Development in Esophagectomy for Esophageal Cancer: The Current Standing Point of Robotic Surgery
Simple Summary
Abstract
1. Introduction
2. Development of Esophagectomy and LN Dissection for EC
3. Oncological Aspects of EC and Lymphadenectomy
4. Minimally Invasive Surgery for EC
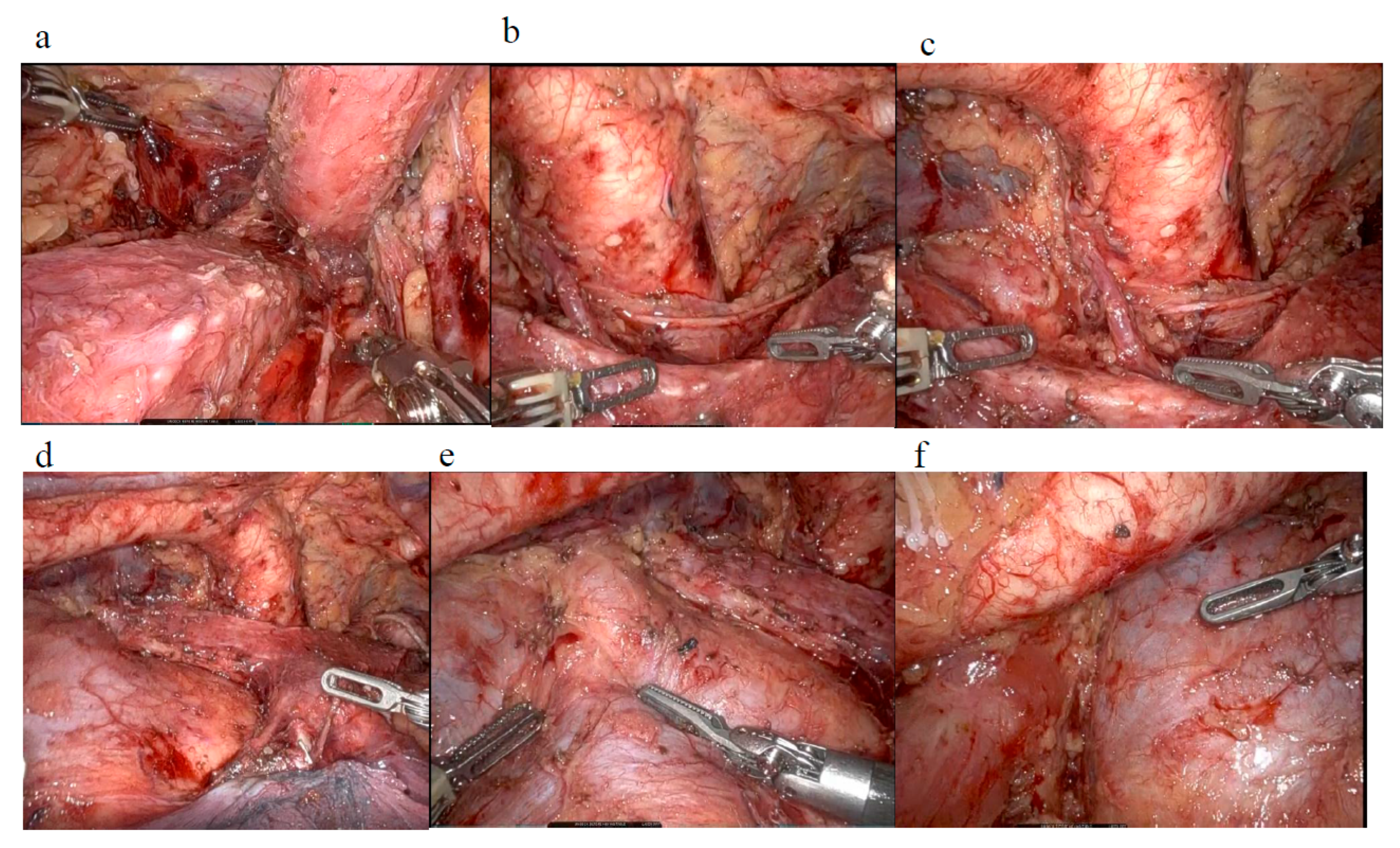
5. Robot-Assisted Minimally Invasive Surgery for EC
| Trial (Ref) (Recruitment) | Country | Approach | Number of Patients | Operation | Type of Carcinoma | Endpoint | Result | p Value |
|---|---|---|---|---|---|---|---|---|
| ROBOT trial [50] | Netherlands | RAMIE | 54 | McKnown | AC | Postoperative complications | 32 (59%) | 0.02 |
| (2012–2016) | OE | 55 | SCC | (modified Clavien-Dindo classification grade 2–5) | 44 (80%) | |||
| RAMIE trial [54] | China | RAMIE | 181 | McKnown | SCC | Achievement rate of the LND | 79.5% | 0.001 |
| (2017–2019) | VATS | 177 | along the left RLN | 67.6% (NAC cases) | ||||
| 5-yr OS (primary endpoint) | Ongoing | |||||||
| REVATE trial [53] | Taiwan | RAMIE | 51 | McKnown | SCC | Success rate of the LND along | 88.3% | <0.001 |
| (2018–2022) | China | VATS | 51 | the left RLN | 69% | |||
| ROBOT-2 trial [52] | Germany | RAMIE | 109 | Ivor-Lewis | AC | Total number of resected LN | Ongoing | - |
| (2021–ongoing) | Netherlands | VATS | 109 | |||||
| Switzerland |
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takeuchi, H.; Fujii, H.; Ando, N.; Ozawa, S.; Saikawa, Y.; Suda, K.; Oyama, T.; Mukai, M.; Nakahara, T.; Kubo, A.; et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann. Surg. 2009, 249, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, Y.; Kato, K.; Igaki, H.; Ito, Y.; Nozaki, I.; Daiko, H.; Yano, M.; Udagawa, H.; Nakagawa, S.; Takagi, M.; et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: From the results of JCOG0502, a prospective multicenter study. Ann. Surg. 2016, 264, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Tsurumaru, M.; Udagawa, H.; Kajiyama, Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann. Surg. 1994, 220, 364–372; discussion 372–373. [Google Scholar] [CrossRef]
- Ito, S.; Takahashi, A.; Ueno, H.; Takiguchi, S.; Kajiwara, Y.; Kakeji, Y.; Eguchi, S.; Goi, T.; Saiura, A.; Sasaki, A.; et al. Annual report on National Clinical Database 2021 for gastroenterological surgery in Japan. Ann. Gastroenterol. Surg. 2025, 9, 32–59. [Google Scholar] [CrossRef]
- Matsuda, S.; van der Sluis, P.; Kumamaru, H.; Kingma, F.; Kawakubo, H.; Ruurda, J.; Watanabe, M.; Miyata, H.; Lagarde, S.; Takeuchi, H.; et al. Oesophagectomy in the East versus the West: Comparison of two national audit databases. Br. J. Surg. 2025, 112, znaf035. [Google Scholar] [CrossRef]
- van Hillegersberg, R.; Boone, J.; Draaisma, W.A.; Broeders, I.A.M.J.; Giezeman, M.J.M.M.; Borel Rinkes, I.H.M. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg. Endosc. 2006, 20, 1435–1439. [Google Scholar] [CrossRef]
- Czerny, J. Neue Operationen-Resektion Des Oesophagus. Zentralbl Chir. 1877, 4, 433–434. [Google Scholar]
- Mikulicz, J.V. Ein Fall von Resection Des Carcinomatosen Oesophagus Mit Plastischem Ersatz Des Excidirten Stukes. Prag. Med. Wochr. 1886, 11, 93–94. [Google Scholar]
- Wookey, H. Surgical Treatment Carcinoma Pharynx Upper Esophagus. Surg. Gynecol. Obstet. 1942, 75, 499. [Google Scholar]
- Seidenberg, B.; Rosenak, S.S.; Hurwitt, E.S.; Som, M.L. Immediate reconstruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann. Surg. 1959, 149, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Torek, F. First Successful Case Resection Tho Racic Portion Esophagus Carcinoma. Surg. Gynecol. Obstet. 1913, 16, 614–617. [Google Scholar]
- Osawa, T. Esophageal Surgery. Nippon Geka Gakkai Zasshi. 1933, 34, 1319–1590. [Google Scholar]
- Lewis, I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br. J. Surg. 1946, 34, 18–31. [Google Scholar] [CrossRef]
- Nakayama, K. Approach to midthoracic esophageal carcinoma for its radical surgical treatment. Surgery 1954, 35, 574–589. [Google Scholar] [PubMed]
- Logan, A. The surgical treatment of carcinoma of the esophagus and Cardia. J. Thorac. Cardiovasc. Surg. 1963, 46, 150–161. [Google Scholar] [CrossRef]
- Skinner, D.B. En bloc resection for neoplasms of the esophagus and cardia. J. Thorac. Cardiovasc. Surg. 1983, 85, 59–71. [Google Scholar] [CrossRef]
- Tachimori, Y.; Nagai, Y.; Kanamori, N.; Hokamura, N.; Igaki, H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis. Esophagus. 2011, 24, 33–38. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Udagawa, H.; Ueno, M.; Shinohara, H.; Haruta, S.; Lee, S.; Momose, K.; Tsurumaru, M. Should lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 2014, 11, 204–210. [Google Scholar] [CrossRef]
- Ohkura, Y.; Ueno, M.; Iizuka, T.; Udagawa, H. Effectiveness of lymphadenectomy along the thoracic duct for radical esophagectomy. Esophagus 2022, 19, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Defize, I.L.; Gorgels, S.M.C.; Mazza, E.; Schurink, B.; Strignano, P.; Catalano, G.; Brosens, L.A.; Chiusa, L.; Bleys, R.L.; Mook, S.; et al. The presence of metastatic thoracic duct lymph nodes in Western esophageal cancer patients: A multinational observational study. Ann. Thorac. Surg. 2022, 113, 429–435. [Google Scholar] [CrossRef]
- Matsuda, S.; Kawakubo, H.; Takeuchi, H.; Mayanagi, S.; Irino, T.; Fukuda, K.; Nakamura, R.; Wada, N.; Kitagawa, Y. Prognostic impact of thoracic duct lymph node metastasis in esophageal squamous cell carcinoma. Ann. Gastroenterol. Surg. 2021, 5, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Takeuchi, H.; Kawakubo, H.; Shimada, A.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Kameyama, K.; Kitagawa, Y. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine 2016, 95, e3839. [Google Scholar] [CrossRef]
- Matsuda, S.; Kawakubo, H.; Takeuchi, H.; Hayashi, M.; Mayanagi, S.; Takemura, R.; Irino, T.; Fukuda, K.; Nakamura, R.; Wada, N.; et al. Minimally invasive oesophagectomy with extended lymph node dissection and thoracic duct resection for early-stage oesophageal squamous cell carcinoma: Extended minimally invasive oesophagectomy for squamous cell carcinoma. Br. J. Surg. 2020, 107, 705–711. [Google Scholar] [CrossRef]
- Oshikiri, T.; Numasaki, H.; Oguma, J.; Toh, Y.; Watanabe, M.; Muto, M.; Kakeji, Y.; Doki, Y. Prognosis of patients with esophageal carcinoma after routine thoracic duct resection: A propensity-matched analysis of 12,237 patients based on the Comprehensive Registry of Esophageal Cancer in Japan. Ann. Surg. 2023, 277, e1018–e1025. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; van Berge Henegouwen, M.I.; van Sandick, J.W.; Cuesta, M.A.; van der Peet, D.L.; Heisterkamp, J.; Nieuwenhuijzen, G.A.P.; Rosman, C.; Scheepers, J.J.G.; Sosef, M.N.; et al. Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: Study protocol of a multinational observational study. BMC Cancer 2019, 19, 662. [Google Scholar] [CrossRef]
- Castoro, C.; Scarpa, M.; Cagol, M.; Ruol, A.; Cavallin, F.; Alfieri, R.; Zanchettin, G.; Rugge, M.; Ancona, M.E. Nodal metastasis from locally advanced esophageal cancer: How neoadjuvant therapy modifies their frequency and distribution. Ann. Surg. Oncol. 2011, 18, 3743–3754. [Google Scholar] [CrossRef]
- Van De Ven, C.; Leyn, D.; Coosemans, P.; Van Raemdonck, W.; Lerut, D. Three field lymphadenectomy pattern lymph node spread T3 adenocarcinoma distal esophagus gastro-esophageal junction. Eur. J. Cardiothorac. Surg. 1999, 15, 769–773. [Google Scholar] [CrossRef]
- Yasuda, T.; Yano, M.; Miyata, H.; Yamasaki, M.; Takiguchi, S.; Fujiwara, Y.; Doki, Y. Evaluation of dysphagia and diminished airway protection after three-field esophagectomy and a remedy. World J. Surg. 2013, 37, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, I.; Kato, K.; Igaki, H.; Ito, Y.; Daiko, H.; Yano, M.; Udagawa, H.; Mizusawa, J.; Katayama, H.; Nakamura, K.; et al. Evaluation of safety profile of thoracoscopic esophagectomy for T1bN0M0 cancer using data from JCOG0502: A prospective multicenter study. Surg. Endosc. 2015, 29, 3519–3526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsunoda, S.; Tsubosa, Y.; Sasaki, K.; Machida, R.; Kita, R.; Fukuda, H.; Koyanagi, K.; Takeuchi, H.; Kamei, T.; Mine, S.; et al. A multicenter randomized controlled trial of esophagectomy with or without prophylactic supraclavicular node dissection: A phase 3 trial (JCOG2013, MODERN3). Jpn. J. Clin. Oncol. 2023, 53, 858–862. [Google Scholar] [CrossRef]
- Tachimori, Y.; The Registration Committee for Esophageal Cancer of the Japan Esophageal Society; Ozawa, S.; Numasaki, H.; Ishihara, R.; Matsubara, H.; Muro, K.; Oyama, T.; Toh, Y.; Udagawa, H.; et al. Comprehensive registry of esophageal cancer in japan, 2011. Esophagus 2018, 15, 127–152. [Google Scholar] [CrossRef]
- Cuschieri, A.; Shimi, S.; Banting, S. Endoscopic oesophagectomy through a right thoracoscopic approach. J. R. Coll. Surg. Edinb. 1992, 37, 7–11. [Google Scholar]
- Akaishi, T.; Kaneda, I.; Higuchi, N.; Kuriya, Y.; Kuramoto, J.; Toyoda, T.; Wakabayashi, A. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J. Thorac. Cardiovasc. Surg. 1996, 112, 1533–1540, discussion 1540–1541. [Google Scholar] [CrossRef] [PubMed]
- Biere, S.S.A.Y.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.M.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Straatman, J.; van der Wielen, N.; Cuesta, M.A.; Daams, F.; Roig Garcia, J.; Bonavina, L.; Rosman, C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; van der Peet, D.L. Minimally invasive versus open esophageal resection: Three-year follow-up of the previously reported randomized controlled trial: The TIME trial. Ann. Surg. 2017, 266, 232–236. [Google Scholar] [CrossRef]
- Takeuchi, H.; Miyata, H.; Ozawa, S.; Udagawa, H.; Osugi, H.; Matsubara, H.; Konno, H.; Seto, Y.; Kitagawa, Y. Comparison of short-term outcomes between open and minimally invasive esophagectomy for esophageal cancer using a nationwide database in japan. Ann. Surg. Oncol. 2017, 24, 1821–1827. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Nuytens, F.; Dabakuyo-Yonli, T.S.; Meunier, B.; Gagnière, J.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; Mabrut, J.Y.; et al. Five-Year Survival Outcomes of Hybrid Minimally Invasive Esophagectomy in Esophageal Cancer: Results of the MIRO Randomized Clinical Trial. JAMA Surg. 2021, 156, 323. [Google Scholar] [CrossRef]
- Takeuchi, M.; Endo, H.; Kawakubo, H.; Matsuda, S.; Kikuchi, H.; Kanaji, S.; Kumamaru, H.; Miyata, H.; Ueno, H.; Seto, Y.; et al. No difference in the incidence of postoperative pulmonary complications between abdominal laparoscopy and laparotomy for minimally invasive thoracoscopic esophagectomy: A retrospective cohort study using a nationwide Japanese database. Esophagus 2024, 21, 11–21. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ando, M.; Tsubosa, Y.; Kikuchi, H.; Kawakubo, H.; Noma, K.; Ueno, M.; Tsushima, T.; Bamba, T.; Fujita, T.; et al. A randomized controlled phase III trial comparing thoracoscopic esophagectomy and open esophagectomy for thoracic esophageal cancer: JCOG1409 (MONET trial). J. Clin. Oncol. 2024, 42 (Suppl. S3), 249. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Matsuda, S.; Gotoda, T.; Kato, K.; Wijnhoven, B.; Lordick, F.; Bhandari, P.; Kawakubo, H.; Kodera, Y.; Terashima, M.; et al. Clinical practice guidelines for esophagogastric junction cancer: Upper GI Oncology Summit 2023. Gastric Cancer 2024, 27, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Bodner, J.; Wykypiel, H.; Wetscher, G.; Schmid, T. First experiences with the da Vinci operating robot in thoracic surgery. Eur. J. Cardiothorac. Surg. 2004, 25, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Ishida, Y.; Kawamura, Y.; Inaba, K.; Kanaya, S.; Teramukai, S.; Satoh, S.; Uyama, I. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: Technical report and short-term outcomes. World J. Surg. 2012, 36, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Haj Mohammad, N.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: A randomized controlled trial: A randomized controlled trial. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef]
- de Groot, E.M.; van der Horst, S.; Kingma, B.F.; Goense, L.; van der Sluis, P.C.; Ruurda, J.P.; van Hillegersberg, R. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open esophagectomy: Long-term follow-up of a randomized clinical trial. Dis. Esophagus 2020, 33 (Suppl. S2), doaa079. [Google Scholar] [CrossRef]
- Tagkalos, E.; van der Sluis, P.C.; Berlth, F.; Poplawski, A.; Hadzijusufovic, E.; Lang, H.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Müller-Stich, B.P.; Ruurda, J.P.; et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021, 21, 1060. [Google Scholar] [CrossRef]
- Chao, Y.-K.; Li, Z.; Jiang, H.; Wen, Y.-W.; Chiu, C.-H.; Li, B.; Shang, X.; Fang, T.J.; Yang, Y.; Yue, J.; et al. Multicentre randomized clinical trial on robot-assisted versus video-assisted thoracoscopic oesophagectomy (REVATE trial). Br. J. Surg. 2024, 111, zane143. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B.; Yi, J.; Hua, R.; Chen, H.; Tan, L.; Li, H.; He, Y.; Guo, X.; Sun, Y.; et al. Robot-assisted versus conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: Early results of a multicenter randomized controlled trial: The RAMIE trial. Ann. Surg. 2022, 275, 646–653. [Google Scholar] [CrossRef]
- Nishigori, T.; Kumamaru, H.; Obama, K.; Suda, K.; Tsunoda, S.; Yoda, Y.; Hikage, M.; Shibasaki, S.; Tanaka, T.; Terashima, M.; et al. Short-term outcomes of robot-assisted versus conventional minimally invasive esophagectomy: A propensity score-matched study via a nationwide database. Ann. Gastroenterol. Surg. 2025, 9, 109–118. [Google Scholar] [CrossRef]
- Esagian, S.M.; Ziogas, I.A.; Skarentzos, K.; Katsaros, I.; Tsoulfas, G.; Molena, D.; Karamouzis, M.V.; Rouvelas, I.; Nilsson, M.; Schizas, D. Robot-assisted minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: A systematic review and meta-analysis. Cancers 2022, 14, 3177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, D.; Cao, Y.; Huang, M.; Li, J.; Zhang, J.; Lin, J.; Sarkaria, I.S.; Toni, L.; David, R.; et al. Robotic versus conventional minimally invasive esophagectomy for esophageal cancer: A meta-analysis. Ann. Surg. 2023, 278, 39–50. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Zhang, X.; Yang, H.; Tan, Z.; Lin, Y.; Fu, J. Comparisons of short-term outcomes between robot-assisted and thoraco-laparoscopic esophagectomy with extended two-field lymph node dissection for resectable thoracic esophageal squamous cell carcinoma. J. Thorac. Dis. 2019, 11, 3874–3880. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Chittawadagi, B.; Misra, S.; Ramakrishnan, P.; Chinnusamy, P. Propensity matched analysis of short term oncological and perioperative outcomes following robotic and thoracolaparoscopic esophagectomy for carcinoma esophagus- the first Indian experience. J. Robot. Surg. 2022, 16, 97–105. [Google Scholar] [CrossRef]
- Goense, L.; van der Sluis, P.C.; van der Horst, S.; Tagkalos, E.; Grimminger, P.P.; van Dijk, W.; Ruurda, J.P.; van Hillegersberg, R. Cost analysis of robot-assisted versus open transthoracic esophagectomy for resectable esophageal cancer. Results of the ROBOT randomized clinical trial. Eur. J. Surg. Oncol. 2023, 49, 106968. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Lyu, H.G.; Azimuddin, A.M.; Lu, P.; Ajith, J.; Schmeisser, J.A.; Ninan, E.P.; Lee, K.H.; Badgwell, B.D.; Mansfield, P.; et al. Cost analysis for robotic and open gastrectomy. Ann. Surg. Open. 2024, 5, e396. [Google Scholar] [CrossRef]
- Chiu, P.W.-Y.; de Groot, E.M.; Yip, H.-C.; Egberts, J.-H.; Grimminger, P.; Seto, Y.; Uyama, I.; van der Sluis, P.C.; Stein, H.; Sallum, R.; et al. Robot-assisted cervical esophagectomy: First clinical experiences and review of the literature. Dis. Esophagus 2020, 33 (Suppl. S2), doaa052. [Google Scholar] [CrossRef]
- Fujita, T.; Sato, K.; Fujiwara, N.; Kajiyama, D.; Shigeno, T.; Otomo, M.; Daiko, H. Robot-assisted transcervical esophagectomy with a bilateral cervical approach for thoracic esophagectomy. Surg. Endosc. 2024, 38, 1617–1625. [Google Scholar] [CrossRef]
- Fujita, T.; Sato, K.; Fujiwara, N.; Kajiyama, D.; Kubo, Y.; Daiko, H. Robot-assisted cervical esophagectomy with simultaneous transhiatal abdominal procedure for thoracic esophageal carcinoma. Surg. Endosc. 2024, 38, 6413–6422. [Google Scholar] [CrossRef] [PubMed]
- Hadzijusufovic, E.; Lozanovski, V.J.; Griemert, E.-V.; Bellaio, L.; Lang, H.; Grimminger, P.P. Single-port da Vinci robot-assisted cervical esophagectomy: How to do it. Thorac. Cardiovasc. Surg. 2024, 72, 654–658. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, Y.; Matsuda, S.; Hirata, Y.; Hoshi, Y.; Takeuchi, M.; Kawakubo, H.; Kitagawa, Y. Development in Esophagectomy for Esophageal Cancer: The Current Standing Point of Robotic Surgery. Cancers 2025, 17, 1878. https://doi.org/10.3390/cancers17111878
Morimoto Y, Matsuda S, Hirata Y, Hoshi Y, Takeuchi M, Kawakubo H, Kitagawa Y. Development in Esophagectomy for Esophageal Cancer: The Current Standing Point of Robotic Surgery. Cancers. 2025; 17(11):1878. https://doi.org/10.3390/cancers17111878
Chicago/Turabian StyleMorimoto, Yosuke, Satoru Matsuda, Yuki Hirata, Yuki Hoshi, Masashi Takeuchi, Hirofumi Kawakubo, and Yuko Kitagawa. 2025. "Development in Esophagectomy for Esophageal Cancer: The Current Standing Point of Robotic Surgery" Cancers 17, no. 11: 1878. https://doi.org/10.3390/cancers17111878
APA StyleMorimoto, Y., Matsuda, S., Hirata, Y., Hoshi, Y., Takeuchi, M., Kawakubo, H., & Kitagawa, Y. (2025). Development in Esophagectomy for Esophageal Cancer: The Current Standing Point of Robotic Surgery. Cancers, 17(11), 1878. https://doi.org/10.3390/cancers17111878






