Breast Cancer Orbital Metastases: Clinical and Histopathological Characteristics, Imaging Features, and Disease-Related Survival in a Multicentric Retrospective Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Descriptive Statistics
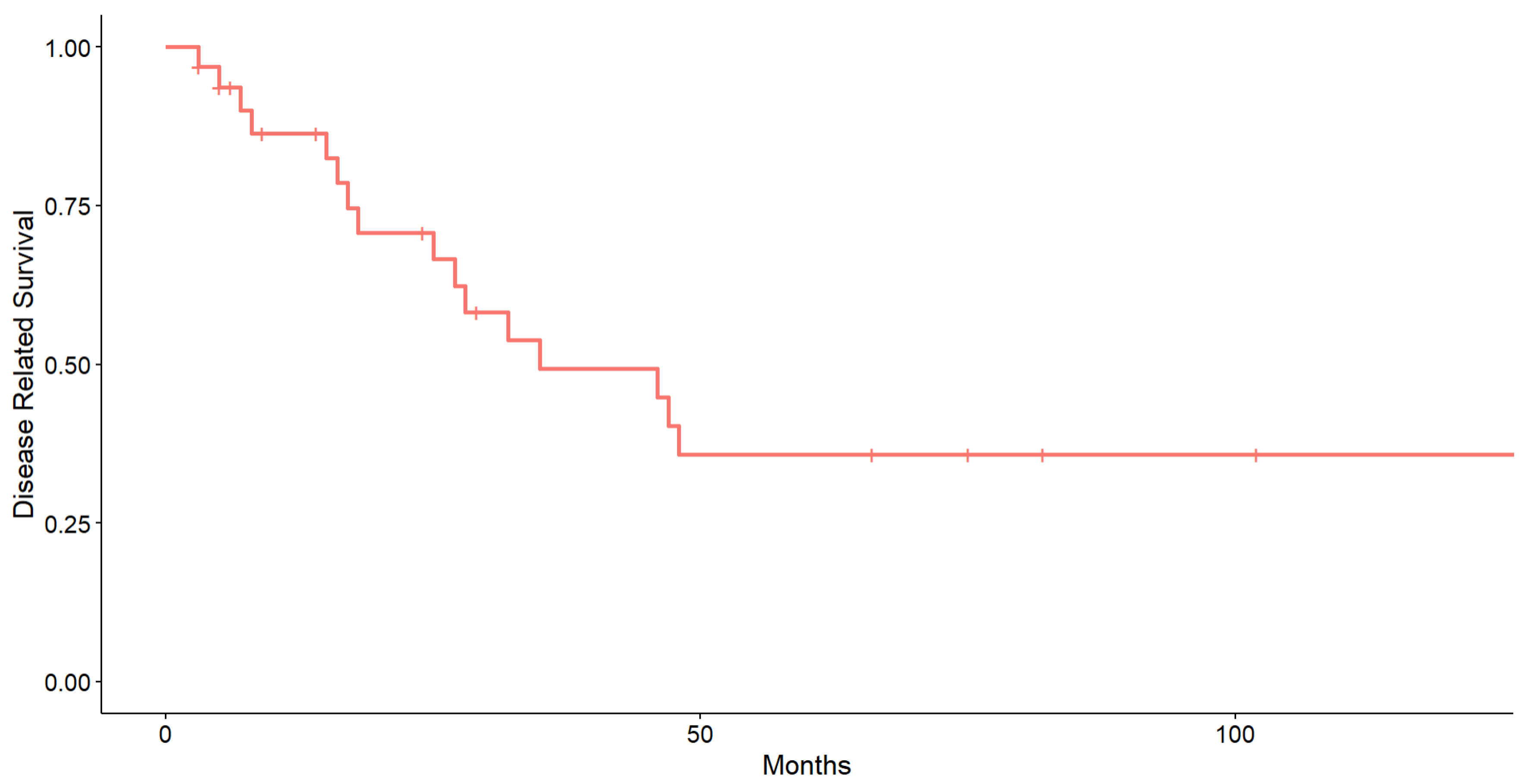
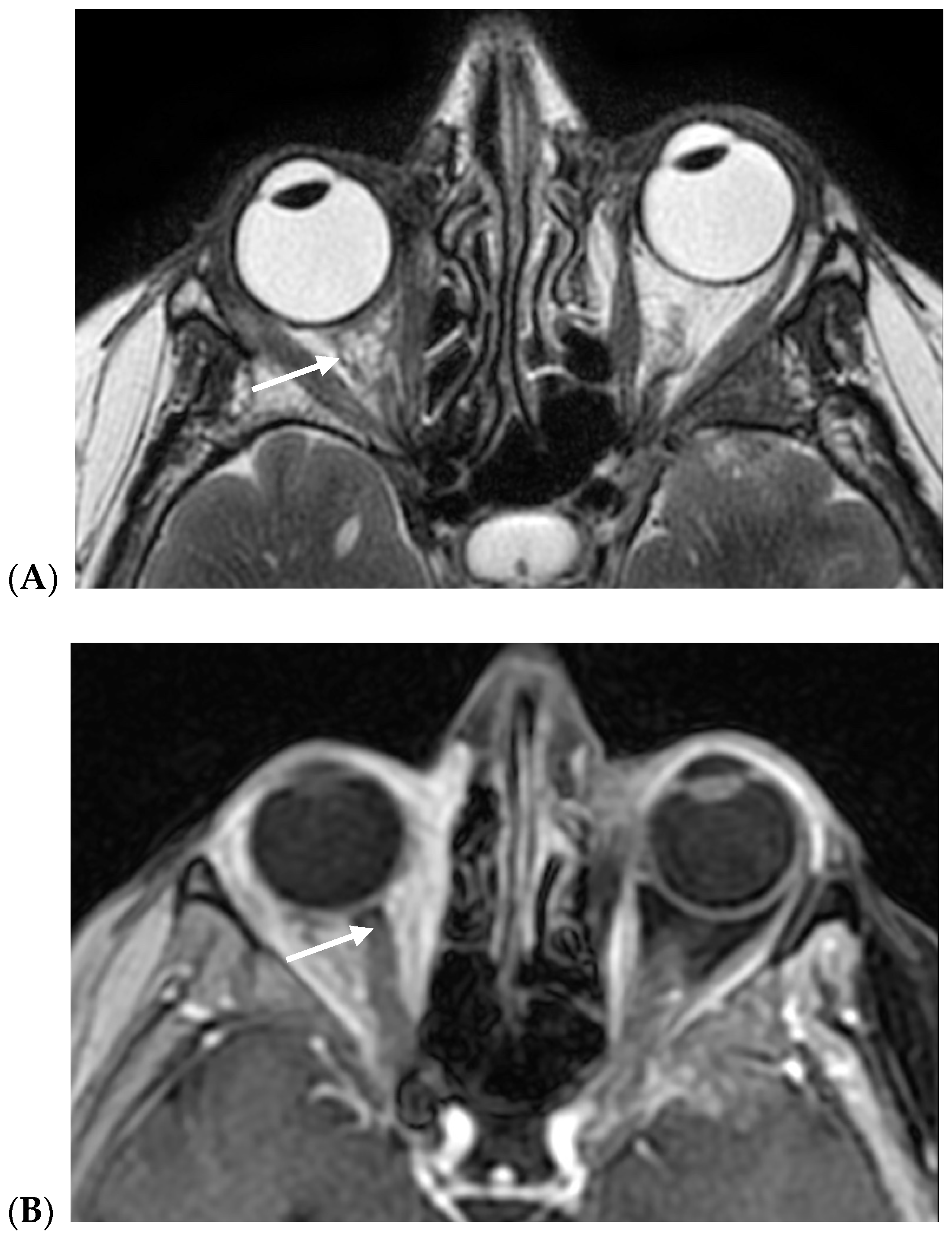
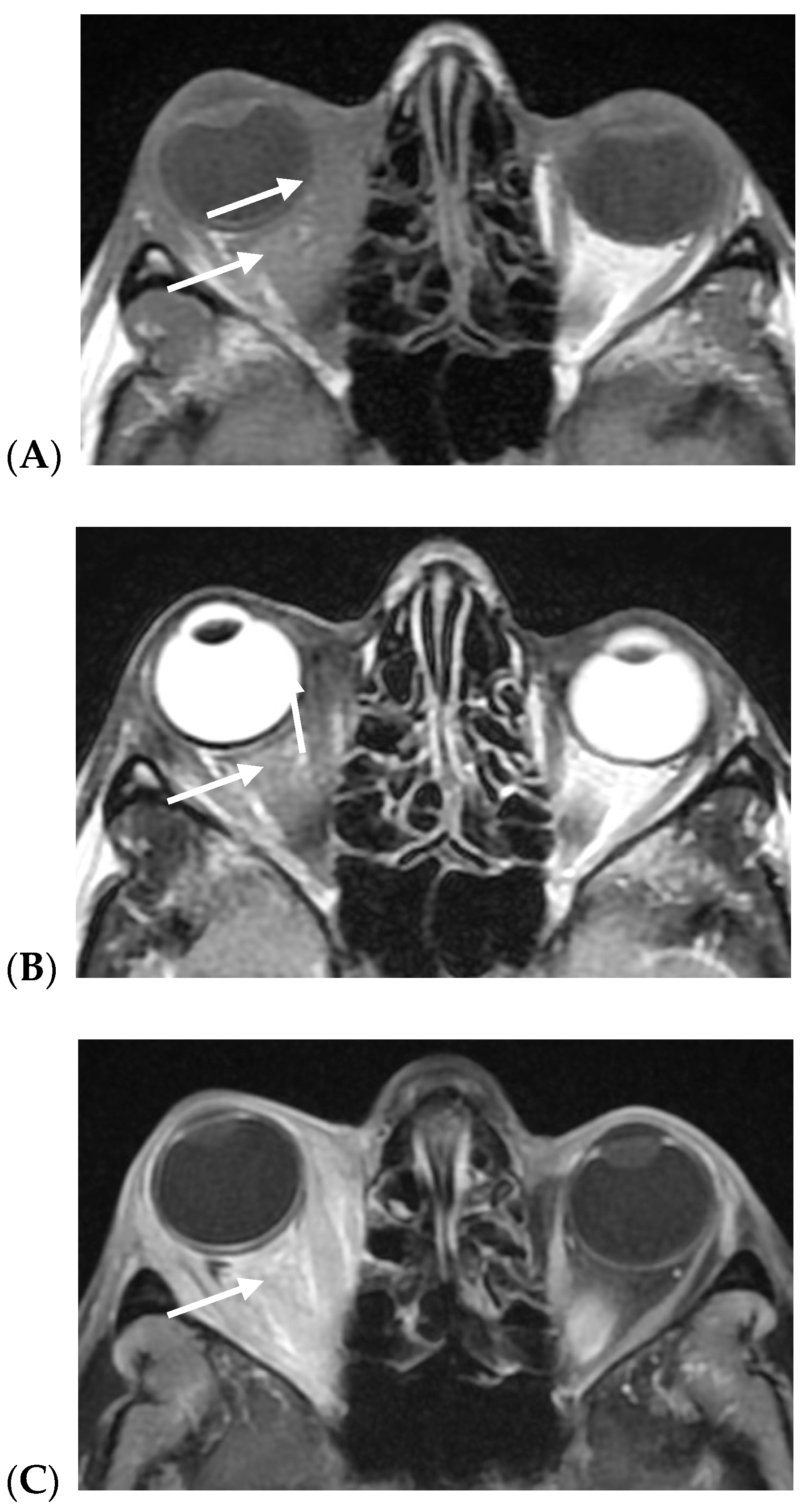
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, R.A.; Rootman, J.; Cline, R.A. Tumors metastatic to the orbit: A changing picture. Surv. Ophthalmol. 1990, 35, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.A.; Archibald, C.W.; Fleming, B.; Ong, L.; O’Donnell, B.; Crampton, J.J.; Selva, D.; McNab, A.A.; Sullivan, T.J. Orbital metastasis: Clinical features, management and outcome. Orbit 2009, 28, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M.; Esmaeli, B. Metastatic tumors of the orbit and ocular adnexa. Curr. Opin. Ophthalmol. 2007, 18, 405–413. [Google Scholar] [CrossRef]
- Oprean, C.M.; Badau, L.M.; Segarceanu, N.A.; Ciocoiu, A.D.; Rivis, I.A.; Vornicu, V.N.; Hoinoiu, T.; Grujic, D.; Bredicean, C.; Dema, A. Unilateral Orbital Metastasis as the Unique Symptom in the Onset of Breast Cancer in a Postmenopausal Woman: Case Report and Review of the Literature. Diagnostics 2021, 11, 725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magliozzi, P.; Strianese, D.; Bonavolont, P.; Ferrara, M.; Ruggiero, P.; Carandente, R.; Bonavolont, G.; Tranfa, F. Orbital metastases in Italy. Int. J. Ophthalmol. 2015, 8, 1018. [Google Scholar] [CrossRef][Green Version]
- Muhd, H.; Zuhaimy, H.; Ismail, M.F.; Arshad, F.; Azmi, S.; Sahak, N.H. Orbital Metastasis as The Initial Presentation of Breast Cancer. Malays. Fam. Physician 2020, 15, 74–78. [Google Scholar] [PubMed] [PubMed Central]
- Grajales-Alvarez, R.; Gutierrez-Mata, A. Orbital metastases from breast cancer: A retrospective analysis of 28 cases. Cancer Treat. Res. Commun. 2020, 24, 100184. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Voutsadakis, I.A.; Papandreou, C.N. Orbital metastasis of breast carcinoma. Breast Cancer 2009, 3, 91–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakobiec, F.A.; Stagner, A.M.; Homer, N.; Yoon, M.K. Periocular Breast Carcinoma Metastases: Predominant Origin from the Lobular Variant. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; Grubb, C.S.; Hwang, M.E.; Tai, C.H.; Wu, C.C.; Jani, A.; Lapa, M.E.; Andrews, J.I.S.; Vanderkelen, S.; Isaacson, S.R.; et al. Breast cancer subtype and stage are prognostic of time from breast cancer diagnosis to brain metastasis development. J. Neurooncol. 2017, 134, 453–463. [Google Scholar] [CrossRef]
- Nakamura, J.; Okuyama, K.; Sato, H.; Yoda, Y.; Kai, K.; Noshiro, H. Repeated changes of the molecular subtype in gastric metastasis from breast cancer: A case report. Mol. Clin. Oncol. 2016, 4, 695–698. [Google Scholar] [CrossRef][Green Version]
- Khan, S.N.; Sepahdari, A.R. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J. Ophthalmol. 2012, 26, 373–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stålhammar, G.; Grossniklaus, H.E. Overrepresentation of human epidermal growth factor receptor 2 positive- and Luminal B breast cancer metastases in the eyes and orbit. Eye 2023, 37, 2499–2504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, J.H.; Karmakar, R.; Mukundan, A.; Thongsit, P.; Chen, M.M.; Chang, W.Y.; Wang, H.C. Systematic meta-analysis of computer-aided detection of breast cancer using hyperspectral imaging. Bioengineering 2024, 11, 1060. [Google Scholar] [CrossRef]
- Savino, G.; Piccinni, F.; Pagliara, M.M.; Sammarco, M.G.; Caputo, C.G.; Moro, A.; Barbera, G.; Tagliaferri, L.; Fionda, B.; Schinzari, G.; et al. Multidisciplinary ocular and periocular cancers meetings: Implementation in a tertiary referral center and analysis over a 12-months period. BMC Ophthalmol. 2022, 22, 497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lancellotta, V.; Guinot, J.L.; Fionda, B.; Rembielak, A.; Di Stefani, A.; Gentileschi, S.; Federico, F.; Rossi, E.; Guix, B.; Chyrek, A.J.; et al. SKIN-COBRA (Consortium for Brachytherapy data Analysis) ontology: The first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer. J. Contemp. Brachytherapy 2020, 12, 105–110. [Google Scholar] [CrossRef]
- Toller, K.K.; Gigantelli, J.W.; Spalding, M.J. Bilateral orbital metastases from breast carcinoma. A case of false pseudotumor. Ophthalmology 1998, 105, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Rakul Nambiar, K.; Ajith, P.S.; Arjunan, A. Unilateral proptosis as the initial manifestation of malignancy. J. Egypt. Natl. Cancer Inst. 2017, 29, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Blohmer, M.; Zhu, L.; Atkinson, J.M.; Beriwal, S.; Rodríguez-López, J.L.; Rosenzweig, M.; Brufsky, A.M.; Tseng, G.; Lucas, P.C.; Lee, A.V.; et al. Patient treatment and outcome after breast cancer orbital and periorbital metastases: A comprehensive case series including analysis of lobular versus ductal tumor histology. Breast Cancer Res. 2020, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Raap, M.; Antonopoulos, W.; Dämmrich, M.; Christgen, H.; Steinmann, D.; Länger, F.; Lehmann, U.; Kreipe, H.; Christgen, M. High frequency of lobular breast cancer in distant metastases to the orbit. Cancer Med. 2014, 4, 104–111. [Google Scholar] [CrossRef]
- Ogueta, S.B.; Schwartz, S.D.; Yamashita, C.K.; Farber, D.B. Estrogen receptor in the human eye: Influence of gender and age on gene expression. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1906–1911. [Google Scholar]
- Colitz, C.M.; Lu, P.; Sugimoto, Y.; Barden, C.A.; Chandler, H.L. Estradiol biosynthesis in canine lens epithelial cells. Curr. Eye Res. 2015, 40, 541–548. [Google Scholar] [CrossRef]
- Garrity, J.A.; Henderson, J.W.; Cameron, J.D. Metastatic Carcinomas. In Henderson’s Orbital Tumors, 4th ed; Raven Press: New York, NY, USA, 2007; pp. 313–326. [Google Scholar]
- Mohadjer, Y.; Holds, J.B. Orbital metastasis as the initial finding of breast carcinoma: A ten-year survival. Ophthalmic Plast. Reconstr. Surg. 2005, 21, 65–66. [Google Scholar] [CrossRef]
- Van Laere, S.J.; Van den Eynden, G.G.; Van der Auwera, I.; Vandenberghe, M.; van Dam, P.; Van Marck, E.A.; van Golen, K.L.; Vermeulen, P.B.; Dirix, L.Y. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res. Treat. 2006, 95, 243–255. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef]
- Guarneri, V.; Giovannelli, S.; Ficarra, G.; Bettelli, S.; Maiorana, A.; Piacentini, F.; Barbieri, E.; Dieci, M.V.; D’Amico, R.; Jovic, G.; et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: Impact on patient management. Oncologist 2008, 13, 838–844. [Google Scholar] [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, J.; Li, S.; Lv, M.; Shen, Y.; Wang, B.; Li, P.; Yi, M.; Zhao, X.; Zhang, L.; et al. Invasive lobular carcinoma of the breast: A special histological type compared with invasive ductal carcinoma. PLoS ONE 2017, 12, e0182397. [Google Scholar] [CrossRef]
- Razem, B.; Slimani, F. An early orbital metastasis from breast cancer: A case report. Int. J. Surg. Case Rep. 2021, 78, 300–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reifler, D.M. Orbital metastasis with enophthalmos: A review of the literature. Henry Ford Hosp. Med. J. 1985, 33, 171–179. [Google Scholar] [PubMed]
- Gonçalves, A.C.; Moura, F.C.; Monteiro, M.L. Bilateral progressive enophthalmos as the presenting sign of metastatic breast carcinoma. Ophthalmic Plast. Reconstr. Surg. 2005, 21, 311–313. [Google Scholar] [CrossRef] [PubMed]
- El-Khazen Dupuis, J.; Marchand, M.; Javidi, S.; Nguyen, T.Q.T. Enophthalmos as the Initial Systemic Finding of Undiagnosed Metastatic Breast Carcinoma. Int. Med. Case Rep. J. 2021, 14, 25–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, C.; Strianese, D.; Perrotta, M.; Iuliano, A.; Bernardo, R.; Romeo, V.; Ugga, L.; Brunetti, L.; Tranfa, F.; Elefante, A. Multi-parametric magnetic resonance imaging characterization of orbital lesions: A triple blind study. Semin. Ophthalmol. 2020, 35, 95–102. [Google Scholar] [CrossRef]
- Lanni, V.; Iuliano, A.; Fossataro, F.; Russo, C.; Uccello, G.; Tranfa, F.; Strianese, D.; Vallone, G. The role of ultrasonography in differential diagnosis of orbital lesions. J. Ultrasound 2021, 24, 35–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strianese, D.; Iuliano, A.; Quaranta Leoni, F.M. Secondary Orbital Tumours and Orbital Metastases. In Oculoplastic, Lacrimal and Orbital Surgery; Quaranta Leoni, F.M., Verity, D.H., Paridaens, D., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]

| Overall Population (N = 32) | ||
|---|---|---|
| Age (years) | 62.50 (74.50–57.50) | |
| Eye | RE | 14.00 (43.75%, 95% CI: 28.12–61.91%) |
| LE | 17.00 (53.12%, 95% CI: 37.50–71.29%) | |
| Bilateral | 1.00 (3.12%, 95% CI: 0.00–21.29%) | |
| Age at onset of primitive breast cancer (years) | 55.50 (61.25–45.00) | |
| Breast cancer histotype | Lobular | 23.00 (71.88%, 95% CI: 59.37–88.35%) |
| Mixed | 1.00 (3.12%, 95% CI: 0.00–19.60%) | |
| Ductal | 8.00 (25.00%, 95% CI: 12.50–41.48%) | |
| Orbital metastasis latency (months) | 39.50 (134.00–10.25) | |
| Mortality rate | Alive | 16.00 (50.00%) |
| Dead | 16.00 (50.00%) | |
| Gender | Eye | Primitive Breast Cancer Histotype | Primitive Breast Cancer Age (Years) | Orbital Metastasis Hystotype (Breast Biopsy) | Latency Onset Months | Exopthalmos/ | Mortality | Disease-Related Survival (Months) | Other Clinical Presentations | |
|---|---|---|---|---|---|---|---|---|---|---|
| Enopthalmos | ||||||||||
| Case 1 | F | RE | Lobular | 49 | Lobular | 5 | Exophthalmos | Dead | 28 | |
| Case 2 | F | Bilateral | Lobular | 65 | Lobular | 142 | Exophthalmos | Dead | 18 | Diplopia, eyelid edema, ophthalmoplegia |
| Case 3 | F | RE | Mixed | 67.5 | Lobular | 5 | Enophthalmos | Alive | 29 | Ptosis, ophthalmoplegia |
| Case 4 | F | RE | Lobular | 49 | Lobular | 10 | Exophthalmos | Dead | 25 | Complete ophthalmoplegia |
| Case 5 | F | RE | Lobular | 53 | Lobular | n/a | Enophthalmos | Alive | 9 | |
| Case 6 | F | RE | Lobular | 77 | Lobular | 5 | Exophthalmos | Dead | 32 | |
| Case 7 | F | RE | Lobular | 66.5 | Lobular | 5 | Exophthalmos | Dead | 16 | |
| Case 8 | F | RE | Lobular | 60 | Lobular | 11 | Exophthalmos | Alive | 14 | Periorbital edema |
| Case 9 | F | LE | Lobular | 60 | Lobular | 9 | Exophthalmos | Dead | 17 | Funnel retinal detachment with multiple choroidal lesions |
| Case 10 | F | LE | Lobular | 59 | Lobular | 53 | Enophthalmos | Alive | 3 | Ptosis |
| Case 11 | F | RE | Lobular | 56 | Lobular | 348 | Enophthalmos | Alive | 6 | |
| Case 12 | F | LE | Ductal | 62 | Lobular | n/a | Exophthalmos | Alive | 6 | |
| Case 13 | F | RE | Lobular | 40 | Lobular | 192 | Enophthalmos | Dead | 8 | Enlarged right medial rectus |
| Case 14 | F | LE | Lobular | 45 | Lobular | 138 | Exophthalmos | Dead | 15 | UE hard swelling |
| Case 15 | F | LE | Lobular | 48 | Lobular | 220 | Exophthalmos | Alive | 24 | Ptosis, complete ophthalmoplegia |
| Case 16 | F | RE | Ductal | 78 | Ductal | 76 | Exophthalmos | Dead | 47 | Exotropia |
| Case 17 | F | LE | Ductal | 44 | Ductal | 154 | Exophthalmos | Alive | 66 | Ptosis, diplopia, UE hard swelling |
| Case 18 | F | LE | Ductal | 55 | Ductal | 26 | Exophthalmos | Alive | 146 | - |
| Case 19 | F | LE | Lobular | 32 | Lobular | 106 | Exophthalmos | Alive | 102 | Ptosis, diplopia |
| Case 20 | F | LE | Lobular | 75 | Lobular | 33 | Exophthalmos | Dead | 27 | Complete ophthalmoplegia |
| Case 21 | F | RE | Lobular | 61 | Lobular | 19 | Exophthalmos | Alive | 165 | - |
| Case 22 | F | LE | Ductal | 35 | Ductal | 14 | Exophthalmos | Dead | 5 | Diplopia |
| Case 23 | F | LE | Lobular | 56 | Lobular | 25 | Exophthalmos | Alive | 195 | Ptosis |
| Case 24 | F | RE | Ductal | 35 | Ductal | 166 | Exophthalmos | Dead | 7 | Ptosis, diplopia, UE swelling |
| Case 25 | F | LE | Lobular | 45 | Lobular | 37 | Exophthalmos | Alive | 75 | Ptosis |
| Case 26 | F | LE | Ductal | 53 | Ductal | 94 | Exophthalmos | Dead | 35 | Diplopia |
| Case 27 | F | LE | Ductal | 35 | Ductal | 93 | Exophthalmos | Alive | 211 | Ptosis, diplopia |
| Case 28 | F | LE | Lobular | 42 | Lobular | n\a | Exophthalmos | Dead | 3 | Diplopia, complete ophthalmoplegia |
| Case 29 | F | RE | Lobular | 58 | Lobular | 324 | Exophthalmos | Alive | 5 | Ptosis, exo-ipotropia |
| Case 30 | F | LE | Lobular | 48 | Lobular | 122 | Exophthalmos | Dead | 46 | Hard swelling UE |
| Case 31 | F | RE | Lobular | 77 | Lobular | 1 | Exophthalmos | Dead | 48 | Complete ophthalmoplegia |
| Case 32 | F | LE | Lobular | 60 | Lobular | 42 | Exophthalmos | Alive | 82 | Ptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peschiaroli, S.; Iuliano, A.; Cuffaro, G.; Quaranta Leoni, F.M.; Tartaglione, T.; Pagliara, M.M.; Sammarco, M.G.; Caputo, C.G.; Santoro, A.; Barchitta, M.; et al. Breast Cancer Orbital Metastases: Clinical and Histopathological Characteristics, Imaging Features, and Disease-Related Survival in a Multicentric Retrospective Case Series. Cancers 2025, 17, 1875. https://doi.org/10.3390/cancers17111875
Peschiaroli S, Iuliano A, Cuffaro G, Quaranta Leoni FM, Tartaglione T, Pagliara MM, Sammarco MG, Caputo CG, Santoro A, Barchitta M, et al. Breast Cancer Orbital Metastases: Clinical and Histopathological Characteristics, Imaging Features, and Disease-Related Survival in a Multicentric Retrospective Case Series. Cancers. 2025; 17(11):1875. https://doi.org/10.3390/cancers17111875
Chicago/Turabian StylePeschiaroli, Sofia, Adriana Iuliano, Giovanni Cuffaro, Francesco M. Quaranta Leoni, Tommaso Tartaglione, Monica Maria Pagliara, Maria Grazia Sammarco, Carmela Grazia Caputo, Angela Santoro, Matteo Barchitta, and et al. 2025. "Breast Cancer Orbital Metastases: Clinical and Histopathological Characteristics, Imaging Features, and Disease-Related Survival in a Multicentric Retrospective Case Series" Cancers 17, no. 11: 1875. https://doi.org/10.3390/cancers17111875
APA StylePeschiaroli, S., Iuliano, A., Cuffaro, G., Quaranta Leoni, F. M., Tartaglione, T., Pagliara, M. M., Sammarco, M. G., Caputo, C. G., Santoro, A., Barchitta, M., Lanni, V., Strianese, D., & Savino, G. (2025). Breast Cancer Orbital Metastases: Clinical and Histopathological Characteristics, Imaging Features, and Disease-Related Survival in a Multicentric Retrospective Case Series. Cancers, 17(11), 1875. https://doi.org/10.3390/cancers17111875







