Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs)
Simple Summary
Abstract
1. Introduction
2. Ultrasound in the Evaluation of GEP-NETs
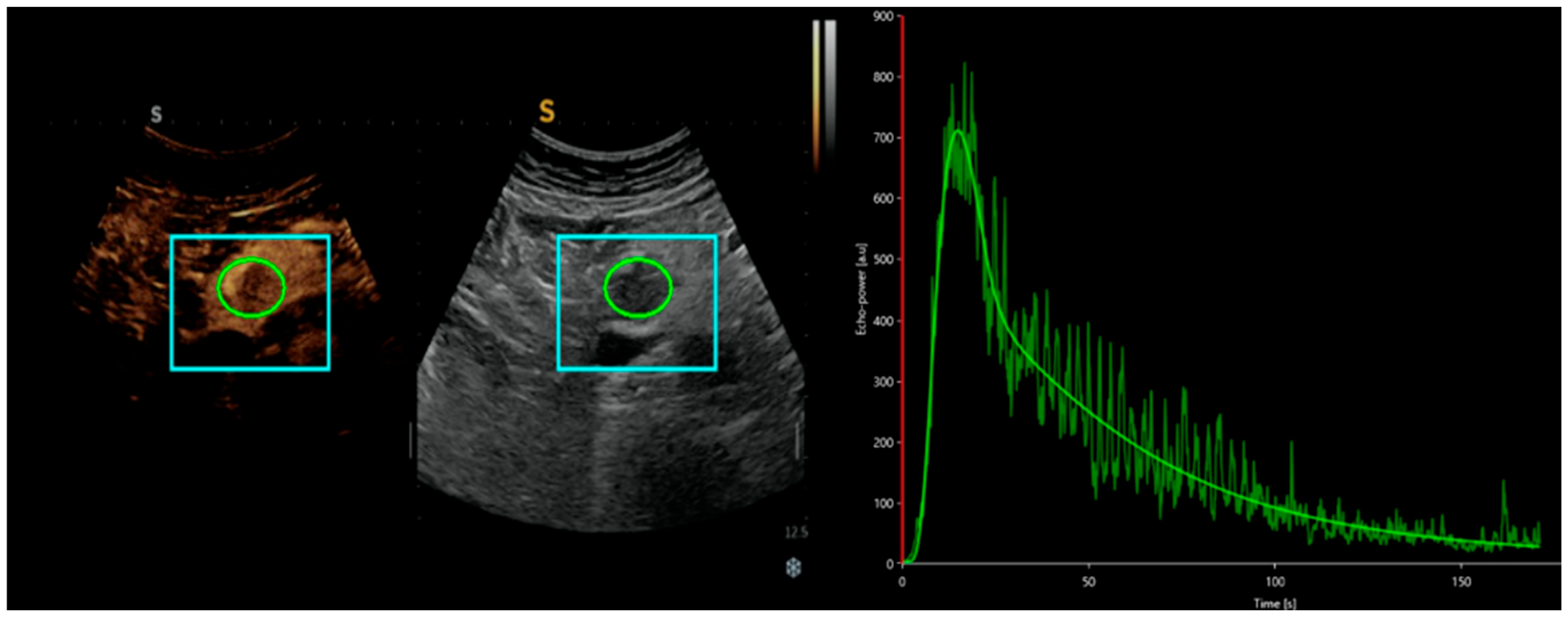
3. Comparison of CEUS and Traditional B-Mode US
3.1. CEUS in the Differential Diagnosis of pNETs and Other Pancreatic Lesions
3.2. CEUS in the Differential Diagnosis of NETs, Liver Metastases, and Other Liver Lesions
3.3. CEUS Characteristics of NETs Associated with Medical Therapies
4. Comparison of Advanced Imaging Techniques: Evaluating the Role of CEUS Versus CE CT Scan, MRI, and PET/CT
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavel, M.; Öberg, K. ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Hofland, J.; Falconi, M. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Ramage, J. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1-G3. J. Neuroendocrinol. 2023, 35, e13306. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Bartsch, D.K. European Neuroendocrine Tumor Society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J. Neuroendocrinol. 2024, 36, e13423. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Pellegrino, F.; Granata, V. Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography 2023, 9, 217–246. [Google Scholar] [CrossRef]
- Chung, Y.E.; Kim, K.W. Contrast-enhanced ultrasonography: Advance and current status in abdominal imaging. Ultrasonography 2015, 34, 3–18. [Google Scholar] [CrossRef]
- Ajmal, S. Contrast-Enhanced Ultrasonography: Review and Applications. Cureus 2021, 13, e18243. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Garcia-Figueiras, R.; Carmona-Bayonas, A.; Sevilla, I.; Teule, A.; Quindos, M.; Grande, E.; Capdevila, J.; Aller, J.; Arbizu, J.; et al. Imaging approaches to assess the therapeutic response of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Current perspectives and future trends of an exciting field in development. Cancer Metastasis Rev. 2015, 34, 823–842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahani, D.V.; Bonaffini, P.A. Gastroenteropancreatic neuroendocrine tumors: Role of imaging in diagnosis and management. Radiology 2013, 266, 38–61. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Blicharz-Dorniak, J. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2017, 68, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, K.; Takushi, Y. Neuroendocrine tumor of the small intestine diagnosed with trans-abdominal ultrasonography: A case report. Int. J. Surg. Case Rep. 2017, 31, 75–78. [Google Scholar] [CrossRef]
- Walczyk, J.; Sowa-Staszczak, A. Diagnostic imaging of gastrointestinal neuroendocrine neoplasms with a focus on ultrasound. J. Ultrason. 2019, 19, 228–235. [Google Scholar] [CrossRef]
- Reddy, Y.; Willert, R.P. Endoscopic ultrasound: What is it and when should it be used? Clin. Med. 2009, 9, 539–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.K. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver 2012, 6, 405–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larghi, A.; Capurso, G.; Carnuccio, A.; Ricci, R.; Alfieri, S.; Galasso, D.; Lugli, F.; Bianchi, A.; Panzuto, F.; De Marinis, L.; et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest. Endosc. 2012, 76, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Ignee, A.; Braden, B.; Barreiros, A.P.; Ott, M.; Hocke, M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin. Gastroenterol. Hepatol. 2008, 6, 590–597.e1. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Larino-Noia, J.; Abdulkader, I.; Forteza, J.; Dominguez-Munoz, J.E. EUS elastography for the characterization of solid pancreatic masses. Gastrointest. Endosc. 2009, 70, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Barthet, M.; Giovannini, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; Koch, S.; Gasmi, M.; Vanbiervliet, G.; Gonzalez, J.M. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy 2019, 51, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Barthet, M.; Giovannini, M.; Gasmi, M.; Lesavre, N.; Boustière, C.; Napoleon, B.; LaQuiere, A.; Koch, S.; Vanbiervliet, G.; Gonzalez, J.M. Long-term outcome after EUS-guided radiofrequency ablation: Prospective results in pancreatic neuroendocrine tumors and pancreatic cystic neoplasms. Endosc. Int. Open. 2021, 9, E1178–E1185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larghi, A.; Rizzatti, G.; Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G. EUS-guided radiofrequency ablation as an alternative to surgery for pancreatic neuroendocrine neoplasms: Who should we treat? Endosc. Ultrasound 2019, 8, 220–226. [Google Scholar] [CrossRef]
- Garg, R.; Mohammed, A.; Singh, A.; Harnegie, M.P.; Rustagi, T.; Stevens, T.; Chahal, P. EUS-guided radiofrequency and ethanol ablation for pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 170–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, D.; Wang, D. Incidental nonfunctioning pancreatic neuroendocrine tumors: Contrast enhanced ultrasound features in diagnosis1. Clin. Hemorheol. Microcirc. 2022, 80, 343–352. [Google Scholar] [CrossRef]
- Serra, C.; Felicani, C. Contrast-enhanced ultrasound in the differential diagnosis of exocrine versus neuroendocrine pancreatic tumors. Pancreas 2013, 42, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Malagò, R.; D’Onofrio, M. Contrast-enhanced sonography of nonfunctioning pancreatic neuroendocrine tumors. AJR 2009, 192, 424–430. [Google Scholar] [CrossRef]
- Dörffel, Y.; Wermke, W. Neuroendocrine tumors: Characterization with contrast-enhanced ultrasonography. Ultraschall Med. 2008, 29, 506–514. [Google Scholar] [CrossRef]
- Bicci, E.; Cozzi, D. Pancreatic neuroendocrine tumours: Spectrum of imaging findings. Gland Surg. 2020, 9, 2215–2224. [Google Scholar] [CrossRef]
- Del Prete, M.; Di Sarno, A. ENETS Centre of Excellence Multidisciplinary Group for Neuroendocrine Tumors in Naples (Italy). Role of contrast-enhanced ultrasound to define prognosis and predict response to biotherapy in pancreatic neuroendocrine tumors. J. Endocrinol. Investig. 2017, 40, 1373–1380. [Google Scholar] [CrossRef]
- Yang, D.H.; Cheng, J. Prediction of Pathological Grades of Pancreatic Neuroendocrine Tumors Based on Dynamic Contrast-Enhanced Ultrasound Quantitative Analysis. Diagnostics 2023, 13, 238. [Google Scholar] [CrossRef]
- Massironi, S.; Conte, D. Contrast-enhanced ultrasonography in evaluating hepatic metastases from neuroendocrine tumours. Dig. Liver Dis. 2010, 42, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Esposto, G.; Santini, P. Dynamic contrast enhanced ultrasound in differential diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2024, 16, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Chammas, M.C.; Bordini, A.L. Contrast-enhanced ultrasonography for the evaluation of malignant focal liver lesions. Ultrasonography 2022, 41, 4–24. [Google Scholar] [CrossRef]
- Ainora, M.E.; Cerrito, L.; Liguori, A.; Mignini, I.; De Luca, A.; Galasso, L.; Garcovich, M.; Riccardi, L.; Ponziani, F.; Santopaolo, F.; et al. Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. Int. J. Mol. Sci. 2023, 24, 8548. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Tana, C.; Caraiani, C.; Dong, Y. Contrast enhanced ultrasound (CEUS) imaging of solid benign focal liver lesions. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- Fang, L.; Huang, B.J.; Ding, H.; Mao, F.; Li, C.L.; Zeng, M.S.; Zhou, J.J.; Chen, Y.; Wang, W.P. Contrast-enhanced ultrasound (CEUS) for the diagnosis of hypoechoic hepatic hemangioma in clinical practice. Clin. Hemorheol. Microcirc. 2019, 72, 395–405. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, P.; Guo, H.; Zhang, N.; Hou, W.J.; Li, X.Y.; Xu, Y. Contrast-enhanced ultrasound for the diagnosis of hepatic adenoma. J. Int. Med. Res. 2011, 3, 920–928. [Google Scholar] [CrossRef]
- Şirli, R.; Sporea, I.; Popescu, A.; Dănilă, M.; Săndulescu, D.L.; Săftoiu, A.; Moga, T.; Spârchez, Z.; Cijevschi, C.; Mihai, C.; et al. Contrast-enhanced ultrasound for the assessment of focal nodular hyperplasia—Results of a multicentre study. Med. Ultrason. 2021, 23, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Stueven, A.K.; Kayser, A. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef]
- Rinke, A.; Krug, S. Neuroendocrine tumours—Medical therapy: Biological. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 79–91. [Google Scholar] [CrossRef]
- Hauser, H.; Gerson, D.S. Systemic Therapies for Metastatic Pancreatic Neuroendocrine Tumors. Curr. Treat Options Oncol. 2019, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Wulfert, S. Contrast-enhanced ultrasound monitoring of perfusion changes in hepatic neuroendocrine metastases after systemic versus selective arterial 177Lu/90Y-DOTATOC and 213Bi-DOTATOC radiopeptide therapy. Exp. Oncol. 2013, 35, 122–126. [Google Scholar] [PubMed]
- Meloni, M.F.; Livraghi, T. Radiofrequency ablation of liver tumors: The role of microbubble ultrasound contrast agents. Ultrasound Q. 2006, 22, 41–47. [Google Scholar] [PubMed]
- Wiggermann, P.; Zuber-Jerger, I. Contrast-enhanced ultrasound improves real-time imaging of ablation region during radiofrequency ablation: Preliminary results. Clin. Hemorheol. Microcirc. 2011, 49, 43–54. [Google Scholar] [CrossRef]
- Roccarina, D.; Garcovich, M. Usefulness of contrast enhanced ultrasound in monitoring therapeutic response after hepatocellular carcinoma treatment. World J. Hepatol. 2015, 7, 1866–1874. [Google Scholar] [CrossRef]
- Jung, E.M.; Ross, C.J. Characterization of microvascularization of liver tumor lesions with high resolution linear ultrasound and contrast enhanced ultrasound (CEUS) during surgery: First results. Clin. Hemorheol. Microcirc. 2010, 46, 89–99. [Google Scholar] [CrossRef]
- Piscaglia, F.; Bolondi, L. The safety of Sonovue in abdominal applications: Retrospective analysis of 23188 investigations. Ultrasound Med. Biol. 2006, 32, 1369–1375. [Google Scholar] [CrossRef]
- Ter Haar, G. Safety and bio-effects of ultrasound contrast agents. Med. Biol. Eng. Comput. 2009, 47, 893–900. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T. Detection of liver metastases from endocrine tumors: A prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef]
- Yu, R.; Wachsman, A. Imaging of neuroendocrine tumors: Indications, interpretations, limits, and pitfalls. Endocrinol. Metab. Clin. N. Am. 2017, 46, 795–814. [Google Scholar] [CrossRef]
- Besa, C.; Ward, S. Neuroendocrine liver metastases: Value of apparent diffusion coefficient and enhancement ratios for characterization of histopathologic grade. J. Magn. Reson. Imaging. 2016, 44, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Ebner, R.; Sheikh, G.T. ESR Essentials: Role of PET/CT in neuroendocrine tumors-practice recommendations by the European Society for Hybrid, Molecular and Translational Imaging. Eur. Radiol. 2025, 35, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Parihar, A.S. Somatostatin Receptor Imaging and theranostics: Current practice and future prospects. J. Nucl. Med. Oct. 2021, 62, 1323–1329. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. Feb. 2010, 1, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Pavlakis, N. Dual Somatostatin Receptor/FDG PET/CT Imaging in metastatic neuroendocrine tumours: Proposal for a Novel Grading Scheme with Prognostic significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Ambrosini, V.; Caplin, M. Use and perceived utility of [(18) F]FDG PET/CT in neuroendocrine neoplasms: A consensus report from the European Neuroendocrine Tumor Society (ENETS) Advisory Board Meeting 2022. J. Neuroendocrinol. 2024, 36, e13359. [Google Scholar] [CrossRef]
- De Mestier, L.; Dromain, C. Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: State of the art. Endocr. Relat. Cancer 2014, 21, R105–R120. [Google Scholar] [CrossRef]
- Westwood, M.; Joore, M. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2013, 17, 1–243. [Google Scholar]
| Location | Margins | Echogenicity | Echotexture | Color Doppler |
|---|---|---|---|---|
| Stomach (Gastric NETs) | Well-defined, sometimes polypoid | Hypoechoic compared to the gastric wall | Homogeneous in well-differentiated NETs, heterogeneous in high-grade NETs | Moderate to high vascularization |
| Small Intestine (Enteric NETs, si-NET) | Well-defined, sometimes with a peritumoral hypoechoic halo | Hypoechoic compared to the intestinal wall | Homogeneous in low-grade NETs, heterogeneous in aggressive NETs | Marked intralesional vascularization |
| Colon-Rectum (Colorectal NETs) | Well-circumscribed, often small (<1 cm) | Hypoechoic or isoechoic | Homogeneous in low-grade NETs, heterogeneous in poorly differentiated NETs | Moderate vascularization |
| Pancreas (Pancreatic NETs, pNETs) | Well-defined, sometimes lobulated | Hypoechoic compared to pancreatic parenchyma | Homogeneous in well-differentiated forms, heterogeneous in high-grade NETs | Increased intralesional vascularization, often intense |
| Type of Lesion | Arterial Phase | Portal/Late Phase | Additional Considerations |
|---|---|---|---|
| Pancreatic Adenocarcinoma | Hypoenhancement (reduced contrast) | Persistent hypoenhancement | Often associated with necrotic areas |
| Pancreatic Neuroendocrine Tumor (pNET) G1/G2 | Hyperenhancement or Isoenhancement | May retain a higher signal than pancreatic parenchyma | Hypervascular lesion; higher TIC parameters (PE, AUC, mTT) |
| Pancreatic Neuroendocrine Tumor (pNET) G3 | Hypoenhancement | Persistent hypoenhancement | Lower TIC parameters (rPE, rAUC) compared to G1/G2 pNETs |
| Pancreatic Neuroendocrine Carcinomas (pNEC) | Hypoenhancement | Persistent hypoenhancement | Lower TIC parameters (rPE, rAUC) compared to G1/G2 pNETs |
| Liver metastases from gastrointestinal NETs | Increased arterial enhancement | Persistent hypervascularization | Pattern similar to HCC, opposite to other liver metastases |
| Lesion Type | Arterial Phase | Portal Phase | Late Phase | Typical Appearance |
|---|---|---|---|---|
| Hepatic Hemangioma | Peripheral nodular enhancement (discontinuous) | Progressive centripetal fill-in | Complete fill-in (isodense with liver) | Well-defined, slow filling from periphery to center |
| Hepatic Adenoma | Hyperenhancing (often heterogeneous) | Becomes iso- or hypoattenuating | No washout (hypodense) | Hypervascular lesion with early enhancement and washout |
| Focal Nodular Hyperplasia (FNH) | Homogeneous intense enhancement, central scar hypodense | Isoattenuating | Central scar enhances (hyperintense) | Spoke-wheel arterial pattern, central scar visible in delayed phase |
| Hepatocellular Carcinoma (HCC) | Arterial hyperenhancement (early, intense) | Washout (hypodense relative to liver) | Late and mild washout | Classic pattern: arterial hyperenhancement + portal/late phase washout |
| Intrahepatic Cholangiocarcinoma (iCC) | Mild peripheral enhancement | Progressive centripetal enhancement | Marked washout | Peripheral rim enhancement with delayed fibrous core uptake |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galasso, L.; Maratta, M.G.; Sardaro, V.; Esposto, G.; Mignini, I.; Borriello, R.; Gasbarrini, A.; Ainora, M.E.; Schinzari, G.; Zocco, M.A. Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs). Cancers 2025, 17, 1879. https://doi.org/10.3390/cancers17111879
Galasso L, Maratta MG, Sardaro V, Esposto G, Mignini I, Borriello R, Gasbarrini A, Ainora ME, Schinzari G, Zocco MA. Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs). Cancers. 2025; 17(11):1879. https://doi.org/10.3390/cancers17111879
Chicago/Turabian StyleGalasso, Linda, Maria Grazia Maratta, Valeria Sardaro, Giorgio Esposto, Irene Mignini, Raffaele Borriello, Antonio Gasbarrini, Maria Elena Ainora, Giovanni Schinzari, and Maria Assunta Zocco. 2025. "Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs)" Cancers 17, no. 11: 1879. https://doi.org/10.3390/cancers17111879
APA StyleGalasso, L., Maratta, M. G., Sardaro, V., Esposto, G., Mignini, I., Borriello, R., Gasbarrini, A., Ainora, M. E., Schinzari, G., & Zocco, M. A. (2025). Role of B-Mode and Contrast-Enhanced Ultrasound in the Diagnostic Workflow of Gastro-Entero-Pancreatic Neuroendocrine Tumors (GEP-NETs). Cancers, 17(11), 1879. https://doi.org/10.3390/cancers17111879







