Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Statistical Analysis
3. Results
3.1. Treatment with Intravenous Iron
3.2. Clinical Outcomes
3.3. Transfusion Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IDA | Iron Deficiency Anaemia |
| Hb | Haemoglobin |
| CRC | Colorectal Cancer |
| POAS | Perioperative Anaemia Service |
| IV | Iron Intravenous Iron |
| Fe | Ferritin |
| TSAT % | Transferrin Saturation % |
| GICU | General Intensive Care Unit |
| UHS | University Hospital Southampton NHS Foundation Trust |
References
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Weng, M.; Zhang, J. Preoperative anaemia and long-term survival in patients undergoing colorectal cancer surgery: A retrospective cohort study. World J. Surg. Oncol. 2023, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Richards, T.; Klein, A.; Wood, E.M.; Wallace, S.; Shulman, M.A.; Martin, C.; Bellomo, R.; Corcoran, T.B.; Peyton, P.J.; et al. Postoperative anaemia and patient-centred outcomes after major abdominal surgery: A retrospective cohort study. Br. J. Anaesth. 2022, 129, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Hochrieser, H.; Posch, M.; Metnitz, B.; Rhodes, A.; Moreno, R.P.; Pearse, R.M.; Metnitz, P. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br. J. Anaesth. 2014, 113, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. J. Br. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; Apelseth, T.O.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836. [Google Scholar] [PubMed]
- Maclean, B.; Weyand, A.; Lim, J.; Richards, T. Preoperative iron therapy: Where are we? Best Pract. Res. Clin. Anaesthesiol. 2023, 37, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Corwin, H.L.; Meier, J.; Auerbach, M.; Bisbe, E.; Blitz, J.; Erhard, J.; Faraoni, D.; Farmer, S.L.; Frank, S.M.; et al. Recommendations from the international consensus conference on anaemia management in surgical patients (ICCAMS). Ann. Surg. 2023, 277, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Shander, A.; Knight, K.; Thurer, R.; Adamson, J.; Spence, R. Prevalence and outcomes of anaemia in surgery: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 58S–69S. [Google Scholar] [CrossRef]
- Henry, D.H.; Dahl, N.V.; Auerbach, M.; Tchekmedyian, S.; Laufman, L.R. Intravenous Ferric Gluconate Significantly Improves Response to Epoetin Alfa Versus Oral Iron or No Iron in Anaemc Patients with Cancer Receiving Chemotherapy. Oncologist 2007, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Moncur, A.; Chowdhary, M.; Chu, Y.; Francis, N.K. Impact and outcomes of postoperative anaemia in colorectal cancer patients: A systematic review. Color. Dis. 2021, 23, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Lederhuber, H.; Massey, L.H.; Abeysiri, S.; Roman, M.A.; Rajaretnam, N.; McDermott, F.D.; Miles, L.F.; Smart, N.J.; Richards, T. Preoperative intravenous iron and the risk of blood transfusion in colorectal cancer surgery: Meta-analysis of randomized clinical trials. Br. J. Surg. 2024, 111, 320. [Google Scholar] [CrossRef] [PubMed]
- National Bowel Cancer Audit. Trust Results for University Hospital Southampton NHS Foundation Trust, 2013–2023. Available online: https://www.nboca.org.uk/trust-results/trust/university-hospital-southampton-nhs-foundation-trust (accessed on 5 March 2025).
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Rao Baikady, R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Intra-venous Iron Factsheet. 2011. Available online: https://www.transfusionguidelines.org/document-library/documents/intravenous-iv-iron-factsheet/download-file/rtc-sw_blood_cons_iron.pdf (accessed on 1 March 2025).
- Wilson, M.J.; Dekker, J.W.; Bruns, E.; Borstlap, W.; Jeekel, J.; Zwaginga, J.J.; Schipperus, M. Short-term effect of preoperative intravenous iron therapy in colorectal cancer patients with anemia: Results of a cohort study. Transfusion 2018, 58, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Kangaspunta, M.; Mäkijärvi, J.; Koskensalo, S.; Kokkola, A.; Arkkila, P.; Scheinin, T.; Rasilainen, S. Preoperative intravenous iron treatment reduces postoperative complications and postoperative anemia in preoperatively anemic patients with colon carcinoma. Int. J. Color. Dis. 2022, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Naoum, F.A. Iron deficiency in cancer patients. Rev. Bras. Hematol. Hemoter. 2016, 38, 325–330. [Google Scholar] [CrossRef]
- Johansson, P.; Rasmussen, A.; Thomsen, L. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: A randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015, 109, 257–266. [Google Scholar]
- Tiglis, M.; Cobilinschi, C.; Mirea, L.E.; Băetu, A.E.; Peride, I.; Neagu, T.P.; Niculae, A.; Checherită, I.A.; Grintescu, I.M. The importance of iron administration in correcting anaemia after major surgery. J. Crit. Care Med. 2021, 7, 184–191. [Google Scholar] [CrossRef]
- Brookes, M.J.; Boult, J.; Roberts, K.; Cooper, B.T.; Hotchin, N.A.; Matthews, G.; Iqbal, T.; Tselepis, C. A role for iron in wnt signalling. Oncogene 2008, 27, 966–975. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Z.; Wang, Z.; Miao, Z.; Lv, Z.; Li, X.; Yang, J.; Xu, H. Iron-deficiency anaemia: A predictor of diminished disease-free survival of T3N0M0 stage colon cancer. J. Surg. Oncol. 2012, 105, 371–375. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Not Anaemic (n = 99) | Anaemic, Treated (n = 220) | Anaemic, Not Treated (n = 88) | p-Value * | |
|---|---|---|---|---|---|
| Patient Demographics | |||||
| Age | 65.24 (11.78) | 71.02 (12.34) | 72.35 (12.42) | 0.393 | |
| Sex (% Female) | 29 (29) | 106 (48) | 54 (61) | 0.036 | |
| BMI | 28.77 (4.67) | 27.43 (5.76) | 26.37 (5.94) | 0.1537 | |
| Surgical Procedure | 0.137 | ||||
| Abdominal-perineal excision of rectum, n (%) | 11 (11.1) | 8 (3.6) | 6 (6.8) | ||
| Anterior Resection, n (%) | 42 (42.4) | 54 (24.5) | 32 (36.4) | ||
| Left Hemicolectomy, n (%) | 5 (5.1) | 14 (6.4) | 3 (3.4) | ||
| Pan-Proctocolectomy, n (%) | 1 (1.0) | 2 (0.9) | 1 (1.1) | ||
| Proctectomy, n (%) | 1 (1.0) | 0 (0) | 1 (1.1) | ||
| Right Hemicolectomy, n (%) | 36 (36.0) | 135 (61.4) | 43 (48.9) | ||
| Colectomy, n (%) | 3 (3.0) | 7 (3.2) | 2 (2.3) | ||
| Surgical Approach | 0.544 | ||||
| Laparoscopic, n (%) | 75 (75.8) | 147 (66.8) | 60 (68.2) | ||
| Open Surgery, n (%) | 24 (24.2) | 70 (31.8) | 28 (31.8) | ||
| Robotic Surgery, n (%) | 0 (0) | 3 (1.4) | 0 (0) | ||
| Comorbidities | |||||
| Diabetes Mellitus, n (%) | 12 (12.1) | 46 (20.9) | 12 (13.6) | 0.140 | |
| Hypertension, n (%) | 34 (34.3) | 93 (42.3) | 44 (50) | 0.218 | |
| Ischaemic Heart Disease, n (%) | 7 (7.1) | 15 (6.8) | 5 (5.7) | 0.715 | |
| Cerebrovascular Disease, n (%) | 3 (3.0) | 7 (3.2) | 8 (9.1) | 0.030 | |
| COPD, n (%) | 5 (5.1) | 18 (8.2) | 9 (10.2) | 0.566 | |
| Anaemic (Treated) | Anaemic (Untreated) | Not Anaemic | |
|---|---|---|---|
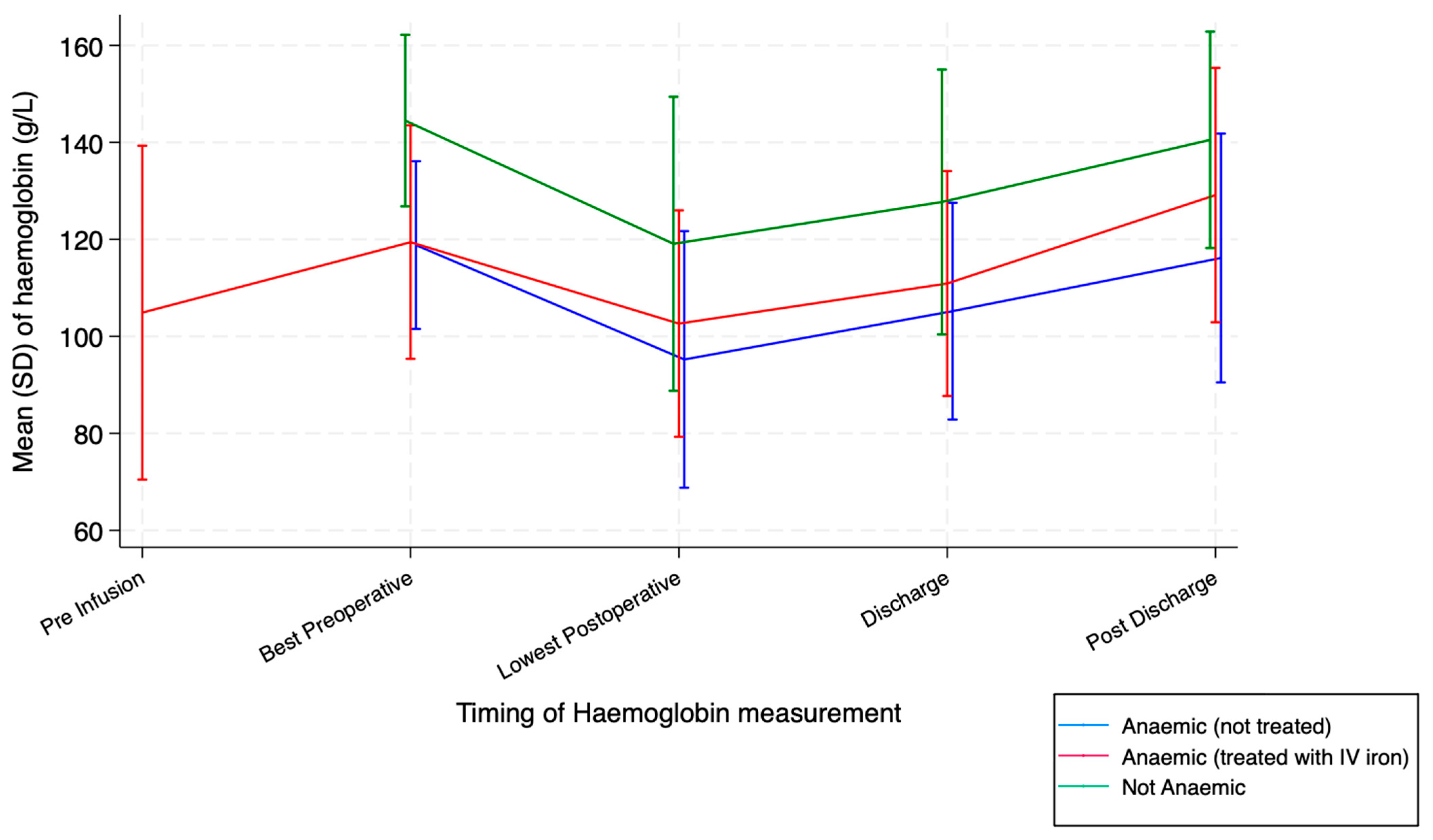
| Pre-infusion Hb (mean [SD], g/L) | 104.90 (17.57) | ||
| Pre-infusion TSAT (mean [SD], %) | 12.43 (8.34) | ||
| Pre-infusion ferritin (median [IQR], ng/mL) | 14 (8, 30) | ||
| Best Hb pre-operatively (mean [SD], g/L) | 119.43 (12.28) | 118.83 (8.82) | 144.51 (9.02) |
| Lowest Hb post-operatively (mean [SD], g/L) | 102.63 (11.91) | 95.24 (13.50) | 119.09 (15.47) |
| Hb at discharge (mean [SD], g/L) | 110.91 (11.83) | 105.20 (11.40) | 127.72 (13.94) |
| Hb post-discharge (mean [SD], g/L) | 129.15 (13.38) | 116.16 (13.09) | 140.53 (11.39) |
| Number of Patients | Hazard Ratio (95% Confidence Interval) | p-Value | ||
|---|---|---|---|---|
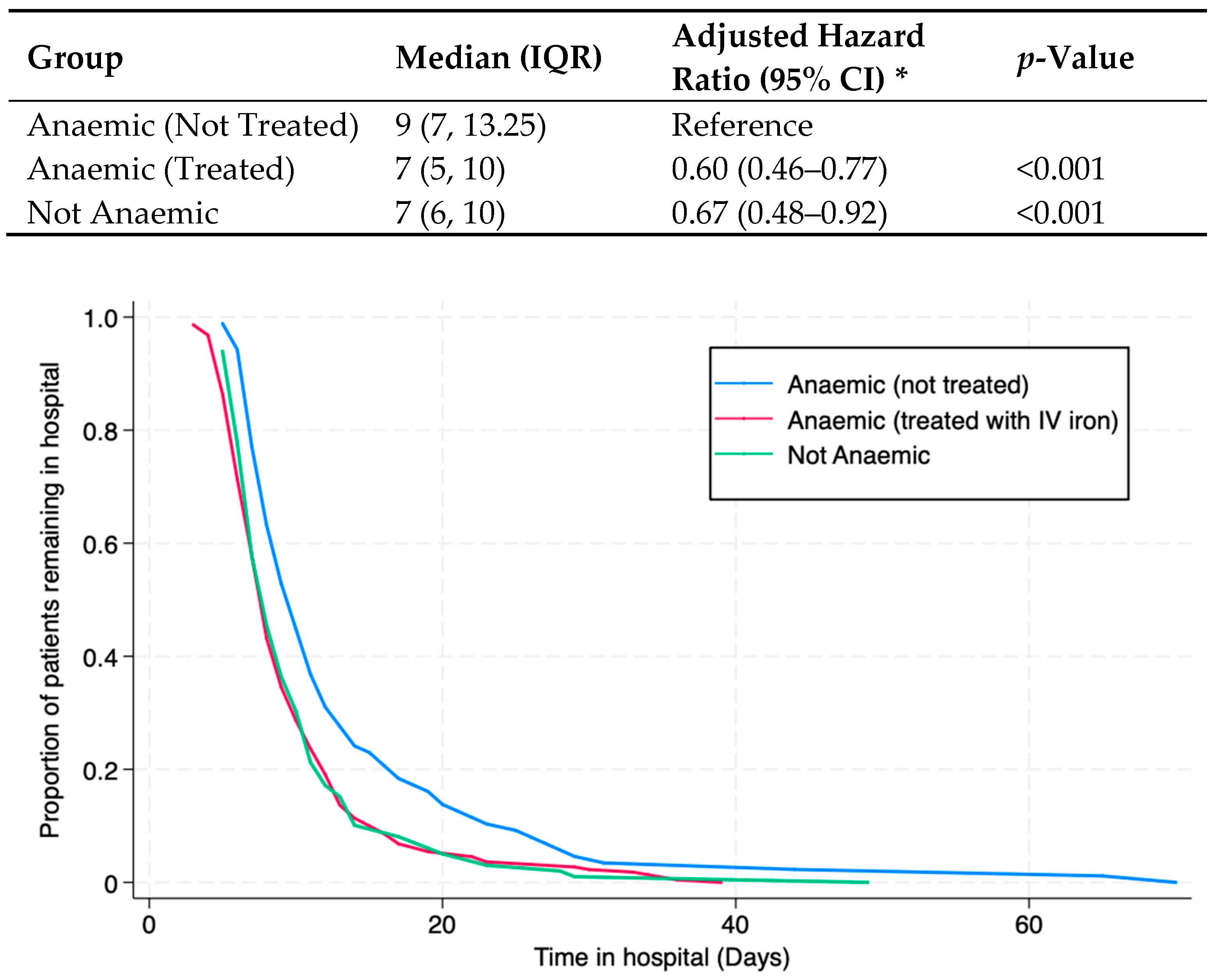
| Anaemia treated versus not treated | ||||
| Unadjusted | 308 | 0.63 (0.49 to 0.81) | <0.001 | |
| Adjusted for age and sex | 308 | 0.60 (0.46 to 0.77) | <0.001 | |
| Adjusted for above and body mass index | 301 | 0.60 (0.46 to 0.78) | <0.001 | |
| Adjusted for above and surgical details | 301 | 0.61 (0.47 to 0.80) | <0.001 | |
| Adjusted for above and comorbidities | 301 | 0.59 (0.45 to 0.78) | <0.001 | |
| Not anaemic versus non-treated anaemia | ||||
| Unadjusted | 187 | 0.64 (0.48 to 0.86) | 0.003 | |
| Adjusted for age and sex | 187 | 0.67 (0.48 to 0.92) | 0.015 | |
| Adjusted for above and body mass index | 184 | 0.69 (0.49 to 0.96) | 0.027 | |
| Adjusted for above and surgical details | 184 | 0.66 (0.46 to 0.94) | 0.021 | |
| Adjusted for above and comorbidities | 184 | 0.66 (0.45 to 0.95) | 0.026 | |
| Age- and Sex-Adjusted Models | Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|
| Anaemia Treated versus Not Treated | |||
| Perioperative blood transfusion | 0.35 (0.18 to 0.66) | 0.001 | |
| Unplanned admission to ICU | 0.49 (0.16 to 1.46) | 0.198 | |
| Emergency return to theatre | 0.37 (0.07 to 1.95) | 0.243 | |
| Unplanned readmission, 30 days | 0.93 (0.23 to 3.75) | 0.924 | |
| Unplanned readmission, 8 weeks | 0.94 (0.35 to 2.55) | 0.903 | |
| Not Anaemic versus Not Treated | |||
| Perioperative blood transfusion | 0.20 (0.07 to 0.55) | 0.002 | |
| Unplanned admission to ICU | 0.22 (0.04 to 1.25) | 0.087 | |
| Emergency return to theatre | 0.32 (0.04 to 2.28) | 0.253 | |
| Unplanned readmission, 30 days | 1.35 (0.23 to 7.92) | 0.738 | |
| Unplanned readmission, 8 weeks | 1.22 (0.33 to 4.49) | 0.765 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritche, D.; Wensley, F.; Johnson, Y.L.; Robins, C.; Wakatsuki, M.; Fecher-Jones, I.C.; Sheppard, L.; West, M.A.; Aarvold, A.; Edwards, M.R.; et al. Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis. Cancers 2025, 17, 1877. https://doi.org/10.3390/cancers17111877
Fritche D, Wensley F, Johnson YL, Robins C, Wakatsuki M, Fecher-Jones IC, Sheppard L, West MA, Aarvold A, Edwards MR, et al. Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis. Cancers. 2025; 17(11):1877. https://doi.org/10.3390/cancers17111877
Chicago/Turabian StyleFritche, Dominic, Frances Wensley, Yanika L. Johnson, Callum Robins, Mai Wakatsuki, Imogen C. Fecher-Jones, Lisa Sheppard, Malcolm A. West, Alice Aarvold, Mark R. Edwards, and et al. 2025. "Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis" Cancers 17, no. 11: 1877. https://doi.org/10.3390/cancers17111877
APA StyleFritche, D., Wensley, F., Johnson, Y. L., Robins, C., Wakatsuki, M., Fecher-Jones, I. C., Sheppard, L., West, M. A., Aarvold, A., Edwards, M. R., Grocott, M. P. W., Plumb, J., & Levett, D. Z. H. (2025). Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis. Cancers, 17(11), 1877. https://doi.org/10.3390/cancers17111877







