Surgery for T1/2N0 Oropharyngeal Carcinoma Is a Better Treatment Option than Radiotherapy—A Long-Term Follow-Up Study from a Single Japanese High-Volume Cancer Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluation
- Anterior wall tumors: intrinsic/extrinsic tongue musculature
- Lateral wall tumors: pharyngeal constrictor muscles or prevertebral fascia
- Superior wall tumors: soft palate musculature or palatal aponeurosis
- Posterior wall tumors: posterior pharyngeal wall musculature or prevertebral fascia
2.3. Treatment
2.4. Follow-Up
2.5. Statistical Analysis
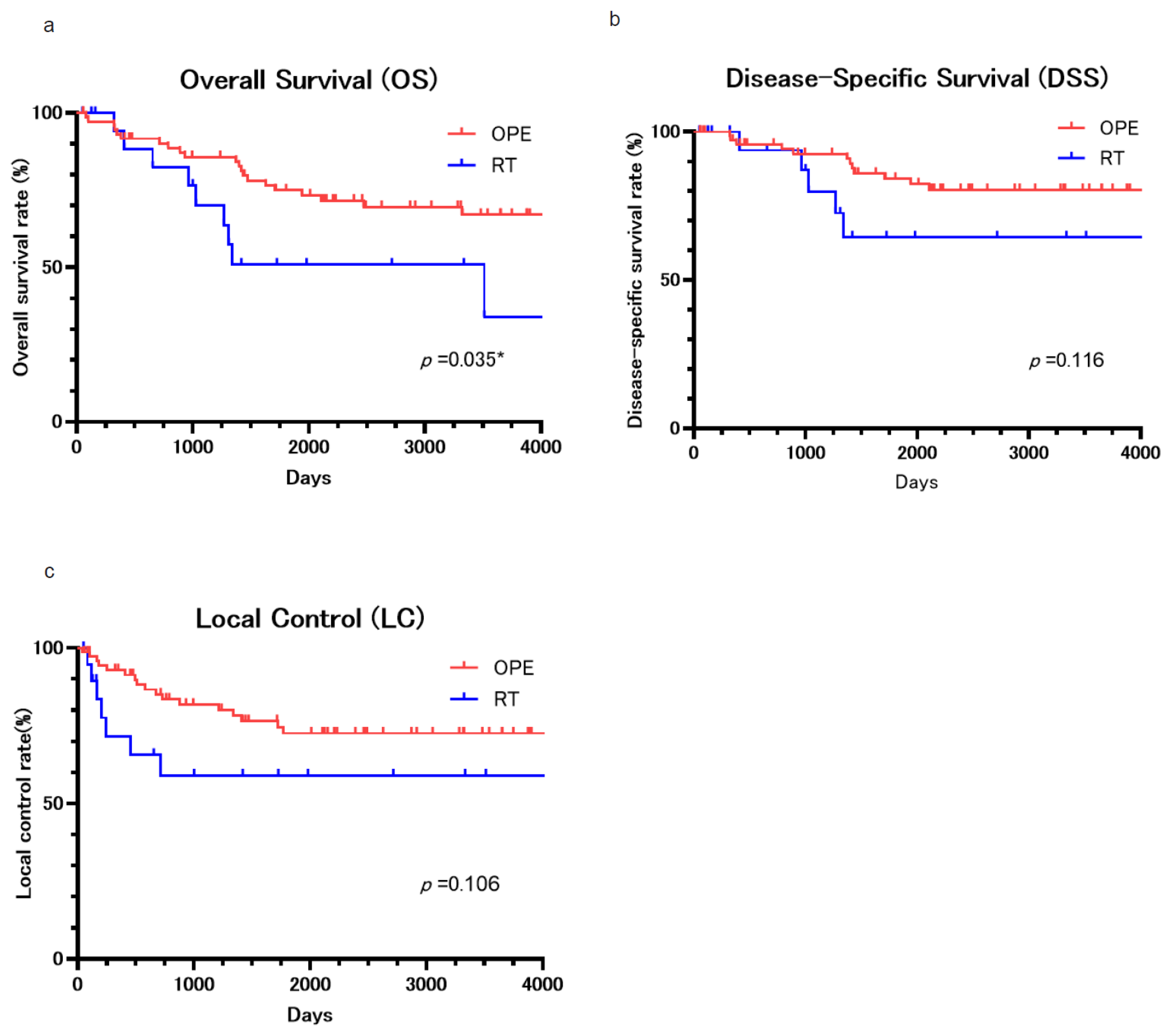
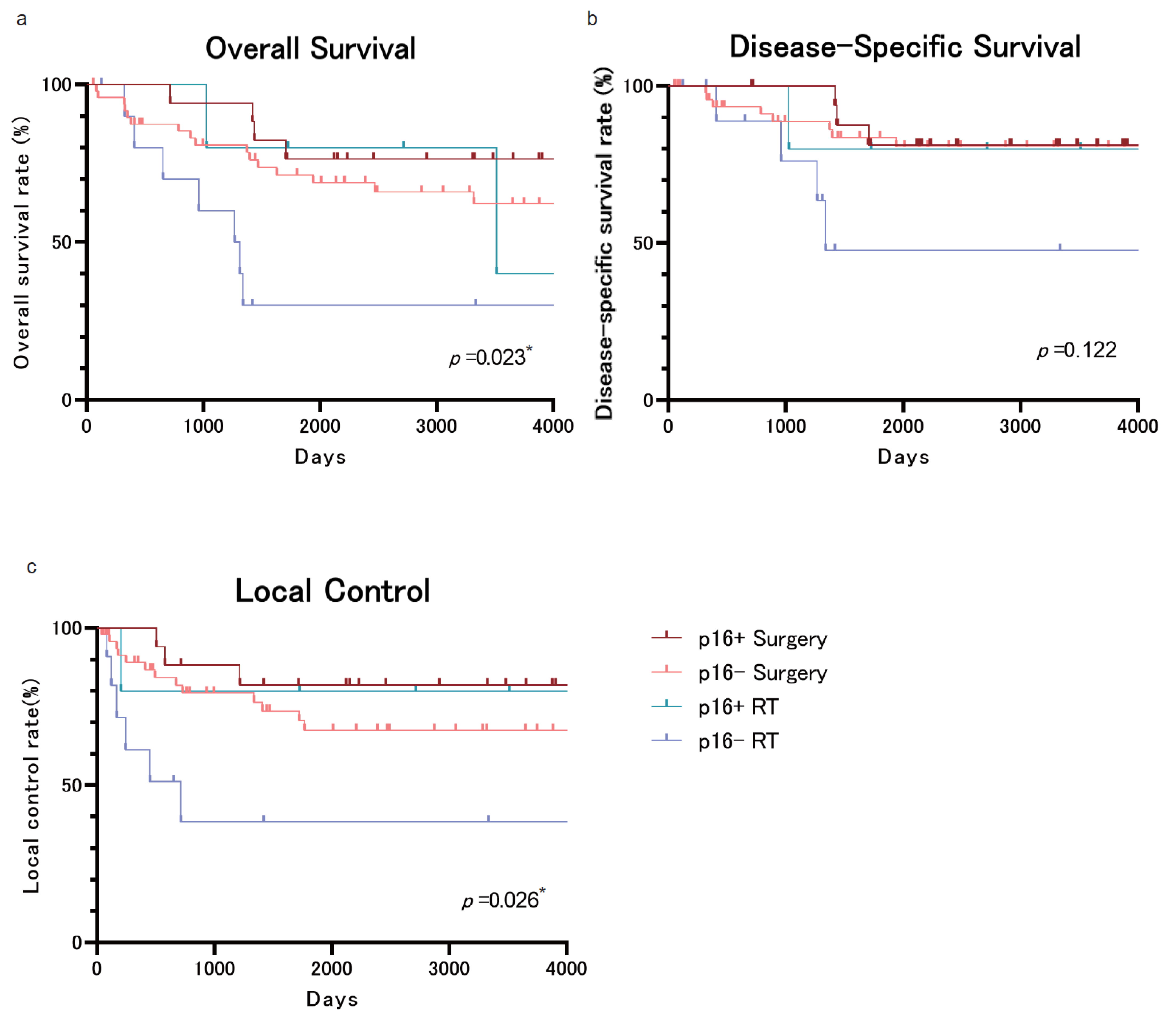
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Scott-Wittenborn, N.; D’Souza, G.; Tewari, S.; Rooper, L.; Troy, T.; Drake, V.; Bigelow, E.O.; Windon, M.J.; Ryan, W.R.; Ha, P.K.; et al. Prevalence of human papillomavirus in head and neck cancers at tertiary care centers in the United States over time. Cancer 2022, 128, 1767–1774. [Google Scholar] [CrossRef]
- Fakhry, C.; Krapcho, M.; Eisele, D.W.; D’Souza, G. Head and neck squamous cell cancers in the United States are rare and the risk now is higher among white individuals compared with black individuals. Cancer 2018, 124, 2125–2133. [Google Scholar] [CrossRef]
- Zhang, Y.; Fakhry, C.; D’Souza, G. Projected Association of Human Papillomavirus Vaccination with Oropharynx Cancer Incidence in the US, 2020–2045. JAMA Oncol. 2021, 7, e212907. [Google Scholar] [CrossRef]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the incidence of head and neck cancer by subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Duray, A.; Descamps, G.; Decaestecker, C.; Remmelink, M.; Sirtaine, N.; Lechien, J.; Ernoux-Neufcoeur, P.; Bletard, N.; Somja, J.; Depuydt, C.E.; et al. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope 2012, 122, 1558–1565. [Google Scholar] [CrossRef]
- Garnaes, E.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborg-Barnkob, B.; Hoegdal, E.; Lajer, C.B.; Andersen, E.; Specht, L.; et al. Increasing incidence of base of tongue cancers from 2000 to 2010 due to HPV: The largest demographic study of 210 Danish patients. Br. J. Cancer 2015, 113, 131–134. [Google Scholar] [CrossRef]
- Garnaes, E.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborg-Barnkob, B.; Hoegdall, E.; Krenk, L.; Josiassen, M.; Lajer, C.B.; et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: The largest registry-based study to date. Int. J. Cancer 2015, 136, 2196–2203. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Brizel, D.M. Different strokes for different folks: New paradigms for staging oropharynx cancer. J. Clin. Oncol. 2015, 33, 817–818. [Google Scholar] [CrossRef]
- Huang, S.H.; Xu, W.; Waldron, J.; Siu, L.; Shen, X.; Tong, L.; Ringash, J.; Bayley, A.; Kim, J.; Hope, A.; et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 2015, 33, 836–845. [Google Scholar] [CrossRef]
- Molteni, G.; Bassani, S.; Arsie, A.E.; Zampieri, E.; Mannelli, G.; Orlandi, E.; Bossi, P.; Virgilio, A.D. Role of TORS as De-Escalation Strategy in HPV-Related Oropharyngeal Cancer, What We Need to Know. Healthcare 2024, 12, 1014. [Google Scholar] [CrossRef]
- Parsons, J.T.; Mendenhall, W.M.; Stringer, S.P.; Amdur, R.J.; Hinerman, R.W.; Villaret, D.B.; Moore-Higgs, G.J.; Greene, B.D.; Speer, T.W.; Cassisi, N.J.; et al. Squamous cell carcinoma of the oropharynx: Surgery, radiation therapy, or both. Cancer 2002, 94, 2967–2980. [Google Scholar] [CrossRef]
- Hicks, W.L., Jr.; Kuriakose, M.A.; Loree, T.R.; Orner, J.B.; Schwartz, G.; Mullins, A.; Donaldson, C.; Winston, J.M.; Bakamjian, V.Y. Surgery versus radiation therapy as single-modality treatment of tonsillar fossa carcinoma: The Roswell Park Cancer Institute experience (1971–1991). Laryngoscope 1998, 108, 1014–1019. [Google Scholar] [CrossRef]
- Cosmidis, A.; Rame, J.P.; Dassonville, O.; Temam, S.; Massip, F.; Poissonnet, G.; Poupart, M.; Marandas, P.; Raucourt, D.D. T1-T2 NO oropharyngeal cancers treated with surgery alone. A GETTEC study. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2004, 261, 276–281. [Google Scholar]
- Dabas, S.; Gupta, K.; Ranjan, R.; Sharma, A.K.; Shukla, H.; Dinesh, A. Oncological outcome following de-intensification of treatment for stage I and II HPV negative oropharyngeal cancers with transoral robotic surgery (TORS): A prospective trial. Oral Oncol. 2017, 69, 80–83. [Google Scholar] [CrossRef]
- Laccourreye, O.; Castelnau-Marchand, P.; Rubin, F.; Badoual, C.; Halimi, P.; Giraud, P. The keys to conservative treatment of early-stage squamous cell carcinoma of the tonsillar region. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Amdur, R.J.; Stringer, S.P.; Villaret, D.B.; Cassisi, N.J. Radiation therapy for squamous cell carcinoma of the tonsillar region: A preferred alternative to surgery? J. Clin. Oncol. 2000, 18, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Hinerman, R.W.; Malyapa, R.S.; Werning, J.W.; Lansford, C.D.; Villaret, D.B. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am. J. Clin. Oncol. 2006, 29, 290–297. [Google Scholar] [CrossRef]
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Peterson, D.E.; Doerr, W.; Hovan, A.; Pinto, A.; Saunders, D.; Elting, L.S.; Spijkervet, F.K.L.; Brennan, M.T. Osteoradionecrosis in cancer patients: The evidence base for treatment-dependent frequency, current management strategies, and future studies. Support. Care Cancer 2010, 18, 1089–1098. [Google Scholar] [CrossRef]
- Tan, W.; Bui, R.; Ranasinghe, V.J.; Coblens, O.; Shabani, S. Transoral Robotic Surgery for Oropharyngeal and Hypopharyngeal Squamous Cell Carcinoma. Cureus 2024, 16, e57186. [Google Scholar] [CrossRef]
- Howard, J.; Masterson, L.; Dwivedi, R.C.; Riffat, F.; Benson, R.; Jefferies, S.; Jani, P.; Tysome, J.R.; Nutting, C. Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma. Cochrane Database Syst. Rev. 2016, 12, Cd010963. [Google Scholar] [CrossRef]
- Galati, L.T.; Myers, E.N.; Johnson, J.T. Primary surgery as treatment for early squamous cell carcinoma of the tonsil. Head Neck 2000, 22, 294–296. [Google Scholar] [CrossRef]
- Regueiro, C.A.; Aragón, G.; Millán, I.; Valcárcel, F.J.; de la Torre, A.; Magallón, R. Prognostic factors for local control, regional control and survival in oropharyngeal squamous cell carcinoma. Eur. J. Cancer 1994, 30, 2060–2067. [Google Scholar] [CrossRef]
- Johansen, L.V.; Grau, C.; Overgaard, J. Squamous cell carcinoma of the oropharynx—An analysis of treatment results in 289 consecutive patients. Acta. Oncol. 2000, 39, 985–994. [Google Scholar]
- Perez, C.A.; Carmichael, T.; Devineni, V.R.; Simpson, J.R.; Frederickson, J.; Sessions, D.; Spector, G.; Finberg, B. Carcinoma of the tonsillar fossa: A nonrandomized comparison of irradiation alone or combined with surgery: Long-term results. Head Neck 1991, 13, 282–290. [Google Scholar] [CrossRef]
- Lusinchi, A.; Wibault, P.; Marandas, P.; Kunkler, I.; Eschwege, F. Exclusive radiation therapy: The treatment of early tonsillar tumors. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 273–277. [Google Scholar] [CrossRef]
- Holliday, M.A.; Tavaluc, R.; Zhuang, T.; Wang, H.; Davidson, B. Oncologic benefit of tonsillectomy in stage I and II tonsil cancer: A surveillance epidemiology and end results database review. JAMA Otolaryngol. Head Neck Surgj. 2013, 139, 362–366. [Google Scholar] [CrossRef]
- Hughes, R.T.; Levine, B.J.; May, N.; Shenker, R.F.; Yang, J.H.; Lanier, C.M.; Frizzell, B.A.; Greven, K.M.; Waltonen, J.D. Survival and Swallowing Function after Primary Radiotherapy versus Transoral Robotic Surgery for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 2023, 85, 284–293. [Google Scholar] [CrossRef]
- Wetzels, J.W.; Merkx, M.A.; de Haan, A.F.; Koole, R.; Speksnijder, C.M. Maximum mouth opening and trismus in 143 patients treated for oral cancer: A 1-year prospective study. Head Neck 2014, 36, 1754–1762. [Google Scholar] [CrossRef]
- Teguh, D.N.; Levendag, P.C.; Voet, P.; van der Est, H.; Noever, I.; de Kruijf, W.; Rooij, P.V.; Schmitz, P.I.M.; Haeijmen, B.J. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck 2008, 30, 622–630. [Google Scholar] [CrossRef]
- Loh, S.Y.; McLeod, R.W.J.; Elhassan, H.A. Trismus following different treatment modalities for head and neck cancer: A systematic review of subjective measures. Eur. Arch. Otorhinolaryngol. 2017, 274, 2695–2707. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lim, J.; Jung, K.W.; Ryu, J.; Won, Y.J. Metachronous Second Primary Malignancies after Head and Neck Cancer in a Korean Cohort (1993–2010). PLoS ONE 2015, 10, e0134160. [Google Scholar] [CrossRef]
- Hosokawa, S.; Takahashi, G.; Okamura, J.; Imai, A.; Mochizuki, D.; Takizawa, Y.; Yamatodani, T.; Misawa, K.; Mineta, H. Risk and prognostic factors for multiple primary carcinomas in patients with head and neck cancer. Jpn. J. Clin. Oncol. 2018, 48, 124–129. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—Systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
| Treatment | ||||
|---|---|---|---|---|
| Surgery (N = 74) | RT (N = 20) | Total (N = 94) | p-Value | |
| Age (median) | 66.5 (35−82) | 77.5 (49−92) | 68 (35−92) | <0.001 *** |
| (range) | ||||
| Gender (%) | ||||
| male | 61 (82.4) | 19 (95.0) | 80 (85.1) | 0.288 |
| female | 13 (17.6) | 1 (5.0) | 14 (14.9) | |
| Primary Tumor (T) (%) | ||||
| T1 | 30 (40.5) | 4 (20.0) | 34 (36.2) | 0.118 |
| T2 | 44 (59.5) | 16 (80.0) | 60 (63.8) | |
| 8th-Edition Stage (%) | ||||
| I | 41 (55.4) | 9 (45.0) | 50 (53.2) | 0.456 |
| II | 33 (44.6) | 11 (55.0) | 44 (46.8) | |
| Subsite (%) | ||||
| lateral | 39 (52.7) | 14 (70.0) | 53 (56.4) | |
| anterior | 10 (13.5) | 2 (10.0) | 12 (12.8) | 0.348 |
| upper | 20 (27.0) | 2 (10.0) | 22 (23.4) | |
| posterior | 5 (6.8) | 2 (10.0) | 7 (7.4) | |
| p16 Status (%) | ||||
| negative | 50 (67.6) | 11 (55.0) | 61 (64.9) | |
| positive | 17 (23.0) | 5 (25.0) | 22 (23.4) | 0.38 |
| unknown | 7 (9.5) | 4 (20.0) | 11 (11.7) | |
| Multiple Cancer (%) | ||||
| none | 32 (43.2) | 8 (40.0) | 40 (42.6) | 1 |
| multiple cancer | 42 (56.8) | 12 (60.0) | 54 (57.4) | |
| Surgery | ||||
|---|---|---|---|---|
| TOS (N = 57) | Open (N = 17) | Total (N = 74) | p-Value | |
| Age (median) | 67 (35−82) | 66 (47−75) | 66.5 (35−82) | 0.689 |
| (range) | ||||
| Gender (%) | ||||
| male | 48 (84.2) | 13 (76.5) | 61 (82.4) | 0.48 |
| female | 9 (15.8) | 4 (23.5) | 13 (17.6) | |
| Primary Tumor (T) (%) | ||||
| T1 | 27 (47.4) | 3 (17.6) | 30 (40.5) | 0.047 * |
| T2 | 30 (52.6) | 14 (82.4) | 44 (59.5) | |
| 8th-edition Stage (%) | ||||
| I | 35 (61.4) | 6 (35.3) | 41 (55.4) | 0.094 |
| II | 22 (38.6) | 11 (64.7) | 33 (44.6) | |
| Subsite (%) | ||||
| lateral | 34 (59.6) | 5 (29.4) | 39 (52.7) | |
| anterior | 1 (1.8) | 9 (52.9) | 10 (13.5) | <0.001 *** |
| upper | 18 (31.6) | 2 (11.8) | 20 (27.0) | |
| posterior | 4 (7.0) | 1 (5.9) | 5 (6.8) | |
| p16 Status (%) | ||||
| negative | 37 (64.9) | 13 (76.5) | 50 (67.5) | |
| positive | 14 (24.6) | 3 (17.6) | 17 (23.0) | 0.755 |
| unknown | 6 (10.5) | 1 (5.9) | 7 (9.5) | |
| Concurrent ND (%) | ||||
| none | 54 (94.7) | 1 (5.9) | 55 (74.3) | <0.001 *** |
| ND | 3 (5.3) | 16 (94.1) | 19 (25.7) | |
| Margin (%) | ||||
| negative | 31 (54.4) | 11 (64.7) | 42 (56.8) | |
| positive | 26 (45.6) | 6 (35.3) | 32 (43.2) | 0.580 |
| Positive Margin (N = 32) | ||||
| horizontal | 23 (88.5) | 4 (66.7) | 27 (84.5) | |
| vertical | 2 (7.7) | 0 (0.0) | 2 (6.2) | 0.112 |
| both | 1 (3.8) | 2 (33.3) | 3 (9.3) | |
| Recurrence (N = 40) | Initial Treatment | Total (N = 94) | ||
|---|---|---|---|---|
| TOS (N = 57) | Open (N = 17) | RT (N = 20) | ||
| Primary lesion (rT) | 10 7 surgery (4 NER, 2 DOD, 1 DOC) 1 RT (DOD) 2 BSC (DOD) | 7 5 surgery (4 NER, 1 DOD, 1 DOC) 1 RT (NER) 1 BSC (DOD) | 7 6 surgery (5 DOD, 1 DOC) 1 BSC (DOD) | 24 19 surgery 2 RT 4 BSC |
| Regional lymph node (rN) | 14 13 surgery (7 NER, 4 DOD, 2 DOC) 1 RT (DOC) | 1 1 surgery (NER) | 0 | 15 14 surgery 1 RT |
| Distant metastasis (rM) | 0 | 0 | 0 | 0 |
| All (rTNM) | 1 BSC (DOD) | 0 | 0 | 1 BSC |
| None | 32 | 9 | 13 | 54 |
| Initial Treatment | |||||
|---|---|---|---|---|---|
| Outcome | TOS (N = 57) | Open (N = 17) | RT (N = 20) | Total (N = 94) | |
| NER | 38 | 15 | 11 | 64 | |
| DOD | Due to the primary lesion | 5 | 1 | 5 | 11 |
| Due to the regional lymph node | 2 | 0 | 0 | 2 | |
| Due to the distant metastases | 3 | 0 | 0 | 3 | |
| DOC | Malignant neoplasm | 7 | 1 | 2 | 10 |
| Other than malignant neoplasm | 2 | 0 | 2 | 4 | |
| Factor | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age | 1.041 | 0.996−1.087 | 0.073 |
| Gender | 1.044 | 0.399−2.732 | 0.931 |
| Primary Tumor (T) | 1.484 | 0.679−3.242 | 0.322 |
| 8th-Edition Stage | 1.997 | 0.969−4.117 | 0.061 |
| p16 Status | 0.560 | 0.228−1.378 | 0.207 |
| Multiple Cancer | 2.62 | 1.124−6.107 | 0.025 * |
| Treatment (Surgery or RT) | 2.271 | 1.034−4.987 | 0.041 * |
| Factor | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Treatment (Surgery or RT) | 2.752 | 1.229−6.163 | 0.014 * |
| 8th-Edition Stage | 1.958 | 0.803−4.776 | 0.139 |
| p16 Status | 0.737 | 0.246−2.210 | 0.586 |
| Head and Neck Region | Other Regions | ||
|---|---|---|---|
| Hypopharynx | 17 | Esophagus | 24 |
| Oral cavity | 10 | Stomach | 12 |
| Nasopharynx | 3 | Lung | 7 |
| Larynx | 2 | Colon | |
| Sinonasal | 2 | Pancreas | |
| Prostate | |||
| Liver, Breast, Ovary for each | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokugo, M.; Shinozaki, T.; Ishii, R.; Ito, Y.; Sakashita, S.; Ishii, G.; Ogawa, T.; Katori, Y.; Matsuura, K.; Hayashi, R. Surgery for T1/2N0 Oropharyngeal Carcinoma Is a Better Treatment Option than Radiotherapy—A Long-Term Follow-Up Study from a Single Japanese High-Volume Cancer Center. Cancers 2025, 17, 1862. https://doi.org/10.3390/cancers17111862
Rokugo M, Shinozaki T, Ishii R, Ito Y, Sakashita S, Ishii G, Ogawa T, Katori Y, Matsuura K, Hayashi R. Surgery for T1/2N0 Oropharyngeal Carcinoma Is a Better Treatment Option than Radiotherapy—A Long-Term Follow-Up Study from a Single Japanese High-Volume Cancer Center. Cancers. 2025; 17(11):1862. https://doi.org/10.3390/cancers17111862
Chicago/Turabian StyleRokugo, Masahiro, Takeshi Shinozaki, Ryo Ishii, Yusuke Ito, Shingo Sakashita, Genichiro Ishii, Takenori Ogawa, Yukio Katori, Kazuto Matsuura, and Ryuichi Hayashi. 2025. "Surgery for T1/2N0 Oropharyngeal Carcinoma Is a Better Treatment Option than Radiotherapy—A Long-Term Follow-Up Study from a Single Japanese High-Volume Cancer Center" Cancers 17, no. 11: 1862. https://doi.org/10.3390/cancers17111862
APA StyleRokugo, M., Shinozaki, T., Ishii, R., Ito, Y., Sakashita, S., Ishii, G., Ogawa, T., Katori, Y., Matsuura, K., & Hayashi, R. (2025). Surgery for T1/2N0 Oropharyngeal Carcinoma Is a Better Treatment Option than Radiotherapy—A Long-Term Follow-Up Study from a Single Japanese High-Volume Cancer Center. Cancers, 17(11), 1862. https://doi.org/10.3390/cancers17111862






