Diagnostic Delay in Soft Tissue Sarcomas: A Review
Simple Summary
Abstract
1. Introduction
2. Theoretical Framework in Diagnostic Delay
3. Elements and Time Intervals: What Is “Delay”?
4. Extent of the Problem
5. What Is the Cause of Delay?
5.1. Tumor Related Causes
5.2. Patient-Related Causes
5.3. General Population-Related Causes
5.4. Primary Care-Related Causes
5.5. Health System-Related Causes
5.6. Sarcoma Team-Related Causes
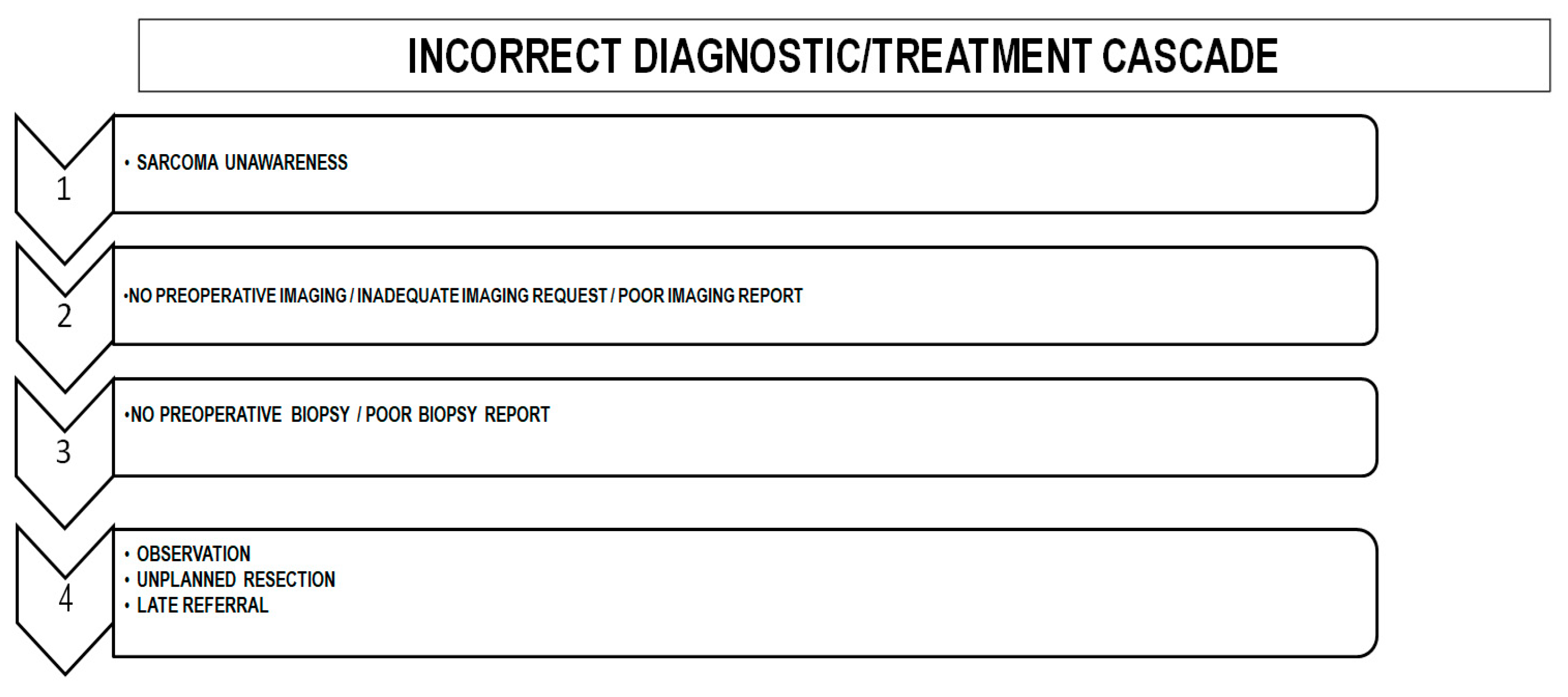
6. Consequences of a Diagnostic/Treatment Delay
6.1. Prognostic Impact of a Late Referral
6.2. Non-Planned Surgical Resection of Sarcomas
6.3. Medico-Legal Impact of Late Referral
6.4. Psychological Impact of Late Referral
7. “Red Flag” Symptoms of STSs
7.1. Size
7.2. Location
7.3. Tumor Growth
7.4. Pain
7.5. Duration of Symptoms
7.6. Predictive Value of Alarm Symptoms
7.7. PC and Alarm Symptoms
8. Referral Criteria
9. Fast-Track Referral Pathways
10. Improving and Promoting Early Referral—General Measures
11. What Are the Results After the Implementation of Referral Clinical Criteria and Fast-Track Referral Pathways?
Has the Implementation of These Guidelines Changed the Referral and Characteristics of STSs Seen by the Sarcoma MDT?
12. Avoiding Collapse—How to Filter Massive Referrals
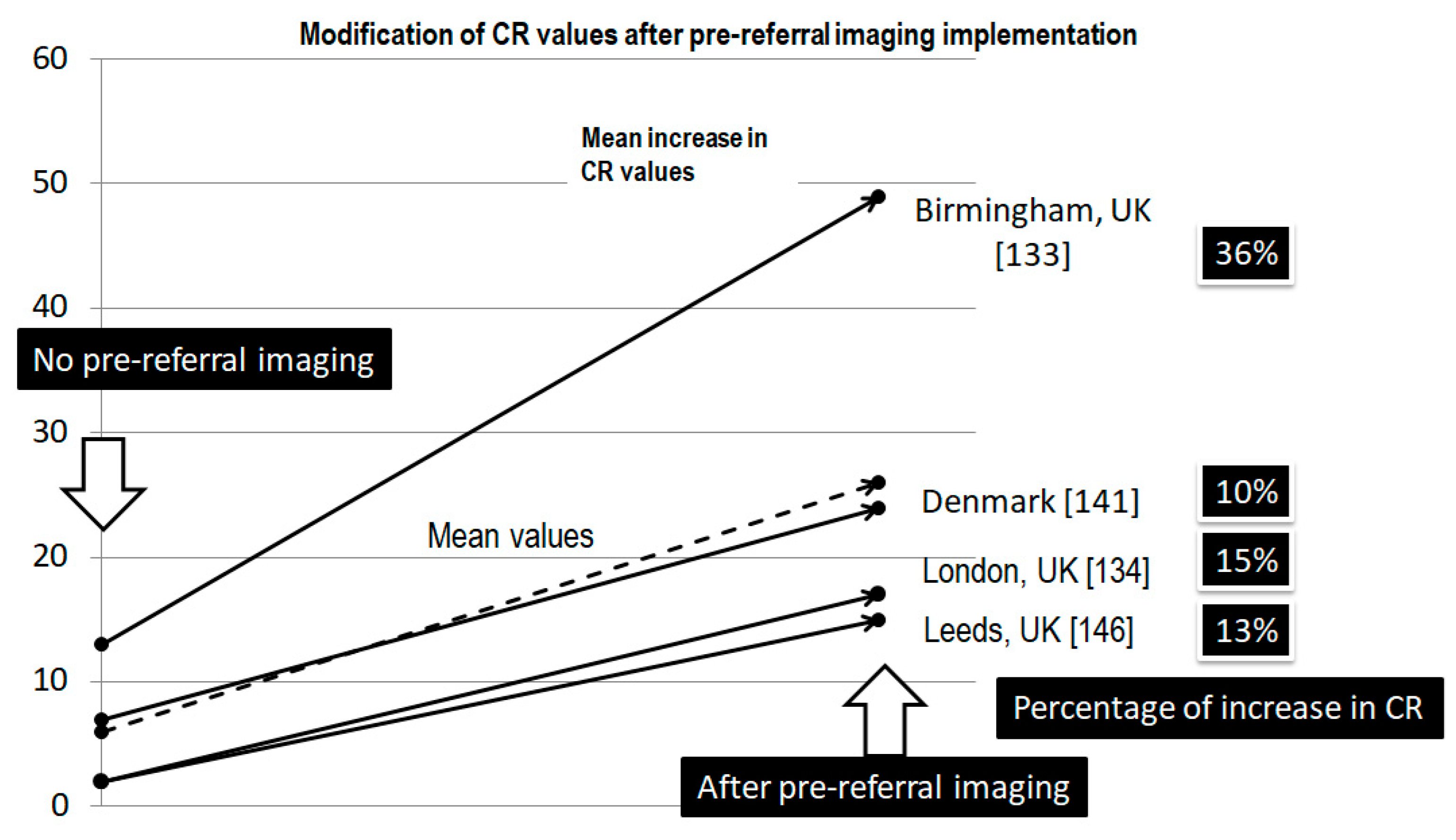
12.1. Pre-Referral Imaging
12.2. Diagnostic Triage
12.3. Telemedicine
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AYA | Adolescents and young adults |
| BS | Bone sarcoma |
| CR | Conversion rate |
| CPP | Cancer Patient Pathway |
| CT | Chemotherapy |
| DFS | Dermatofibrosarcoma |
| DFS | Disease-free survival |
| DM | Distant metastasis |
| DMFS | Distant metastasis-free survival |
| DMR | Distant metastasis rate |
| DR | Detection rate |
| DSS | Disease-specific survival |
| EP | Emergency presentation |
| FDS | Faster diagnostic standard |
| FSG | French Sarcoma Group |
| GIST | Gastrointestinal stromal tumor |
| GP | General practitioner |
| HG | High grade |
| H/N | Head/neck |
| KS | Kaposi’s sarcoma |
| LG | Low grade |
| LMS | Leiomyosarcoma |
| LRFS | Local recurrence-free survival |
| m | Months |
| MDT | Multidisciplinary team |
| MRI | Magnetic resonance imaging |
| NPRs | Non-planned resections |
| MFS | Metastasis-free survival |
| NCDB | National Cancer Database |
| NICE | National Institute for Care and Care Excellence |
| OpS | Orthopedic surgeon |
| OS | Overall survival |
| Path | Pathology |
| PPV | Positive predictive value |
| RT | Radiotherapy |
| RTD | Route to diagnosis |
| RPS | Retroperitoneal sarcoma |
| Rx | Radiology |
| SC | Subcutaneous |
| SDTM | Sarcoma diagnostic triage meeting |
| SSMN | Scottish Sarcoma Managed Clinical Network |
| S/SD | Size/symptom duration ratio |
| SS | Synovial sarcoma |
| STS | Soft tissue sarcoma |
| SUK20S | Sarcoma UK 2020 survey |
| T0 | Patient’s delay |
| T1 | General practitioner’s delay |
| T2 | Non-specialist’s delay |
| TTI | Time to treatment initiation |
| T/Extr | Trunk/extremities |
| 2WW | Two Weeks Wait |
| WD_LPS | Well-differentiated liposarcoma |
| w | Week |
| w/o | Without |
References
- Delays Cost Lives. A Call to Policy Makers to Improve Early Diagnosis of Sarcoma. Sarcoma UK. The Bone & Soft Tissue Cancer Charity. Available online: https://sarcoma.org.uk/wp-content/uploads/2022/05/early_diagnosis_rgb_single_pages_update_2.pdf (accessed on 23 January 2025).
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Bacon, A.; Wong, K.; Fernando, M.S.; Rous, B.; Hill, R.J.W.; Collins, S.D.; Broggio, J.; Strauss, S.J. Incidence and survival of soft tissue sarcoma in England between 2013 and 2017, an analysis from the National Cancer Registration and Analysis Service. Int. J. Cancer 2023, 152, 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Mannan, K.; Briggs, T.W. tissue tumours of the extremities. BMJ 2005, 331, 590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heidi Buvarp Dyrop. From symptoms to diagnosis of sarcoma—Revealing the diagnostic pathway. Ph.D. Dissertation, Health, Aarhus University, Aarhus, Denmark, 2016. Available online: https://www.ortopaedi.dk/wp-content/uploads/2016/12/Dyrup.pdf (accessed on 1 January 2025).
- Smolle, M.A.; Leithner, A.; Grimer, R.J. Evaluating the British sarcoma referral form. Ann. R. Coll. Surg. Engl. 2015, 97, 434–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, L.; Sansom, N.; Hemphill, S.; Bradley, S.H.; Shinkins, B.; Wheatstone, P.; Hamilton, W.; Neal, R.D. Trends and variation in urgent referrals for suspected cancer 2009/2010–2019/2020. Br. J. Gen. Pract. 2021, 72, 34–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dwivedi, A.K.; Dwivedi, S.N.; Deo, S.; Shukla, R.; Pandey, A.; Dwivedi, D.K. An epidemiological study on delay in treatment initiation of cancer patients. Health 2012, 4, 66–79. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Lyratzopoulos, G. Progress and priorities in reducing the time to cancer diagnosis. Br. J. Cancer 2023, 128, 468–470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tope, P.; Farah, E.; Ali, R.; El-Zein, M.; Miller, W.H.; Franco, E.L. The impact of lag time to cancer diagnosis and treatment on clinical outcomes prior to the COVID-19 pandemic: A scoping review of systematic reviews and meta-analyses. Elife 2023, 12, e81354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neal, R.D. Do diagnostic delays in cancer matter? Br. J. Cancer 2009, 101 (Suppl. S2), S9–S12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olesen, F.; Hansen, R.P.; Vedsted, P. Delay in diagnosis: The experience in Denmark. Br. J. Cancer 2009, 101 (Suppl. S2), S5–S8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, F.; Webster, A.; Scott, S.; Emery, J. The Andersen Model of Total Patient Delay: A systematic review of its application in cancer diagnosis. J. Health Serv. Res. Policy 2012, 17, 110–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, B.L.; Cacioppo, J.T. Delay in seeking a cancer diagnosis: Delay stages and psychophysiological comparison processes. Br. J. Soc. Psychol. 1995, 34 Pt 1, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Safer, M.A.; Tharps, Q.J.R.; Jackson, T.C.; Levknthal, H. Determinants of three stages of delay in seeking care at a medical clinic. Med. Care 1979, 17, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.E.; Walter, F.M.; Webster, A.; Sutton, S.; Emery, J. The model of pathways to treatment: Conceptualization and integration with existing theory. Br. J. Health Psychol. 2013, 18, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pascua, L.R.; Sánchez-Herráez, S.; Casas-Ramos, P.; Izquierdo-García, F.J.; Maderuelo-Fernández, J.A. Circuito de asistencia a pacientes con sarcomas de partes blandas de las extremidades: Un tortuoso y lento camino hasta las unidades de referencia [Health care circuit for patients with soft tissue sarcomas of the extremities. A tortuous and slow road to referral units]. Rev. Española Cirugía Ortopédica Traumatol. 2014, 58, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl. S1), S92–S107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coxon, D.; Campbell, C.; Walter, F.M.; Scott, S.E.; Neal, R.D.; Vedsted, P.; Emery, J.; Rubin, G.; Hamilton, W.; Weller, D. The Aarhus statement on cancer diagnostic research: Turning recommendations into new survey instruments. BMC Health Serv. Res. 2018, 18, 677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schärer, M.; Heesen, P.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B.; Swiss Sarcoma Network. Benchmarking Time-to-Treatment Initiation in Sarcoma Care Using Real-World-Time Data. Cancers 2023, 15, 5849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
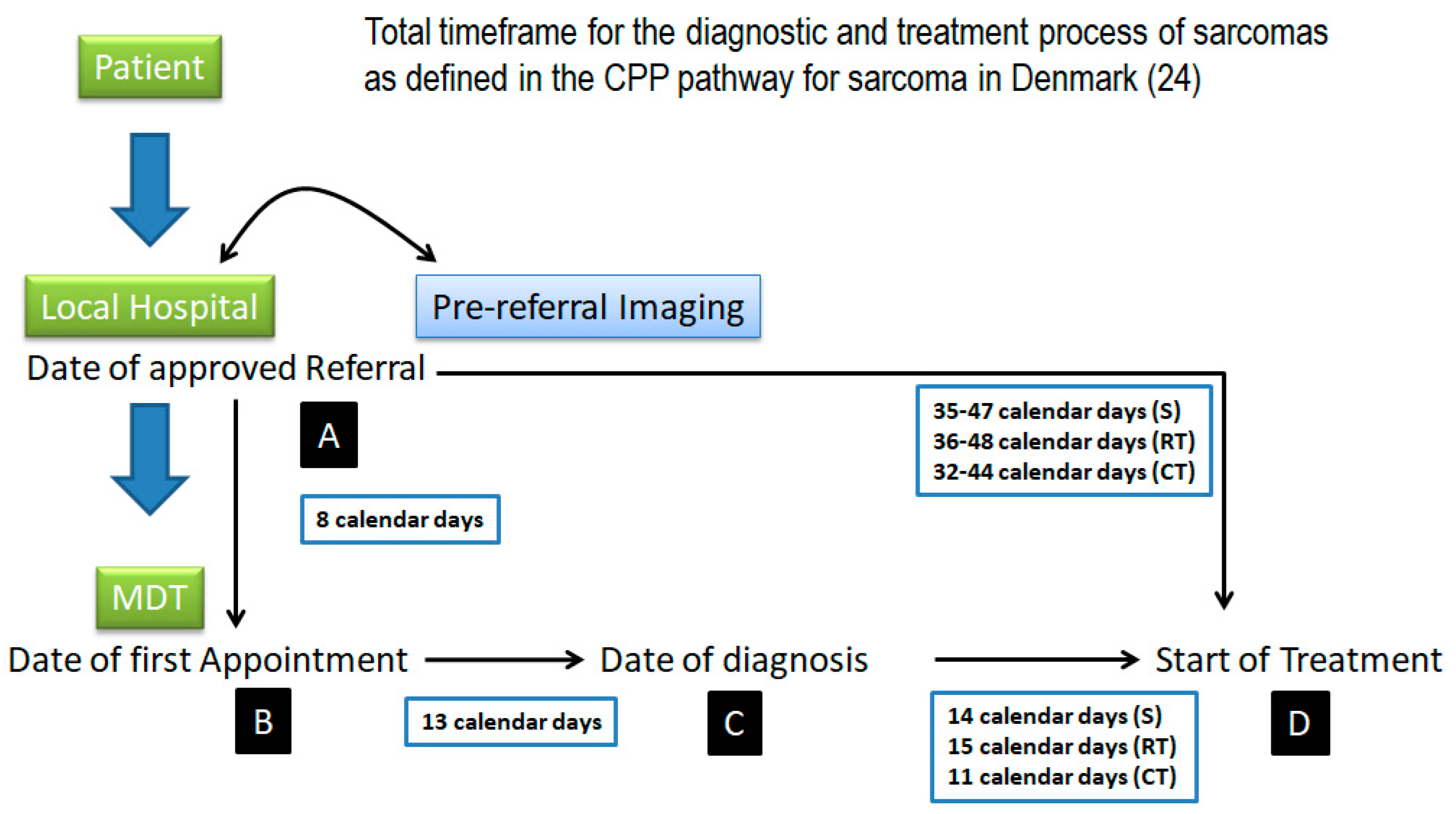
- Sundhedsstyrelsen. Pakkeforløb for Sarkomer i Knogle og Bløddele, Version: 1.1; Sundhedsstyrelsen: København, Denmark, 2009. [Google Scholar]
- Thorn, A.; Seem, K.M.; Talman, M.-L.; Engelmann, B.E.; Sørensen, M.S.; Aggerholm-Pedersen, N.; Baad-Hansen, T.; Petersen, M.M. The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study. Cancers 2024, 16, 4077. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Thomas, J.M. Delay in referral to a specialist soft-tissue sarcoma unit. Eur. J. Surg. Oncol. 2005, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Stas, M.; De Wever, I. Delay in diagnosis of soft tissue sarcomas. Eur. J. Surg. Oncol. 2003, 29, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-García, J.; Lacerenza, S.; Hindi, N.; García, I.C.; Marquina, G.; Cano, J.M.C.; Trufero, J.M.; Tripero, A.R.S.; García, T.L.; Rioboo, M.J.C.; et al. Delays in diagnosis and surgery of sarcoma patients during the COVID-19 outbreak in Spain. Ther. Adv. Med. Oncol. 2024, 16, 17588359231220611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elyes, M.; Heesen, P.; Schelling, G.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B.; Swiss Sarcoma Network. Enhancing Healthcare for Sarcoma Patients: Lessons from a Diagnostic Pathway Efficiency Analysis. Cancers 2023, 15, 4892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soomers, V.L.M.N.; Husson, O.; Desar, I.M.E.; van de Sande, M.A.J.; de Haan, J.J.; Verhoef, C.; Vriens, I.J.H.; van Houdt, W.J.; van de Poll-Franse, L.; van der Graaf, W.T.A. Patient and diagnostic intervals of survivors of sarcoma: Results from the SURVSARC study. Cancer 2020, 126, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Drabbe, C.; Grünhagen, D.J.; Van Houdt, W.J.; Braam, P.M.; Soomers, V.L.M.N.; Van der Hage, J.A.; De Haan, J.J.; Keymeulen, K.B.M.I.; Husson, O.; Van der Graaf, W.T.A. Diagnosed with a Rare Cancer: Experiences of Adult Sarcoma Survivors with the Healthcare System-Results from the SURVSARC Study. Cancers 2021, 13, 679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soomers, V.; Husson, O.; Young, R.; Desar, I.; Van der Graaf, W. The sarcoma diagnostic interval: A systematic review on length, contributing factors and patient outcomes. ESMO Open 2020, 5, e000592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarcoma UK. Impact of Sarcoma. National sarcoma Survey 2020; Technical Report; The Bone & Soft tissue Cancer Charity: London, UK. Available online: https://sarcoma.org.uk/wp-content/uploads/2022/04/impact_of_sarcoma_2020_national_sarcoma_survey_-_technical_report_-_accessible.pdf (accessed on 14 February 2025).
- Lyratzopoulos, G.; Saunders, C.L.; A Abel, G.; McPhail, S.; Neal, R.D.; Wardle, J.; Rubin, G.P. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br. J. Cancer 2015, 112 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, S.; Clark, S.E.; Gerrand, C.; Gilchrist, K.; Lawal, M.; Maio, L.; Martins, A.; Storey, L.; Taylor, R.M.; Wells, M.; et al. Patients’ Experiences of a Sarcoma Diagnosis: A Process Mapping Exercise of Diagnostic Pathways. Cancers 2023, 15, 3946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dyrop, H.B.; Vedsted, P.; Rædkjær, M.; Safwat, A.; Keller, J. Routes to Diagnosis for Suspected Sarcoma: The Impact of Symptoms and Clinical Findings on the Diagnostic Process. Sarcoma 2016, 2016, 8639272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, T.; Matsumine, A.; Matsubara, T.; Asanuma, K.; Uchida, A.; Sudo, A. The symptom-to-diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J. Surg. Oncol. 2011, 104, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Seinen, J.; Almquist, M.; Styring, E.; Rydholm, A.; Nilbert, M. Delays in the management of retroperitoneal sarcomas. Sarcoma 2010, 2010, 702573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, G.D.; Smith, G.; Dramis, A.; Grimer, R.J. Delays in referral of soft tissue sarcomas. Sarcoma 2008, 2008, 378574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, J.H.; Rahman, J.; Deo, S.; Flint, M. Effects of time to treatment initiation on outcomes for soft tissue sarcomas. J. Surg. Oncol. 2023, 127, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Fujiwara, T.; Healey, J.H. Patients with an increased time to treatment initiation have a poorer overall survival after definitive surgery for localized high-grade soft-tissue sarcoma in the extremity or trunk: Report from the National Cancer Database. Bone Jt. J. 2021, 103-B, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Curtis, G.L.; Lawrenz, J.M.; George, J.; Styron, J.F.; Scott, J.; Shah, C.; Shepard, D.R.; Rubin, B.; Nystrom, L.M.; Mesko, N.W. Adult soft tissue sarcoma and time to treatment initiation: An analysis of the National Cancer Database. J. Surg. Oncol. 2018, 117, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.; Horrill, T.; Mollison, A.; Stringer, E.; Lambert, L.K.; Stajduhar, K. Barriers to cancer treatment for people experiencing socioeconomic disadvantage in high-income countries: A scoping review. BMC Health Serv. Res. 2024, 24, 670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Styring, E.; Rydholm, A.; von Steyern, F.V. Better referral of soft tissue sarcoma. Surgeon 2012, 10, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Nandra, R.; Hwang, N.; Matharu, G.; Reddy, K.; Grimer, R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ann. R. Coll. Surg. Engl. 2015, 97, 425–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almuammar, A.; Dryden, C.; Burr, J.A. Factors associated with late presentation of cancer: A limited literature review. J. Radiother. Pract. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Syros, A.; Baron, M.C.; Adalbert, J.; Remer, H.B.; Heng, M.; Crawford, B. Barriers to care for musculoskeletal sarcoma patients: A public health perspective. Front. Public Health 2024, 12, 1399471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- 2017-Lay-Report-Lucy-Allison-AYA-Sarcoma-Project.pdf. Available online: https://childcancernetwork.org.nz/wp-content/uploads/2017/06/2017-Lay-Report-Lucy-Allison-AYA-Sarcoma-Project.pdf (accessed on 13 March 2025).
- Miller, B.J.; Gao, Y.; Duchman, K.R. Socioeconomic measures influence survival in osteosarcoma: An analysis of the National Cancer Data Base. Cancer Epidemiol. 2017, 49, 112–117. [Google Scholar] [CrossRef]
- Smartt, A.A.B.; Jang, E.S.; Tyler, W.K. Is there an association between insurance status and survival and treatment of primary bone and extremity soft-tissue sarcomas? A SEER database study. Clin. Orthop. Relat. Res. 2020, 478, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.S.; Hammoor, B.; Enneking, F.K.; Chan, C.M.; Spiguel, A.R.; Gibbs, C.P.; Scarborough, M.T.; Tyler, W.K. Effect of insurance status on mortality in adults with sarcoma of the extremities and pelvis: A SEER-Medicare study. J. Am. Acad. Orthop. Surg. 2023, 31, e14–e22. [Google Scholar] [CrossRef]
- Malik, A.T.M.; Alexander, J.; Khan, S.N.; Scharschmidt, T.J. Has the affordable care act been associated with increased insurance coverage and early-stage diagnoses of bone and soft-tissue sarcomas in adults? Clin. Orthop. Relat. Res. 2021, 479, 493–502. [Google Scholar] [CrossRef]
- Alcindor, T.; Dumitra, S.; Albritton, K.; Thomas, D.M. Disparities in Cancer Care: The Example of Sarcoma-In Search of Solutions for a Global Issue. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wendt, R.; Gao, Y.; Miller, B.J. Rural patients are at risk for increased stage at presentation and diminished overall survival in osteosarcoma. Cancer Epidemiol. 2019, 61, 119–123. [Google Scholar] [CrossRef]
- Sasi, A.; Ganguly, S.; Biswas, B.; Pushpam, D.; Kumar, A.; Agarwala, S.; Alam Khan, S.; Kumar, V.S.; Deo, S.; Sharma, D.N.; et al. Determinants and impact of diagnostic interval in bone sarcomas: A retrospective cohort study. Pediatr. Blood Cancer 2023, 70, e30135. [Google Scholar] [CrossRef]
- Fayet, Y.; Tétreau, R.; Honoré, C.; Le Nail, L.-R.; Dalban, C.; Gouin, F.; Causeret, S.; Piperno-Neumann, S.; Mathoulin-Pelissier, S.; Karanian, M.; et al. Determinants of the access to remote specialised services provided by national sarcoma reference centres. BMC Cancer 2021, 21, 631. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Song, Y.; Holt, G.E. Effect of marital status on treatment and survival of extremity soft tissue sarcoma. Ann. Oncol. 2014, 25, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Alamanda, V.K.; Song, Y.; Schwartz, H.S.; Holt, G.E. Racial disparities in extremity soft-tissue sarcoma outcomes: A Nationwide analysis. Am. J. Clin. Oncol. 2015, 38, 595–599. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Sherwani, M.; Khan, A.; Zahid, M.; Abbas, M.; Asif, N. Neglected orthopedic oncology--causes, epidemiology and challenges for management in developing countries. Indian J. Cancer 2015, 52, 325–329. [Google Scholar] [CrossRef]
- Costas-Muniz, R.; Leng, J.; Aragones, A.; Ramirez, J.; Roberts, N.; Mujawar, M.I.; Gany, F. Association of socioeconomic and practical unmet needs with self-reported nonadherence to cancer treatment appointments in low-income Latino and Black cancer patients. Ethn. Health 2016, 21, 118–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fossum, C.C.; Breen, W.G.; Sun, P.Y.; Retzlaff, A.A.; Okuno, S.H. Assessment of Familiarity with Work-up Guidelines for Bone and Soft Tissue Sarcoma Among Primary Care Practitioners in Minnesota. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 143–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chotel, F.; Unnithan, A.; Chandrasekar, C.R.; Parot, R.; Jeys, L.; Grimer, R.J. Variability in the presentation of synovial sarcoma in children: A plea for greater awareness. J. Bone Jt. Surg. Br. 2008, 90, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Lyratzopoulos, G.; A Abel, G.; McPhail, S.; Neal, R.D.; Rubin, G.P. Measures of promptness of cancer diagnosis in primary care: Secondary analysis of national audit data on patients with 18 common and rarer cancers. Br. J. Cancer 2013, 108, 686–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyratzopoulos, G.; Wardle, J.; Rubin, G. Rethinking diagnostic delay in cancer: How difficult is the diagnosis? BMJ 2014, 349, g7400. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, S.C.; A Abel, G.; Lyratzopoulos, G. Pre-referral GP consultations in patients subsequently diagnosed with rarer cancers: A study of patient-reported data. Br. J. Gen. Pract. 2016, 66, e171–e181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zakkak, N.; Barclay, M.E.; Swann, R.; McPhail, S.; Rubin, G.; Abel, G.A.; Lyratzopoulos, G. The presenting symptom signatures of incident cancer: Evidence from the English 2018 National Cancer Diagnosis Audit. Br. J. Cancer 2024, 130, 297–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rafiq, M.; de Boer, J.; Mar, J.; Desai, J.; Bae, S.; E Gyorki, D.; Di Bella, C.; Lyratzopoulos, G.; Lewin, J.H.; Emery, J. Clinical activity in general practice before sarcoma diagnosis: An Australian cohort study. Br. J. Gen. Pract. 2024, 74, e508–e516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holthuis, E.I.; van der Graaf, W.T.A.; Drabbe, C.; van Houdt, W.J.; Schrage, Y.M.; Hartman, T.C.O.; Uijen, A.A.; Bos, I.; Heins, M.; Husson, O.; et al. The prediagnostic general practitioner care of sarcoma patients: A real-world data study. J. Surg. Oncol. 2024, 130, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ogura, K.; Hayakawa, K.; Ikuta, K.; Nezu, Y.; Miwa, S.; Yoshida, S.; Nakai, S.; Kinoshita, H.; Kawabata, Y.; et al. Real-world Referral Pattern of Unplanned Excision in Patients with Soft-tissue Sarcoma: A Multicenter Study Conducted by the Bone and Soft-tissue Tumor Study Group of the Japan Clinical Oncology Group. Vivo 2024, 38, 2712–2717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Symonds, R.P. Cancer biology may be more important than diagnostic delay. BMJ 2002, 325, 774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rougraff, B.T.; Davis, K.; Lawrence, J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin. Orthop. Relat. Res. 2007, 462, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Takashi, H.; Kensaku, A.; Taniguchi, Y.; Yonezawa, H.; et al. Delayed Initiation of Treatment Is Associated with Metastasis of Soft-tissue Sarcoma. Anticancer. Res. 2020, 40, 7009–7015. [Google Scholar] [CrossRef] [PubMed]
- Featherall, J.; Curtis, G.L.; Lawrenz, J.M.; Jin, Y.; George, J.; Scott, J.; Shah, C.; Shepard, D.; Rubin, B.P.; Nystrom, L.M.; et al. Time to treatment initiation and survival in adult localized, high-grade soft tissue sarcoma. J. Surg. Oncol. 2019, 120, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Tsukushi, S.; Arai, E.; Kozawa, E.; Futamura, N.; Ishiguro, N.; Nishida, Y. Association of short duration from initial symptoms to specialist consultation with poor survival in soft-tissue sarcomas. Am. J. Clin. Oncol. 2015, 38, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Rougraff, B.T.; Lawrence, J.; Davis, K. Length of symptoms before referral: Prognostic variable for high-grade soft tissue sarcoma? Clin. Orthop. Relat. Res. 2012, 470, 706–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saithna, A.; Pynsent, P.B.; Grimer, R.J. Retrospective analysis of the impact of symptom duration on prognosis in soft tissue sarcoma. Int. Orthop. 2008, 32, 381–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gofman, A.; Issakov, J.; Kollender, Y.; Soyfer, V.; Dadia, S.; Jiveliouk, I.; Flusser, G.; Bickels, J.; Meller, I.; Merimsky, O. Synovial sarcoma of the extremities and trunk: A long-lasting disease. Oncol. Rep. 2007, 18, 1577–1581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruka, W.; Emrich, L.J.; Driscoll, D.L.; Karakousis, C.P. Tumor size/symptom duration ratio as a prognostic factor in patients with high-grade soft tissue sarcomas. Eur. J. Cancer Clin. Oncol. 1988, 24, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- E Giuliano, A.; Eilber, F.R. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J. Clin. Oncol. 1985, 3, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Grignol, V.P.; Lopez-Aguiar, A.G. The Implications of an Unplanned Sarcoma Excision (the “Whoops” Operation). Surg. Clin. N. Am. 2022, 102, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pretell-Mazzini, J.; Barton, M.D., Jr.; Conway, S.A.; Temple, H.T. Unplanned excision of soft-tissue sarcomas: Current concepts for management and prognosis. J. Bone Jt. Surg. 2015, 97, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Larios, F.; Gonzalez, M.R.; Ruiz-Arellanos, K.; E Silva, G.A.; Pretell-Mazzini, J. Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?-A Systematic Review and Meta-Analysis. Cancers 2024, 16, 443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belzarena, A.C.; Binitie, O.; Letson, G.D.; Joyce, D.M. Unplanned Sarcoma Excisions: Understanding How They Happen. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2024, 8, e23.00176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venkatesan, M.; Richards, C.; McCulloch, T.; Perks, A.; Raurell, A.; Ashford, R.U.; East Midlands Sarcoma Service. Inadvertent surgical resection of soft tissue sarcomas. Eur. J. Surg. Oncol. 2012, 38, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Schärer, M.; Hösli, P.; Heesen, P.; Schelling, G.; Obergfell, T.; Nydegger, K.N.; Studer, G.; Bode-Lesniewska, B.; Fuchs, B.; Swiss Sarcoma Network. Integrated Care in Specialized Networks: Leveraging Early Referrals to Reduce Local Recurrence in Soft Tissue Sarcoma. Cancers 2024, 16, 3616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davidson, D. CORR Insights®: Delayed Diagnosis Is the Primary Cause of Sarcoma Litigation: Analysis of Malpractice Claims in the United States. Clin. Orthop. Relat. Res. 2020, 478, 2254–2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jena, A.B.; Seabury, S.; Lakdawalla, D.; Chandra, A. Malpractice risk according to physician specialty. N. Engl. J. Med. 2011, 365, 629–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mello, M.M.; Chandra, A.; Gawande, A.A.; Studdert, D.M. National costs of the medical liability system. Health Aff. 2010, 29, 1569–1577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesko, N.W.; Mesko, J.L.; Gaffney, L.M.; Halpern, J.L.; Schwartz, H.S.; Holt, G.E. Medical malpractice and sarcoma care—A thirty-three year review of case resolutions, inciting factors, and at risk physician specialties surrounding a rare diagnosis. J. Surg. Oncol. 2014, 110, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.D.; Sargazi, N.; Yin, Q.; Chandrasekar, C.R. Delayed diagnosis in primary care-The main cause of sarcoma litigation in the United Kingdom. J. Surg. Oncol. 2016, 113, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Ross, A. Litigation in musculoskeletal oncology. Bone Jt. 360 2015, 4, 35–36. [Google Scholar] [CrossRef]
- Hwang, R.; Park, H.Y.; Sheppard, W.; Bernthal, N.M. Delayed Diagnosis Is the Primary Cause of Sarcoma Litigation: Analysis of Malpractice Claims in the United States. Clin. Orthop. Relat. Res. 2020, 478, 2239–2253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, W.; Kichena, S.; Eckhoff, M.D.; Childs, B.R.; Rajani, R.; Wells, M.E.; Kelly, S.P. Critical Review of Oncologic Medical Malpractice Claims Against Orthopaedic Surgeons. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2023, 7, e22.00169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayram, S.; Oduncu, M.; Beşkoç, C.; Atan, Y. Orthopedic Surgeons at Greater Risk of Malpractice Claims for Treatment of Primary Malignant Bone and Soft Tissue Tumors Compared with Metastatic Bone Disease. J. Surg. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Legal Insights into Sarcoma: Addressing Diagnostic Delays and Negligence in Treatment. Available online: https://www.inneg.co.uk/2024/03/07/sarcoma-delays-and-negligence (accessed on 21 April 2025).
- Cutts, S.; Andrea, F.; Piana, R.; Haywood, R. The management of soft tissue sarcomas. Surgeon 2012, 10, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, A.H.G.; Woollard, A.; Ware, H.; Gikas, P. Soft tissue sarcoma: Recognizing a rare disease. Clevel. Clin. J. 2022, 89, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Church, D.J.; Krumme, J.; Kotwal, S. Evaluating Soft-Tissue Lumps and Bumps. Mo. Med. 2017, 114, 289–294. [Google Scholar] [PubMed] [PubMed Central]
- Kolovich, G.G.; Wooldridge, A.N.; Christy, J.M.; Crist, M.K.; Mayerson, J.L.; Scharschmidt, T.J. A retrospective statistical analysis of high-grade soft tissue sarcomas. Med. Oncol. 2012, 29, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J. Size matters for sarcomas! Ann. R. Coll. Surg. Engl. 2006, 88, 519–524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nandra, R.; Forsberg, J.; Grimer, R. If your lump is bigger than a golf ball and growing, think Sarcoma. Eur. J. Surg. Oncol. 2015, 41, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Gassert, F.G.; Gassert, F.T.; Specht, K.; Knebel, C.; Lenze, U.; Makowski, M.R.; von Eisenhart-Rothe, R.; Gersing, A.S.; Woertler, K. Soft tissue masses: Distribution of entities and rate of malignancy in small lesions. BMC Cancer 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pham, K.; Ezuddin, N.S.; Pretell-Mazzini, J.; Subhawong, T.K. Small soft tissue masses indeterminate at imaging: Histological diagnoses at a tertiary orthopedic oncology clinic. Skelet. Radiol. 2019, 48, 1555–1563. [Google Scholar] [CrossRef]
- Obaid, H.; Vassos, N.; Adams, S.J.; Bryce, R.; Donuru, A.; Sinclair, N. Development of a risk assessment model to differentiate malignant and benign musculoskeletal soft-tissue masses on magnetic resonance imaging. J. Med Imaging Radiat. Oncol. 2020, 64, 9–17. [Google Scholar] [CrossRef]
- Datir, A.; James, S.; Ali, K.; Lee, J.; Ahmad, M.; Saifuddin, A. MRI of soft-tissue masses: The relationship between lesion size, depth, and diagnosis. Clin. Radiol. 2008, 63, 373–378. [Google Scholar] [CrossRef]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.M.; Singer, S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416–421; discussion 421–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NICE. Guidance on Cancer Studies, Improving Outcomes for People with Sarcoma; National Institute for Health and Clinical Excellence: London, UK, 2006. [Google Scholar]
- Styring, E.; Billing, V.; Hartman, L.; Nilbert, M.; Seinen, J.; Veurink, N.; von Steyern, F.V.; Rydholm, A. Simple guidelines for efficient referral of soft-tissue sarcomas: A population-based evaluation of adherence to guidelines and referral patterns. J. Bone Jt. Surg. 2012, 94, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Elyes, M.; Heesen, P.; Schelling, G.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B.; On Behalf Of The Swiss Sarcoma Network. Pain Accelerates, Swelling and Sensory Disturbances Delay Diagnosis in Mesenchymal Tumors. Cancers 2025, 17, 510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grimer, R. Early symptoms of bone and soft tissue sarcomas: Could they be diagnosed earlier? Ann. R. Coll. Surg. Engl. 2012, 94, 261–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, C.J.; Pynsent, P.B.; Grimer, R.J. Clinical features of soft tissue sarcomas. Ann. R. Coll. Surg. Engl. 2001, 83, 203–205. [Google Scholar] [PubMed] [PubMed Central]
- Hussein, R.; Smith, M.A. Soft tissue sarcomas: Are current referral guidelines sufficient? Ann. R. Coll. Surg. Engl. 2005, 87, 171–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshi, M.; Oebisu, N.; Iwai, T.; Shimatani, A.; Ban, Y.; Takada, N.; Yao, H.; Nakamura, H. Review of the referral documents of patients with malignant soft tissue tumors. Sci. Rep. 2022, 12, 19527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institute for Health and Excellence (NICE): Referral guidelines for suspected cancer—NICE guideline 27. National Institute for Health and Excellence (NICE) 2015. Available online: https://www.nice.org.uk/guidance/ng12 (accessed on 12 March 2025).
- McCullough, A.L.; Scotland, T.R.; Dundas, S.R.; Boddie, D.E. The impact of a managed clinical network on referral patterns of sarcoma patients in Grampian. Scott. Med. J. 2014, 59, 108–113. [Google Scholar] [CrossRef]
- Jansen-Landheer, M.L.; Krijnen, P.; Oostindier, M.J.; Kloosterman-Boele, W.; Noordijk, E.; Nooij, M.; Steup, W.; Taminiau, A.; Vree, R.; Hogendoorn, P.; et al. Improved diagnosis and treatment of soft tissue sarcoma patients after implementation of national guidelines: A population-based study. Eur. J. Surg. Oncol. 2009, 35, 1326–1332. [Google Scholar] [CrossRef]
- The Japanese Orthopaedic Association. Clinical Practice Guidelines on the Management of Soft Tissue Tumors, 1st ed.; Nankodo: Tokyo, Japan, 2020. [Google Scholar]
- Dyrop, H.B.; Vedsted, P.; Safwat, A.; Maretty-Nielsen, K.; Hansen, B.H.; Jørgensen, P.H.; Baad-Hansen, T.; Keller, J. Alarm symptoms of soft-tissue and bone sarcoma in patients referred to a specialist center. Acta Orthop. 2014, 85, 657–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NHS, The NHS Cancer Plan. Department of Health: The NHS Cancer Plan. In A Plan for Investment, A Plan for Reform; Department of Health: London, UK, 2000. Available online: https://www.nuffieldtrust.org.uk/sites/default/files/2019-11/nhsplan.pdf (accessed on 12 March 2025).
- Suspected Cancer: Recognition and Referral. NICE Guideline. Available online: https://www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-pdf-1837268071621 (accessed on 24 March 2025).
- NHS Cancer Programme: Faster Diagnosis Framework. Available online: https://www.england.nhs.uk/wp-content/uploads/2019/07/B1332-NHS-Cancer-Programme-Faster-Diagnosis-Framework-v5.pdf (accessed on 13 March 2025).
- Prades, J.; A Espinàs, J.; Font, R.; Argimon, J.M.; Borràs, J.M. Implementing a Cancer Fast-track Programme between primary and specialised care in Catalonia (Spain): A mixed methods study. Br. J. Cancer 2011, 105, 753–759. [Google Scholar] [CrossRef]
- Martinez, M.T.; Gonzalez, I.; Tarazona, N.; Roselló, S.; Saiz, R.; Sanmartín, A.; Martínez-Agulló, A.; Caballero, A.; Mas, P.; Franco, J.; et al. Implementation and assessment of a fast-track programme to improve communication between primary and specialized care in patients with suspected cancer: How to shorten time between initial symptoms of cancer, diagnosis and initiation of treatment. Clin. Transl. Oncol. 2015, 17, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Vallverdu-Cartie, H.; Comajuncosas-Camp, J.; Orbeal-Saenz, R.A.; Negre, J.L.L.; Garriga, P.J.G.; Fraile, J.J.; Bosch, J.H.; Pradell, C.S.; Alsina, S.T.; Bosch, J.U.; et al. Results of implementation of a fast track pathway for diagnosis of colorectal cancer. Rev. Española Enfermedades Dig. 2011, 103, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, A.N.; Van Unnik, J.A.; Keus, R.B. Diagnosis and treatment of soft tissue tumours: The dutch nationwide-accepted consensus. Sarcoma 1998, 2, 183–191. [Google Scholar] [CrossRef]
- Swann, R.; McPhail, S.; Witt, J.; Shand, B.; A Abel, G.; Hiom, S.; Rashbass, J.; Lyratzopoulos, G.; Rubin, G.; National Cancer Diagnosis Audit Steering Group. Diagnosing cancer in primary care: Results from the National Cancer Diagnosis Audit. Br. J. Gen. Pract. 2018, 68, e63–e72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swann, R.; McPhail, S.; A Abel, G.; Witt, J.; Wills, L.; Hiom, S.; Lyratzopoulos, G.; Rubin, G. National Cancer Diagnosis Audits for England 2018 versus 2014: A comparative analysis. Br. J. Gen. Pract. 2023, 73, e566–e574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyratzopoulos, G.; Vedsted, P.; Singh, H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br. J. Cancer 2015, 112 (Suppl. S1), S84–S91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarcoma Policy CheckList. Feb-2017. Available online: https://www.healthpolicypartnership.com/app/uploads/The-sarcoma-policy-checklist.pdf (accessed on 21 April 2025).
- Malik, A.; Wigney, L.; Murray, S.; Gerrand, C.H. The effectiveness of “two-week” referrals for suspected bone and soft tissue sarcoma. Sarcoma 2007, 2007, 23870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, W.S.J.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A.; Jeys, L. ‘’Two-week waits”-are they leading to earlier diagnosis of soft-tissue sarcomas? Sarcoma 2010, 2010, 312648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pencavel, T.D.; Strauss, D.C.; Thomas, G.P.; Thomas, J.M.; Hayes, A.J. Does the two-week rule pathway improve the diagnosis of soft tissue sarcoma? A retrospective review of referral patterns and outcomes over five years in a regional sarcoma centre. Ann. R. Coll. Surg. Engl. 2010, 92, 417–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szucs, Z.; Davidson, D.; Wong, H.H.; Horan, G.; Bearcroft, P.W.P.; Grant, I.; Grimer, R.; Hopper, M.A.; Hatcher, H.; Earl, H. A Comprehensive Single Institutional Review of 2 Years in a Designated Fast-Track Sarcoma Diagnostic Clinic Linked with a Sarcoma Specialist Advisory Group: Meeting the Target but Failing the Task? Sarcoma 2016, 2016, 6032606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerrand, C.; Francis, M.; Dennis, N.; Charman, J.; Lawrence, G.; Evans, T.; Grimer, R. Routes to diagnosis for sarcoma—Describing the sarcoma patient journey. Eur. J. Surg. Oncol. 2015, 41, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.; Oliver, S.E.; Hall, G.; Allgar, V.; Melling, P.; Bolton, E.; Atkin, K.; Denton, D.; Forbes, S.; Green, T.; et al. Patient non-attendance at urgent referral appointments for suspected cancer and its links to cancer diagnosis and one year mortality: A cohort study of patients referred on the Two Week Wait pathway. Cancer Epidemiol. 2019, 63, 101588. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Johnson, G.D.; Grimer, R.J.; Wilson, S. Trends in presentation of bone and soft tissue sarcomas over 25 years: Little evidence of earlier diagnosis. Ann. R. Coll. Surg. Engl. 2011, 93, 542–547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujiwara, T.; Grimer, R.J.; Evans, S.; Rincon, M.R.M.; Tsuda, Y.; Le Nail, L.-R.; Abudu, S. Impact of NICE guidelines on the survival of patients with soft-tissue sarcomas. Bone Jt. J. 2021, 103-B, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Hernández, M.; Casas Ramos, P.; Saldaña Díaz, A.; Mellado Romero, M.A.; Puertas García-Sandoval, J.P.; Ortiz Cruz, E.J. The computer application «SCAE» in the early diagnosis of musculoskeletal cancer in the healthcare area of the Hospital Universitario 12 de Octubre. Analysis of its effectiveness and proposals for improvement. Rev. Española Cirugía Ortopédica Traumatología 2022, 66, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Dyrop, H.B.; Safwat, A.; Vedsted, P.; Maretty-Nielsen, K.; Hansen, B.H.; Jørgensen, P.H.; Baad-Hansen, T.; Bünger, C.; Keller, J. Cancer Patient Pathways shortens waiting times and accelerates the diagnostic process of suspected sarcoma patients in Denmark. Health Policy 2013, 113, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Smith, L. Urgent cancer referrals: How well are they working and can they be improved? Br. J. Gen. Pract. 2021, 71, 390–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noebauer-Huhmann, I.-M.; Vanhoenacker, F.M.; Vilanova, J.C.; Tagliafico, A.S.; Weber, M.-A.; Lalam, R.K.; Grieser, T.; Nikodinovska, V.V.; de Rooy, J.W.J.; Papakonstantinou, O.; et al. Soft tissue tumor imaging in adults: European Society of Musculoskeletal Radiology-Guidelines 2023-overview, and primary local imaging: How and where? Eur. Radiol. 2024, 34, 4427–4437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakkaraju, A.; Sinha, R.; Garikipati, R.; Edward, S.; Robinson, P. Ultrasound for initial evaluation and triage of clinically suspicious soft-tissue masses. Clin. Radiol. 2009, 64, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, E.; Bhuva, S.; Gupta, H.; Robinson, P. Assessment of referrals into the soft tissue sarcoma service: Evaluation of imaging early in the pathway process. Sarcoma 2012, 2012, 781723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dyrop, H.B.; Vedsted, P.; Rædkjær, M.; Safwat, A.; Keller, J. Imaging investigations before referral to a sarcoma center delay the final diagnosis of musculoskeletal sarcoma. Acta Orthop. 2017, 88, 211–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballhause, T.M.; Reiter, A.; Korthaus, A.; Frosch, K.-H.; Schlickewei, C.W.; Priemel, M.H. Diagnostic delay in soft tissue tumors: A single-center study of a university cancer center with a focus on health services research. BMC Health Serv. Res. 2022, 22, 452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, A.; Botchu, R.; Ashford, R.U.; Rennie, W.J. Diagnostic triage for sarcoma: An effective model for reducing referrals to the sarcoma multidisciplinary team. Br. J. Radiol. 2015, 88, 20150037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alanie, O.M.; Mahendra, A.; Mackinnon, M.; McCleery, M.; Nicholas, C.; Gupta, S. A virtual multi-disciplinary meeting is a cost-effective method of triaging referrals to a regional musculoskeletal oncology service. Scott. Med J. 2021, 66, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Nannini, S.; Penel, N.; Bompas, E.; Willaume, T.; Kurtz, J.E.; Gantzer, J. Shortening the Time Interval for the Referral of Patients with Soft Tissue Sarcoma to Expert Centers Using Mobile Health: Retrospective Study. JMIR mHealth uHealth 2022, 10, e40718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsagkaris, C.; Trygonis, N.; Spyrou, V.; Koulouris, A. Telemedicine in Care of Sarcoma Patients beyond the COVID-19 Pandemic: Challenges and Opportunities. Cancers 2023, 15, 3700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuchs, B.; Studer, G.; Bode-Lesniewska, B.; Heesen, P.; On Behalf Of The Swiss Sarcoma Network. The Next Frontier in Sarcoma Care: Digital Health, AI, and the Quest for Precision Medicine. J. Pers. Med. 2023, 13, 1530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


|
|
|
|
|
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year [Reference] | Database, Country | Years of Analysis, n | Type of Sarcoma | Patient Interval (T0) | Primary Care Interval | Secondary Care Interval | Tertiary Care Interval | Total Interval |
| Carrillo- García, 2024 [28] | Multi institutional (5), Spain | 2018 (7 m) n = 182 | BS: 19.2% STS: 80.8% | From symptoms to diagnosis: 83 days (5–328) From symptoms to surgery: 54.5 days (2–331) | ||||
| Elyes, 2023 [29] | Swiss Sarcoma Board (MDT/SSN), Switzerland | 2018–2021 n = 1028 | STS and BS benign tumors | 8.8 w | 0.4 w (0–1.4) | 4.3 w (2.1–9.1) | 1.3 w (−0.6–3.4) | 23.3 w (10.4–59.4) |
| Soomers, 2020 [30] Cas Drabbe, 2021 [31] | Netherland Cancer Registry (NCR)/SURVSARC Study, The Netherlands | 2008–2016 n = 1099 | STS: 76% BS: 24% | >1 m: 60% >3 m: 36% >1 y: 15% | <2 w: 51% >2 w: 49% | <1 m: 64% 1–3 m: 23% | <1 m: 85% 1–2: 30% | Diagnostic interval: ≥1 m: 55% ≥3 m: 28% ≥1 y: 5% |
| Soomers, 2020 [32] | Systematic review of PubMed, Medline, Embase, Web of Science, and Cochrane Library | From 1945 36 studies n = 16,845 | STS and BS | - | 0.1–13.3 w | 1.1–6.9 w | 2.1–7.9 w | 4.3–614.9 w |
| Sarcoma UK, 2020 [33] | Anonymous online survey via UK’s Sarcoma Networks | 2020 n = 117 | STS: 79% BS: 16% GIST: 5% | <2 w: 33% 2–4 w: 20% 4 w–3 m: 22% >3 m: 25% | - | - | - | Diagnostic interval: <2 w: 8% >3 m: 47% |
| Lyratzopoulos, 2015 [34] | English National Audit of Cancer Diagnosis in PC, UK | 2009–2010 n = 72 | STS & BS | 45 days | 45 days Pre-referral interval: 90 days | |||
| (b) | ||||||||
| Author, Year [Reference] | Centre, Country | Years of Analysis, n | Type of Sarcoma | Patient Interval | Primary Care Interval | Secondary Care Interval | Tertiary Care Interval | Total Interval |
| Sam Martin, 2023 [35] | Sarcoma Unit, Royal National Orthopaedic Hospital, Stanmore, UK | 2017 (5 m) n = 78 | BS: 21 STS: 41 H/N: 9 GIST: 7 | 14 days (1–4971) | Diagnostic interval: 86 days (1–1276) | |||
| Dyrop, 2016 [36] | Aarhus Sarcoma Centre, Aarhus, Denmark | 2014–2015 n = 102 | STS | 77 days (11–261) | 17 days (1–56) | 29 days (15–56) | 17 days (10–24) | Diagnostic interval: 65 days (42–133) Total interval: 176 days (883–673) |
| Ramos- Pascua, 2014 [18] | Servicio de Cirugía Ortopédica y Traumatología, León, Spain | 2006–2012 n = 112 | Primary STS T/E | 289 days | 80 days | 173 days | 46 days | - |
| Nakamura, 2011 [37] | Department of Orthopaedic Surgery, Mie University Graduate School, Japan | 2001–2009 n = 100 | Primary STS No WD_LPS No DFS | 3 m (1–72) | - | - | - | From symptoms to diagnosis: 6 m (1–72) TTI: 2 w |
| Seinen, 2010 [38] | Department of Oncology, Skäne University Hospital, Sweden | 2003–2009 n = 33 | Primary RPS LPS (40%) LMS (24%) | 23 days (0–524) | 15 days (0–244) | 36 days (2–1223) | 55 days (1–483) | - |
| Johnson, 2008 [39] | Royal Orthopaedic Hospital, Birmingham, UK | 2005 n = 162 | STS | 1.3 w | 2.4 w | 9.1 w | - | Median time from symptoms to referral to sarcoma specialist: 40.4 w (mean: 2.3 w) |
| Clark, 2005 [26] | Soft-Tissue Sarcoma Unit of the Royal Marsden Hospital, London, UK | 2003–2004 n = 159 | STS and BS | - | 14 m (4–96) | Hospital/specialist level delay 21 m | - | |
| Brouns, 2003 [27] | Department of Surgical Oncology, University Hospital Gasthuisberg, Leuven, Belgium | 1999–2001 n = 100 | STS and GIST | 4 m (1–240) | HG STS: 85% < 6 m, 50% w/o delay LG STS: 45% > 6 m, 50% w/o delay From first consultation to diagnosis: 6 m (2–79) | |||
| Author, Year (Reference) | Database, Country | Years of Analysis, n | Type of Sarcoma | TTI | TTI Increase |
|---|---|---|---|---|---|
| Schärer, 2023 [22] | Swiss sarcoma Network (SSN/MDT/SB), Switzerland | 2018–2022 n = 266 | S/D_STS (80.1%) BS (19.9%) | S_STS: 42 days (27–71) D_STS: 28 days (18–48) | - |
| Ryu, 2023 [40] | New Zealand Sarcoma Multidisciplinary Meeting (NZ-MDM), New Zealand | 2011–2020 n = 223 | STS | Median: 33 days | Median TTI 2011–2015: 27 days (11.5–37) Median TTI 2016–2020: 35 days (21–47) |
| Ogura, 2021 [41] | National Cancer Database (NCDB), US | 2004–2015 n = 23,786 | HG-STS T/E | Median: 14 days (0–32) Mean: 20.9 ± 27.5 days | Median TTI in 2004: 11 days Median TTI in 2015: 17 days |
| Curtis, 2018 [42] | National Cancer Database (NCDB), US | 2004–2013 n = 41,529 | STS | Median: 22 days Mean: 29.7 days | Median TTI in 2004: 20 days Median TTI in 2013: 26 days |
| Tumor-related causes | Malignant vs. benign tumors |
| Anatomic location | |
| Deep | |
| Grade | |
| Histology | |
| Stage | |
| Patient-related causes | Patient’s characteristics DM, tobacco Mental condition Age |
| Socio-economic status Private vs. others Income status Housing | |
| Geographical barriers Time or distance from reference center | |
| Socio-cultural barriers Race Educational status Immigrants | |
| General population-related causes | Lack of awareness |
| Primary care-related causes | Lack of initial suspicion Misinterpretation of imaging/biopsy |
| Health system-related causes | Lack of communication/relationships Lack of clinical guidelines Lack of continuity of care |
| Sarcoma team-related causes |
| Author, Year, [Reference] | Period of Time, n | Histology | Variable Measured | Results | Waiting Time Paradox |
|---|---|---|---|---|---|
| Ryu, 2023 [40] | 2011–2020 n = 223 | HG T/Extr No WD_LPS No SC-STS | TTI | TTI > 30 days DMFS rate 63% vs. TTI < 30 days DMFS rate 50% (p = 0.03) LRFS/DSS: ns | Yes |
| Ogura, 2021 [41] | 2004–2015 n = 23,786 (NCDB) | HG T/Extr | TTI | TTI: 0–30 days, OS: 57.7%/TTI: 31–60 days, OS: 53.9%, HR: 1.08 TTI: 61–90 days, OS: 50.6%, HR: 1.11/TTI > 91 days, OS: 49.9%, HR: 1.22 (p < 0.05) | No |
| Araki, 2020 [72] | 210–2017 n = 153 | STS T/Extr No LG | T0, T1/2 | T0 < 5 months 5-years MFS 95% vs. 49% (>5 months), p = 0.02 T1/2 ≤ 29 days 5-years MFS 95% vs. 49% (>29 days), p = 0.01 | No No |
| Featherall, 2019 [73] | 2004–2012 n = 8648 (NCDB) | HG localized STS | TTI | Linear association with OS Minimal HR (0.64): 42 days | No |
| Nandra, 2015 [45] | 1985–2010 n = 4945 | STS: 46% | T0 | LG: 44 weeks (mortality: 2.3%, HR: 8.52) vs. 20 weeks (HG) (mortality: 17%), p < 0.0001 | Yes |
| Urakawa, 2015 [74] | 2001–2011 n = 152 | No DFS No WD_LPS | T0+T1+T2 | 5-year OS (≥6 months): 95% vs. 66% (<6 months), p = 0.016 | Yes |
| Rougraff, 2012 [75] | 1992–2007 n = 381 | STS T/Extr | Symptoms- treatment | No relationship with size, OS, or metastases | Yes |
| Nakamura, 2011 [37] | 2001–2009 n = 100 | No WD_LPS | Symptoms- diagnosis | 5-year OS (<6 months): 77% vs. 59.7% (>6 months) 5-year DMR (<6 months): 76.5% vs. 38.8% (>6 months) | No Yes |
| Rougraff, 2007 [71] | 1992–2003 n = 624 | STS/BS T/Extr | Symptoms- diagnosis | HG: 17 months vs. 29.4 months (LG). No relationship with Size, OS, Location or Metastases Q4: 5-year OS 84% vs. 65% (HR: 0.6), p < 0.05 Q4: 5-year DFS 81% vs. 60% (HR: 0.5), p < 0.05 | Yes No No |
| Saithna, 2007 [76] | 25 years period n = 1349 | STS | Symptoms- diagnosis S/SD | Additional week of symptoms improves the monthly OS by 0.2% S/SD ratio impacts OS with a HR: 1.4 (p < 0.0001) | Yes No |
| Gofman, 2007 [77] | 1991–2004 n = 73 | SS T/Extr | Symptoms- diagnosis | Delayed ≤1 year was superior to a delayed >1 years in terms of systemic control (HR:0.33), p = 0.037. OS was not affected | No |
| Ruka, 1988 [78] | 1950–1984 n = 267 | HG STS No visceral No DFS, KS No desmoids | Symptoms- treatment S/SD | Duration of symptoms without relationship with OS 5-year OS in S/SD ratio ≤ 1: 41% (49 months) vs. 28% (21 months) in S/SD > 1, p = 0.02 5-year DFS in S/SD ratio ≤ 1: 45% (40 months) vs. 31% (8 months) in S/SD > 1, p = 0.019 5-year DM in S/SD ratio ≤ 1: 28% (17 months) vs. 44% (48 months) in S/SD > 1, p = 0.0034 | Yes Yes Yes |
| Soomers, 2020 [32] | 1945–present 36 studies n = 16,845 | STS and BS | Total interval (10 retrospective studies) | No effect on survival: 5 studies Improved survival rate with longer total interval (26 vs. 20 weeks): 1 study (STS) Improved survival rates with shorter total interval: 3 studies | Yes Yes No |
| Author, Year [Reference] | Database, Country, Years Reviewed, Number of Claims | Type of Sarcoma | Plaintiff (%) | Alleged Negligence | Negligence Proven | Average Indemnity Payment |
|---|---|---|---|---|---|---|
| Mesko, 2014 [89] | LexisNexis®, USA 1980–2012 n = 216 | Extremity STS (59.7%) BS (40.3%) | PC (34.4%) OpS (22.8%) Rx (12.4%) | Delay in diagnosis (81%) Unnecessary amputation (10.6%) | 62% | USD 2,302,483 (65,076– 12,661,611) |
| Ross, 2015 [91] | NHSLA, UK (England and Wales) 2003–2012 n = 69 | NA | OpS (100%) | Delay in diagnosis (23%) | 36.2% | GBP 116,502 |
| Harrison, 2016 [90] | NHSLA, UK (England and Wales) 1995–2010 n = 52 | Orthopedic related | OpS (100%) | Delay in diagnosis (66%) | 71% | GBP 84,000 |
| Hwang, 2020 [92] | Westlaw MDB (USA) 1982–2018 n = 92 | All STS (57%) BS (43%) | PC (26%) OpS (23%) Rx (15%) | Delay in diagnosis (91%) | 30% Settlement:32% | USD 1.9 millions |
| Davis, 2023 [93] | Westlaw MDB (USA) 1980–2021 n = 36 | - | OpS | Failure diagnosis: 42% | Defense: 75% Plaintiff: 17% Settlement: 3% | USD 1,672,500 (134,231– 6,250,000) |
| Bayram, 2024 [94] | UYAP, TRK 2014–2024 n = 70 | Primary STS/BS | OpS (51.7%) Pth (16.4%) Rx (8.2%) | Delay diagnosis and/or diagnostic error: 29.4% | 11 cases (16%) | - |
| UK NICE 2005 [107] | UK NICE 2015 [114] | Sweden 2012 [108] | Scotland SSMCN 2004 [115] | The Netherlands 2005 [116] | Denmark CPP 2009 [24] | UK RNOH 2024 [9] | Japan JOP 2020 [117] | |
|---|---|---|---|---|---|---|---|---|
| Size | >5 cm | >4.3 cm “Golf ball” | >5 cm | >5 cm | >3 cm and complex | >5 cm | >5 cm “Golf ball” | >5 cm |
| Location | Deep | - | Deep | Deep | - | Deep | Deep | Deep |
| Growth | Growing | Growing | - | Growing | - | Growing | Increasing in size | - |
| Pain | Painful lump | - | - | - | - | - | With or without concern in large painless lumps | - |
| Age | - | >50 years | - | - | - | - | - | - |
| Duration | - | Short | - | - | - | Short | - | - |
| Other criteria | - | - | No open biopsy or surgery No imaging | - | Radical excision in case of <3 cm Biopsy in deep >3 cm | Palpable bone tumor Deep and persistent bone pain | Firmer than surrounding tissues Risk factors * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.Á.; Gómez, B.; Díaz-Gómez, D.; López, I.; Lozano, P.; Muñoz, P.; Muñoz-Casares, F.C.; Olivares-Ripoll, V.; Vasques, H.; Asencio-Pascual, J.M. Diagnostic Delay in Soft Tissue Sarcomas: A Review. Cancers 2025, 17, 1861. https://doi.org/10.3390/cancers17111861
Fernández JÁ, Gómez B, Díaz-Gómez D, López I, Lozano P, Muñoz P, Muñoz-Casares FC, Olivares-Ripoll V, Vasques H, Asencio-Pascual JM. Diagnostic Delay in Soft Tissue Sarcomas: A Review. Cancers. 2025; 17(11):1861. https://doi.org/10.3390/cancers17111861
Chicago/Turabian StyleFernández, Juan Ángel, Beatriz Gómez, Daniel Díaz-Gómez, Irene López, Pablo Lozano, Paula Muñoz, Francisco Cristóbal Muñoz-Casares, Vicente Olivares-Ripoll, Hugo Vasques, and José Manuel Asencio-Pascual. 2025. "Diagnostic Delay in Soft Tissue Sarcomas: A Review" Cancers 17, no. 11: 1861. https://doi.org/10.3390/cancers17111861
APA StyleFernández, J. Á., Gómez, B., Díaz-Gómez, D., López, I., Lozano, P., Muñoz, P., Muñoz-Casares, F. C., Olivares-Ripoll, V., Vasques, H., & Asencio-Pascual, J. M. (2025). Diagnostic Delay in Soft Tissue Sarcomas: A Review. Cancers, 17(11), 1861. https://doi.org/10.3390/cancers17111861





