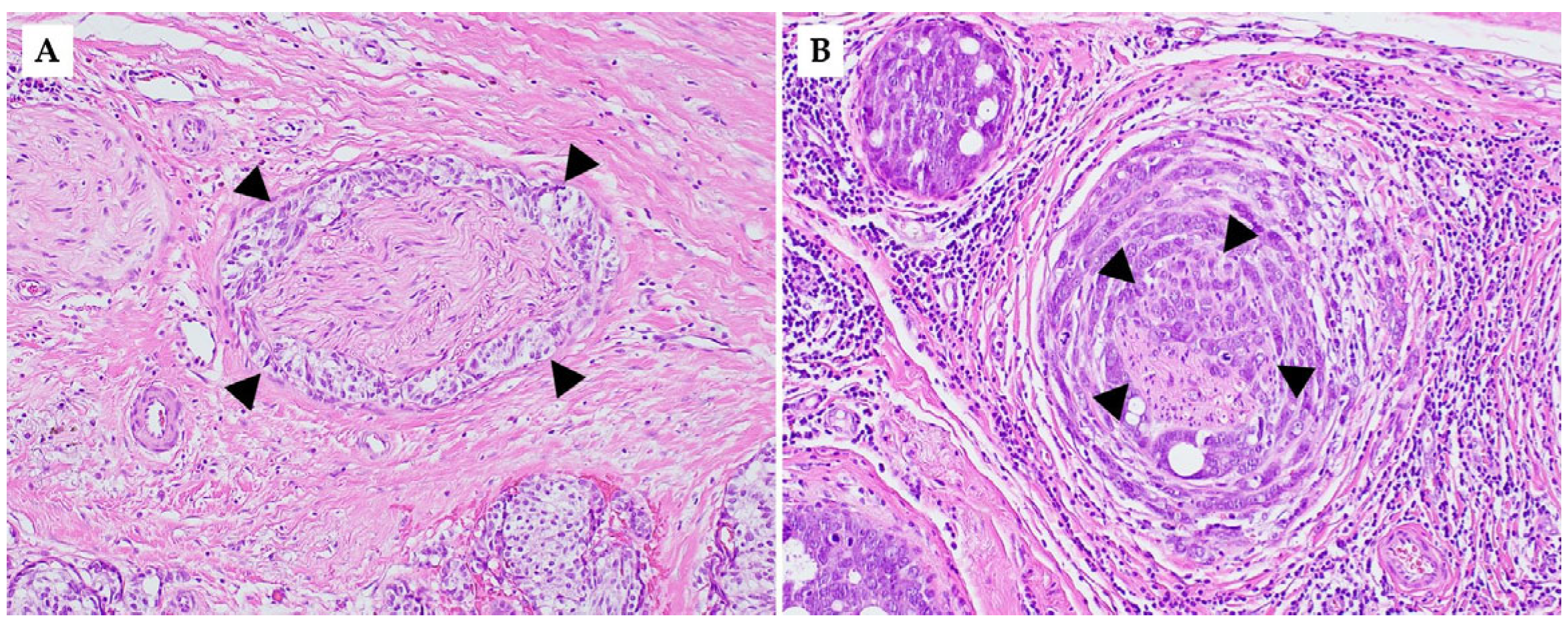
5. Histological Features of Perineural and Intraneural Invasion in Breast Pathology
6. Reporting Perineural Invasion in Breast Cancer
7. Imaging Modalities for Perineural Invasion Detection in Breast Cancer
8. Clinical Manifestations of Perineural Spread in Breast Cancer
8.1. Brachial Plexopathy from Perineural Spread
8.2. Intradural Extramedullary Metastasis via Perineural Invasion
8.3. Optic Nerve Sheath Metastasis
9. Prognostic Significance of PNI in Breast Cancer
9.1. Perineural Invasion and Locoregional Recurrence
9.2. Perineural Invasion Versus Lymphovascular Invasion
10. Therapeutic Implications and Future Directions
10.1. Current Treatment Approaches
10.2. Emerging Targets for Therapy
10.2.1. Integrin Signaling and ECM Stiffness
10.2.2. Neurotrophic Factor Blockade
10.3. Need for Further Research
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Fellegara, G.; Kuhn, E. Perineural and Intraneural “Invasion” in Benign Proliferative Breast Disease. Int. J. Surg. Pathol. 2007, 15, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar] [PubMed]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar] [CrossRef]
- Rodin, A.E.; Larson, D.L.; Roberts, D.K. Nature of the perineural space invaded by prostatic carcinoma. Cancer 1967, 20, 1772–1779. [Google Scholar] [CrossRef]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural Invasion in Squamous Cell Carcinoma of the Head and Neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef]
- Bockman, D.E.; Büchler, M.; Beger, H.G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994, 107, 219–230. [Google Scholar] [CrossRef]
- Nagakawa, T.; Kayahara, M.; Ohta, T.; Ueno, K.; Konishi, I.; Miyazaki, I. Patterns of neural and plexus invasion of human pancreatic cancer and experimental cancer. Int. J. Pancreatol. 1991, 10, 113–119. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Shen, L.; Chen, Z.; Huang, J. Crosstalk between the peripheral nervous system and breast cancer influences tumor progression. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188828. [Google Scholar] [CrossRef]
- Elfituri, O.; Emmadi, R. Perineural and intraneural involvement in ductal carcinoma in-situ of breast: Case report. Pathol. Res. Pract. 2019, 215, 152624. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elhammady, G.; Gass, J.M.; Paramo, J.C.; Poppiti, R.; Alexis, J. PIK3R1, HRAS and AR Gene Alterations Associated with Sclerosing Polycystic Adenoma of the Parotid Gland. Curr. Issues Mol. Biol. 2023, 45, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; Whelan, T.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Braunstein, L.Z.; Taghian, A.G.; Niemierko, A.; Salama, L.; Capuco, A.; Bellon, J.R.; Wong, J.S.; Punglia, R.S.; MacDonald, S.M.; Harris, J.R. Breast-cancer subtype, age, and lymph node status as predictors of local recurrence following breast-conserving therapy. Breast Cancer Res. Treat. 2017, 161, 173–179. [Google Scholar] [CrossRef]
- Narayan, P.; Flynn, J.; Zhang, Z.; Gillespie, E.F.; Mueller, B.; Xu, A.J.; Cuaron, J.; McCormick, B.; Khan, A.J.; Cahlon, O.; et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci. Rep. 2021, 11, 12781. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Jagsi, R.; Raad, R.A.; Goldberg, S.; Sullivan, T.; Michaelson, J.; Powell, S.N.; Taghian, A.G. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: Implications for postmastectomy radiation. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1035–1039. [Google Scholar] [CrossRef]
- Cruveilheir, J. Maladies des nerfs. Anatomie Pathologique du Corps Humain. Paris. JB Bailliere 1842, 2, 35. [Google Scholar]
- Neumann, E. Secundäre Cancroidinfiltration des Nervus mentalis bei einem Fall von Lippencancroid. Arch. Pathol. Anat. Physiol. Klin. Medicin 1862, 24, 201–202. [Google Scholar] [CrossRef]
- Ozaki, H.; Hiraoka, T.; Mizumoto, R.; Matsuno, S.; Matsumoto, Y.; Nakayama, T.; Tsunoda, T.; Suzuki, T.; Monden, M.; Saitoh, Y.; et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg. Today 1999, 29, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Law, W.L.; Chu, K.W. Anterior resection for rectal cancer with mesorectal excision: A prospective evaluation of 622 patients. Ann. Surg. 2004, 240, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Excisional Biopsy Specimens from Patients with Primary Carcinoma of the Colon and Rectum; College of American Pathologists: Northfield, IL, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Gallbladder; College of American Pathologists: Northfield, IL, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Pancreas; College of American Pathologists: Northfield, IL, USA, 2021. [Google Scholar]
- Burgart, L.; Chopp, W.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Stomach; College of American Pathologists: Northfield, IL, USA, 2023. [Google Scholar]
- Beard, C.J.; Chen, M.H.; Cote, K.; Loffredo, M.; Renshaw, A.A.; Hurwitz, M.; D’Amico, A.V. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Tsay, S.H.; Wu, C.C.; Shyr, Y.M.; King, K.L.; Lee, C.H.; Lui, W.Y.; Liu, T.J.; P’Eng, F.K. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann. Surg. 1996, 223, 384–394. [Google Scholar] [CrossRef]
- Ackerman, L.V. Seminar on Lesions of the Breast; American Society of Clinical Pathologists: Chicago, IL, USA, 1957. [Google Scholar]
- Taylor, H.B.; Norris, H.J. Epithelial invasion of nerves in benign diseases of the breast. Cancer 1967, 20, 2245–2249. [Google Scholar] [CrossRef]
- Gobbi, H.; Jensen, R.A.; Simpson, J.F.; Olson, S.J.; Page, D.L. Atypical ductal hyperplasia and ductal carcinoma in situ of the breast associated with perineural invasion. Hum. Pathol. 2001, 32, 785–790. [Google Scholar] [CrossRef]
- Furuhashi, S.; Sakaguchi, T.; Murakami, T.; Fukushima, M.; Morita, Y.; Ikegami, K.; Kikuchi, H.; Setou, M.; Takeuchi, H. Tenascin C in the Tumor-Nerve Microenvironment Enhances Perineural Invasion and Correlates With Locoregional Recurrence in Pancreatic Ductal Adenocarcinoma. Pancreas 2020, 49, 442–454. [Google Scholar] [CrossRef]
- Jeon, H.G.; Bae, J.; Yi, J.-S.; Hwang, I.S.; Lee, S.E.; Lee, E. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int. J. Urol. 2009, 16, 682–686. [Google Scholar] [CrossRef]
- Paner, G.; Srigley, J.; Pettus, J.; Giannico, G.; Sirintrapun, J.; Harik, L. Protocol for the Examination of Prostate Needle Biopsies from Patients with Carcinoma of the Prostate Gland: Specimen Level Reporting; College of American Pathologists: Northfield, IL, USA, 2021. [Google Scholar]
- Rahima, B.; Shingaki, S.; Nagata, M.; Saito, C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 423–431. [Google Scholar] [CrossRef]
- Seethala, R.; Shon, W.; Balzer, B.; Duvvuri, U.; Gharavi, N.; Lydiatt, W. Protocol for the Examination of Specimens from Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck; College of American Pathologists: Northfield, IL, USA, 2022. [Google Scholar]
- Davies, J.D. Neural invasion in benign mammary dysplasia. J. Pathol. 1973, 109, 225–231. [Google Scholar] [CrossRef]
- Gould, V.E.; Rogers, D.R.; Sommers, S.C. Epithelial-nerve intermingling in benign breast lesions. Arch. Pathol. 1975, 99, 596–598. [Google Scholar] [PubMed]
- Doyle, E.M.; Banville, N.; Quinn, C.M.; Flanagan, F.; O’Doherty, A.; Hill, A.D.; Kerin, M.J.; Fitzpatrick, P.; Kennedy, M. Radial scars/complex sclerosing lesions and malignancy in a screening programme: Incidence and histological features revisited. Histopathology 2007, 50, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Cerilli, L.A.; Fechner, R.E. Benign intraneural epithelium in the breast. Arch. Pathol. Lab. Med. 2000, 124, 465. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chen, S.L. Nerve invasion by epithelial cells in benign breast diseases. J. Chin. Med. Assoc. 2009, 72, 150–152. [Google Scholar] [CrossRef]
- Tsang, W.Y.W.; Chan, J.K.C. Neural invasion in intraductal carcinoma of the breast. Hum. Pathol. 1992, 23, 202–204. [Google Scholar] [CrossRef]
- Durieux, N.; Vandenput, S.; Pasleau, F. OCEBM levels of evidence system. Rev. Med. Liege 2013, 68, 644–649. [Google Scholar]
- OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Available online: https://www.cebm.ox.ac.uk/files/levels-of-evidence/cebm-levels-of-evidence-2-1.pdf (accessed on 16 May 2025).
- Cowan, W.K.; Kelly, P.; Sawan, A.; Cunliffe, W.J.; Henry, L.; Higgs, M.J.; Lunt, L.G.; Young, J.R.; Horne, C.H.; Angus, B. The pathological and biological nature of screen-detected breast carcinomas: A morphological and immunohistochemical study. J. Pathol. 1997, 182, 29–35. [Google Scholar] [CrossRef]
- Elmore, J.G.; Moceri, V.M.; Carter, D.; Larson, E.B. Breast carcinoma tumor characteristics in black and white women. Cancer 1998, 83, 2509–2515. [Google Scholar] [CrossRef]
- Ho, C.M.; Mak, C.K.; Lau, Y.; Cheung, W.Y.; Chan, M.C.; Hung, W.K. Skin involvement in invasive breast carcinoma: Safety of skin-sparing mastectomy. Ann. Surg. Oncol. 2003, 10, 102–107. [Google Scholar] [CrossRef]
- Karak, S.G.; Quatrano, N.; Buckley, J.; Ricci, A., Jr. Prevalence and significance of perineural invasion in invasive breast carcinoma. Conn. Med. 2010, 74, 17–21. [Google Scholar]
- Duraker, N.; Çaynak, Z.C.; Türköz, K. Perineural invasion has no prognostic value in patients with invasive breast carcinoma. Breast 2006, 15, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Guan, X.; Ma, M.; Liang, B.; Ren, L.; Liu, Y.; Du, Y.; Jiang, S.-H.; Song, D. Stiffened tumor microenvironment enhances perineural invasion in breast cancer via integrin signaling. Cell. Oncol. 2024, 47, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Wakahara, M.; Ikeda, K.; Umekita, Y. Perineural Invasion Predicts Unfavorable Prognosis in Patients With Invasive Breast Cancer. Cancer Diagn. Progn. 2023, 3, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Roses, D.F.; Bell, D.A.; Flotte, T.J.; Taylor, R.; Ratech, H.; Dubin, N. Pathologic predictors of recurrence in stage 1 (TINOMO) breast cancer. Am. J. Clin. Pathol. 1982, 78, 817–820. [Google Scholar] [CrossRef]
- Mate, T.P.; Carter, D.; Fischer, D.B.; Hartman, P.V.; McKhann, C.; Merino, M.; Prosnitz, L.R.; Weissberg, J.B. A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer 1986, 58, 1995–2002. [Google Scholar] [CrossRef]
- McCready, D.R.; Hanna, W.; Kahn, H.; Chapman, J.-A.; Wall, J.; Fish, E.B.; Lickley, H.L.A. Factors associated with local breast cancer recurrence after lumpectomy alone. Ann. Surg. Oncol. 1996, 3, 358–366. [Google Scholar] [CrossRef]
- McCready, D.R.; Chapman, J.-A.W.; Hanna, W.M.; Kahn, H.J.; Murray, D.; Fish, E.B.; Trudeau, M.E.; Andrulis, I.L.; Lickley, H.L.A. Factors Affecting Distant Disease-Free Survival for Primary Invasive Breast Cancer: Use of a Log-Normal Survival Model. Ann. Surg. Oncol. 2000, 7, 416–426. [Google Scholar] [CrossRef]
- Koca, E.; Kuzan, T.Y.; Dizdar, O.; Babacan, T.; Sahin, I.; Ararat, E.; Altundag, K. Outcomes of locally advanced breast cancer patients with ≥ 10 positive axillary lymph nodes. Med. Oncol. 2013, 30, 615. [Google Scholar] [CrossRef]
- Barb, A.C.; Pasca Fenesan, M.; Pirtea, M.; Margan, M.M.; Tomescu, L.; Melnic, E.; Cimpean, A.M. Tertiary Lymphoid Structures (TLSs) and Stromal Blood Vessels Have Significant and Heterogeneous Impact on Recurrence, Lymphovascular and Perineural Invasion amongst Breast Cancer Molecular Subtypes. Cells 2023, 12, 1176. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Prauzner-Bechcicki, S.; Raczkowska, J.; Madej, E.; Pabijan, J.; Lukes, J.; Sepitka, J.; Rysz, J.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; et al. PDMS substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells. J. Mech. Behav. Biomed. Mater. 2015, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; You, K.; Guo, Y.; Zhang, Y.; Li, Z.; Geng, L. Substrate stiffness affects epithelial-mesenchymal transition of cervical cancer cells through miR-106b and its target protein DAB2. Int. J. Oncol. 2017, 50, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Ngai, D.; Lino, M.; Rothenberg, K.E.; Simmons, C.A.; Fernandez-Gonzalez, R.; Bendeck, M.P. DDR1 (Discoidin Domain Receptor-1)-RhoA (Ras Homolog Family Member A) Axis Senses Matrix Stiffness to Promote Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1763–1776. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Zhang, Y.; He, X.; Chen, X.; Zeng, X.; Liu, F.; Chen, Y.; Chen, J. Targeting Mechanics-Induced Fibroblast Activation through CD44-RhoA-YAP Pathway Ameliorates Crystalline Silica-Induced Silicosis. Theranostics 2019, 9, 4993–5008. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva, Y.; Gillen, K.M.; Gerald, W.; Beggs, H.E.; Reichardt, L.F.; Giancotti, F.G. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J. Clin. Investig. 2009, 119, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Aspord, C.; Pedroza-Gonzalez, A.; Gallegos, M.; Tindle, S.; Burton, E.C.; Su, D.; Marches, F.; Banchereau, J.; Palucka, A.K. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 2007, 204, 1037–1047. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Rossi, A.; Belmonte, B.; Carnevale, S.; Liotti, A.; De Rosa, V.; Jaillon, S.; Piconese, S.; Tripodo, C. Stromal and Immune Cell Dynamics in Tumor Associated Tertiary Lymphoid Structures and Anti-Tumor Immune Responses. Front. Cell Dev. Biol. 2022, 10, 933113. [Google Scholar] [CrossRef]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, Y.; Deng, Y.; Li, J.; Jiang, Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer. Front. Immunol. 2022, 13, 868155. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Villagrana, R.D.; Albores-García, D.; Cervantes-Villagrana, A.R.; García-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef]
- Huang, D.; Su, S.; Cui, X.; Shen, X.; Zeng, Y.; Wu, W.; Chen, J.; Chen, F.; He, C.; Liu, J.; et al. Nerve Fibers in Breast Cancer Tissues Indicate Aggressive Tumor Progression. Medicine 2014, 93, e172. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Y.; Liang, X.; Du, G.; Liu, L.; Lu, L.; Dong, J.; Han, H.; Zhang, G. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 2014, 14, 484. [Google Scholar] [CrossRef]
- Pundavela, J.; Roselli, S.; Faulkner, S.; Attia, J.; Scott, R.J.; Thorne, R.F.; Forbes, J.F.; Bradshaw, R.A.; Walker, M.M.; Jobling, P.; et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol. 2015, 9, 1626–1635. [Google Scholar] [CrossRef]
- Austin, M.; Elliott, L.; Nicolaou, N.; Grabowska, A.; Hulse, R.P. Breast cancer induced nociceptor aberrant growth and collateral sensory axonal branching. Oncotarget 2017, 8, 76606. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.; Zhang, Y.; Han, C.; Zhang, G. Vascular Endothelial Growth Factor Mediates the Sprouted Axonogenesis of Breast Cancer in Rat. Am. J. Pathol. 2021, 191, 515–526. [Google Scholar] [CrossRef]
- Berlingeri-Ramos, A.C.; Detweiler, C.J.; Wagner, R.F., Jr.; Kelly, B.C. Dual S-100-AE1/3 Immunohistochemistry to Detect Perineural Invasion in Nonmelanoma Skin Cancers. J. Ski. Cancer 2015, 2015, 620235. [Google Scholar] [CrossRef][Green Version]
- Scanlon, P.; Tian, J.; Zhong, J.; Silva, I.; Shapiro, R.; Pavlick, A.; Berman, R.; Osman, I.; Darvishian, F. Enhanced immunohistochemical detection of neural infiltration in primary melanoma: Is there a clinical value? Hum. Pathol. 2014, 45, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Gogola, S.; Rejzer, M.; Stoyanov, K.; Gomez, A.S.; Valencia, A.-K.; Cummings, A.; Skerry, T.; Alloush, F.; Aljamal, A.A.; et al. Unraveling the Mysteries of Perineural Invasion in Benign and Malignant Conditions. Curr. Oncol. 2023, 30, 8948–8972. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurti, U.G.; Allison, K.H.; Fitzgibbons, P.L.; Connolly, J.L. Protocol for the Examination of Resection Specimens from Patients with Invasive Carcinoma of the Breast; The College of American Pathologists Location: Northfield, IL, USA, 2024; pp. 1–44. [Google Scholar]
- Doran, S.; Whiriskey, R.; Sheehy, N.; Johnston, C.; Byrne, D. Perineural tumour spread in head and neck cancer: A pictorial review. Clin. Radiol. 2024, 79, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nemzek, W.R.; Hecht, S.; Gandour-Edwards, R.; Donald, P.; McKennan, K. Perineural spread of head and neck tumors: How accurate is MR imaging? AJNR Am. J. Neuroradiol. 1998, 19, 701–706. [Google Scholar]
- Tu, W.; Gottumukkala, R.V.; Schieda, N.; Lavallée, L.; Adam, B.A.; Silverman, S.G. Perineural Invasion and Spread in Common Abdominopelvic Diseases: Imaging Diagnosis and Clinical Significance. RadioGraphics 2023, 43, e220148. [Google Scholar] [CrossRef]
- Crush, A.B.; Howe, B.M.; Spinner, R.J.; Amrami, K.K.; Hunt, C.H.; Johnson, G.B.; Murphy, R.C.; Morreale, R.F.; Peller, P.J. Malignant Involvement of the Peripheral Nervous System in Patients with Cancer: Multimodality Imaging and Pathologic Correlation. RadioGraphics 2014, 34, 1987–2007. [Google Scholar] [CrossRef]
- Hébert-Blouin, M.-N.; Amrami, K.K.; Loukas, M.; Spinner, R.J. A proposed anatomical explanation for perineural spread of breast adenocarcinoma to the brachial plexus. Clin. Anat. 2011, 24, 101–105. [Google Scholar] [CrossRef]
- Rath, T.J.; Policeni, B.; Juliano, A.F.; Agarwal, M.; Block, A.M.; Burns, J.; Conley, D.B.; Crowley, R.W.; Dubey, P.; Friedman, E.R.; et al. ACR Appropriateness Criteria® Cranial Neuropathy: 2022 Update. J. Am. Coll. Radiol. 2022, 19, S266–S303. [Google Scholar] [CrossRef]
- Murthy, N.K.; Amrami, K.K.; Spinner, R.J. Perineural spread to the brachial plexus: A focused review of proposed mechanisms and described pathologies. Acta Neurochir. 2020, 162, 3179–3187. [Google Scholar] [CrossRef]
- Loukas, M.; Hullett, J.; Louis, R.G., Jr.; Holdman, S.; Holdman, D. The gross anatomy of the extrathoracic course of the intercostobrachial nerve. Clin. Anat. 2006, 19, 106–111. [Google Scholar] [CrossRef]
- Jack, M.M.; Smith, B.W.; Capek, S.; Marek, T.; Carter, J.M.; Broski, S.M.; Amrami, K.K.; Spinner, R.J. The spectrum of brachial plexopathy from perineural spread of breast cancer. J. Neurosurg. 2022, 137, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.J.; Yoon, Y.C.; Sung, D.H. Brachial plexopathy due to perineural tumor spread: A retrospective single-center experience of clinical manifestations, diagnosis, treatments, and outcomes. Acta Neurochir. 2024, 166, 490. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Lamont, D.; Muthu, T.; Hussain, Z.; Balakrishnan, V. Metastasis of Breast Cancer to a Lumbar Spinal Nerve Root Ganglion. Spine 2009, 34, E735–E739. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, N.; Ismail, H.; Athar, S.; Ashwood, N. Perineural invasion in intramedullary spinal cord metastasis. Ann. R. Coll. Surg. Engl. 2020, 102, e94–e96. [Google Scholar] [CrossRef]
- Mackel, C.E.; Alsideiri, G.; Papavassiliou, E. Intramedullary-Extramedullary Breast Metastasis to the Caudal Neuraxis Two Decades after Primary Diagnosis: Case Report and Review of the Literature. World Neurosurg. 2020, 140, 26–31. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Mohammad, M.; Khalil, H.; Khouri, T.; Alsweiti, R.; Khzouz, J.; Abu Laban, D.; Jaradat, I.; Ibrahimi, A.K.; Al-Ibraheem, A.; et al. Ocular and Periocular Metastasis in Breast Cancer: Clinical Characteristics, Prognostic Factors and Treatment Outcome. Cancers 2024, 16, 1518. [Google Scholar] [CrossRef]
- Gasperini, J.; Black, E.; Van Stavern, G. Perineural Metastasis of Breast Cancer Treated With Optic Nerve Sheath Fenestration. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 331–333. [Google Scholar] [CrossRef]
- Verma, R.; Chen, K.C.; Ramkumar, H.L.; Goldbaum, M.H.; Shields, C.L. Optic nerve head problem. Surv. Ophthalmol. 2019, 64, 579–583. [Google Scholar] [CrossRef]
- Biswas, J.; Ho, T.C.; Bhavsar, K. Bilateral metastasis to the retina, choroids and optic nerve from breast cancer: A clinicopathological case. Indian J. Ophthalmol. 2007, 55, 71–72. [Google Scholar] [CrossRef]
- Stevenson, M.L.; Criscito, M.C.; Wilken, R.; Doudican, N.A.; Bain, E.E., III; Parashar, B.; Carucci, J.A. Use of Adjuvant Radiotherapy in the Treatment of High-risk Cutaneous Squamous Cell Carcinoma With Perineural Invasion. JAMA Dermatol. 2020, 156, 918–921. [Google Scholar] [CrossRef]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Bauer-Nilsen, K.; McNulty, R.H.; Vicini, F. Novel radiation therapy approaches for breast cancer treatment. Semin. Oncol. 2020, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Al-Hilli, Z.; Vicini, F. Advances in Breast Cancer Radiotherapy: Implications for Current and Future Practice. JCO Oncol. Pract. 2021, 17, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Lakritz, S.; Schreiber, A.R.; Molina, E.; Kabos, P.; Wood, M.; Elias, A.; Kondapalli, L.; Bradley, C.J.; Diamond, J.R. Clinical outcomes of adjuvant taxane plus anthracycline versus taxane-based chemotherapy regimens in older adults with node-positive, triple-negative breast cancer: A SEER-Medicare study. Eur. J. Cancer 2023, 185, 69–82. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Vanhecke, E.; Saule, P.; Mougel, A.; Page, A.; Romon, R.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Nerve Growth Factor Is a Potential Therapeutic Target in Breast Cancer. Cancer Res. 2008, 68, 346–351. [Google Scholar] [CrossRef]
- Zhang, M.; Xian, H.-C.; Dai, L.; Tang, Y.-L.; Liang, X.-H. MicroRNAs: Emerging driver of cancer perineural invasion. Cell Biosci. 2021, 11, 117. [Google Scholar] [CrossRef]
- Nair, R.S.; Kumar, S.; Das, S.; Singh, S.K.; Srivastava, P.; Sondarva, G.; Rao, A.; Sinha, S.C.; Xiong, R.; Bloem, L.; et al. TrkA expression directs the anti-neoplastic activity of MLK3 inhibitors in triple-negative breast cancer. Oncogene 2023, 42, 1132–1143. [Google Scholar] [CrossRef]
- Griffin, N.; Marsland, M.; Roselli, S.; Oldmeadow, C.; Attia, J.; Walker, M.M.; Hondermarck, H.; Faulkner, S. The Receptor Tyrosine Kinase TrkA Is Increased and Targetable in HER2-Positive Breast Cancer. Biomolecules 2020, 10, 1329. [Google Scholar] [CrossRef]
- Santen, R.J.; Song, R.X.; McPherson, R.; Kumar, R.; Adam, L.; Jeng, M.H.; Yue, W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 239–256. [Google Scholar] [CrossRef]
- Haagenson, K.K.; Wu, G.S. The role of MAP kinases and MAP kinase phosphatase-1 in resistance to breast cancer treatment. Cancer Metastasis Rev. 2010, 29, 143–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmad, H.F.; Wegner, C.; Nuraj, J.; Avellan, R.; Gonzalez, J.; Mendez, T.; Jabbour, D.; Gomez-Fernandez, C. Perineural Invasion in Breast Cancer: A Comprehensive Review. Cancers 2025, 17, 1900. https://doi.org/10.3390/cancers17121900
Bahmad HF, Wegner C, Nuraj J, Avellan R, Gonzalez J, Mendez T, Jabbour D, Gomez-Fernandez C. Perineural Invasion in Breast Cancer: A Comprehensive Review. Cancers. 2025; 17(12):1900. https://doi.org/10.3390/cancers17121900
Chicago/Turabian StyleBahmad, Hisham F., Carter Wegner, Joana Nuraj, Rima Avellan, Jeffrey Gonzalez, Teresita Mendez, Diana Jabbour, and Carmen Gomez-Fernandez. 2025. "Perineural Invasion in Breast Cancer: A Comprehensive Review" Cancers 17, no. 12: 1900. https://doi.org/10.3390/cancers17121900
APA StyleBahmad, H. F., Wegner, C., Nuraj, J., Avellan, R., Gonzalez, J., Mendez, T., Jabbour, D., & Gomez-Fernandez, C. (2025). Perineural Invasion in Breast Cancer: A Comprehensive Review. Cancers, 17(12), 1900. https://doi.org/10.3390/cancers17121900







