The Association Between Skeletal Muscle Mass and Surgical Site Infection and Prognosis in Patients Undergoing Free Flap Reconstructive Surgery for Oral Squamous Cell Carcinoma: A Single-Center, Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Study Variables
2.3. Data Analyses
3. Results
3.1. Patient Characteristics
3.2. Clinical Characteristics of Patients Included in the Study Dichotomized by SMI Cutoff Value
3.3. Association Between Clinical Factors and OS and DFS
3.4. Cox Multivariate Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, E.; Chargi, N.; van Gemert, J.T.M.; van Es, R.J.J.; Dieleman, F.J.; Rosenberg, A.J.W.P.; Van Cann, E.M.; de Bree, R. Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral. Oncol. 2020, 101, 104530. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Yamaguchi, T.; Nakamura, H.; Yamatsu, Y.; Hirai, Y.; Shoda, K.; Kurozumi, S.; Ibaragi, S.; Harimoto, N.; Motegi, S.I.; et al. Evaluation of overall and disease-free survival in patients with free flaps for oral cancer resection. Microsurgery 2020, 40, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.P.; Daher, G.S.; Bauman, M.M.J.; Moore, E.J.; Tasche, K.K.; Price, D.L.; Van Abel, K.M. Association of sarcopenia with oncologic outcomes of primary treatment among patients with oral cavity cancer: A systematic review and meta-analysis. Oral. Oncol. 2023, 147, 106608. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Yamaguchi, T.; Nakamura, H.; Suzuki, K.; Harimoto, N.; Shirabe, K.; Yokoo, S. Impact of skeletal muscle mass volume on surgical site infection in free flap reconstruction for oral cancer. Microsurgery 2019, 39, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Chargi, N.; Breik, O.; Forouzanfar, T.; Martin, T.; Praveen, P.; Idle, M.; Parmar, S.; de Bree, R. Association of low skeletal muscle mass and systemic inflammation with surgical complications and survival after microvascular flap reconstruction in patients with head and neck cancer. Head. Neck 2022, 44, 2077–2094. [Google Scholar] [CrossRef] [PubMed]
- Ansari, E.; Ganry, L.; Van Cann, E.M.; de Bree, R. Impact of low skeletal muscle mass on postoperative complications in head and neck cancer patients undergoing free flap reconstructive surgery—A systematic review and meta-analysis. Oral. Oncol. 2023, 147, 106598. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Makiguchi, T.; Yamaguchi, T.; Suzuki, K.; Yokoo, S. Impact of sarcopenia on postoperative surgical site infections in patients undergoing flap reconstruction for oral cancer. Int. J. Oral. Maxillofac. Surg. 2020, 49, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shirakawa, Y.; Tanabe, S.; Sakurama, K.; Noma, K.; Fujiwara, T. Skeletal muscle loss in the postoperative acute phase after esophageal cancer surgery as a new prognostic factor. World J. Surg. Oncol. 2020, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Bonavolontà, P.; Improta, G.; Dell’Aversana Orabona, G.; Goglia, F.; Abbate, V.; Sorrentino, A.; Piloni, S.; Salzano, G.; Iaconetta, G.; Califano, L. Evaluation of sarcopenia and sarcopenic obesity in patients affected by oral squamous cell carcinoma: A retrospective single-center study. J. Craniomaxillofac. Surg. 2023, 51, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ansari, E.; Chargi, N.; van Es, R.J.J.; Dieleman, F.J.; Van Cann, E.M.; de Bree, R. Association of preoperative low skeletal muscle mass with postoperative complications after selective neck dissection. Int. J. Oral. Maxillofac. Surg. 2022, 51, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control 1999, 27, 97–134. [Google Scholar] [CrossRef]
- Vangelov, B.; Bauer, J.; Moses, D.; Smee, R. The effectiveness of skeletal muscle evaluation at the third cervical vertebral level for computed tomography-defined sarcopenia assessment in patients with head and neck cancer. Head. Neck 2022, 44, 1047–1056. [Google Scholar] [CrossRef]
- Yoon, J.K.; Jang, J.Y.; An, Y.S.; Lee, S.J. Skeletal muscle mass at C3 may not be a strong predictor for skeletal muscle mass at L3 in sarcopenic patients with head and neck cancer. PLoS ONE 2021, 16, 0254844. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral. Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bril, S.I.; Wendrich, A.W.; Swartz, J.E.; Wegner, I.; Pameijer, F.; Smid, E.J.; Bol, G.H.; Pothen, A.J.; de Bree, R. Interobserver agreement of skeletal muscle mass measurement on head and neck CT imaging at the level of the third cervical vertebra. Eur. Arch. Otorhinolaryngol. 2019, 276, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, K.; Mizukoshi, A.; Lee, K.; Ikegami, S. Sarcopenia with inflammation as a predictor of survival in patients with head and neck cancer. Auris Nasus Larynx 2021, 48, 1013–1022. [Google Scholar] [CrossRef]
- Sandmael, J.A.; Bye, A.; Solheim, T.S.; Stene, G.B.; Thorsen, L.; Kaasa, S.; Lund, J.Å.; Oldervoll, L.M. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: A pilot randomized trial. Cancer 2017, 123, 4440–4448. [Google Scholar] [CrossRef] [PubMed]
- Wobith, M.; Weimann, A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients 2021, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
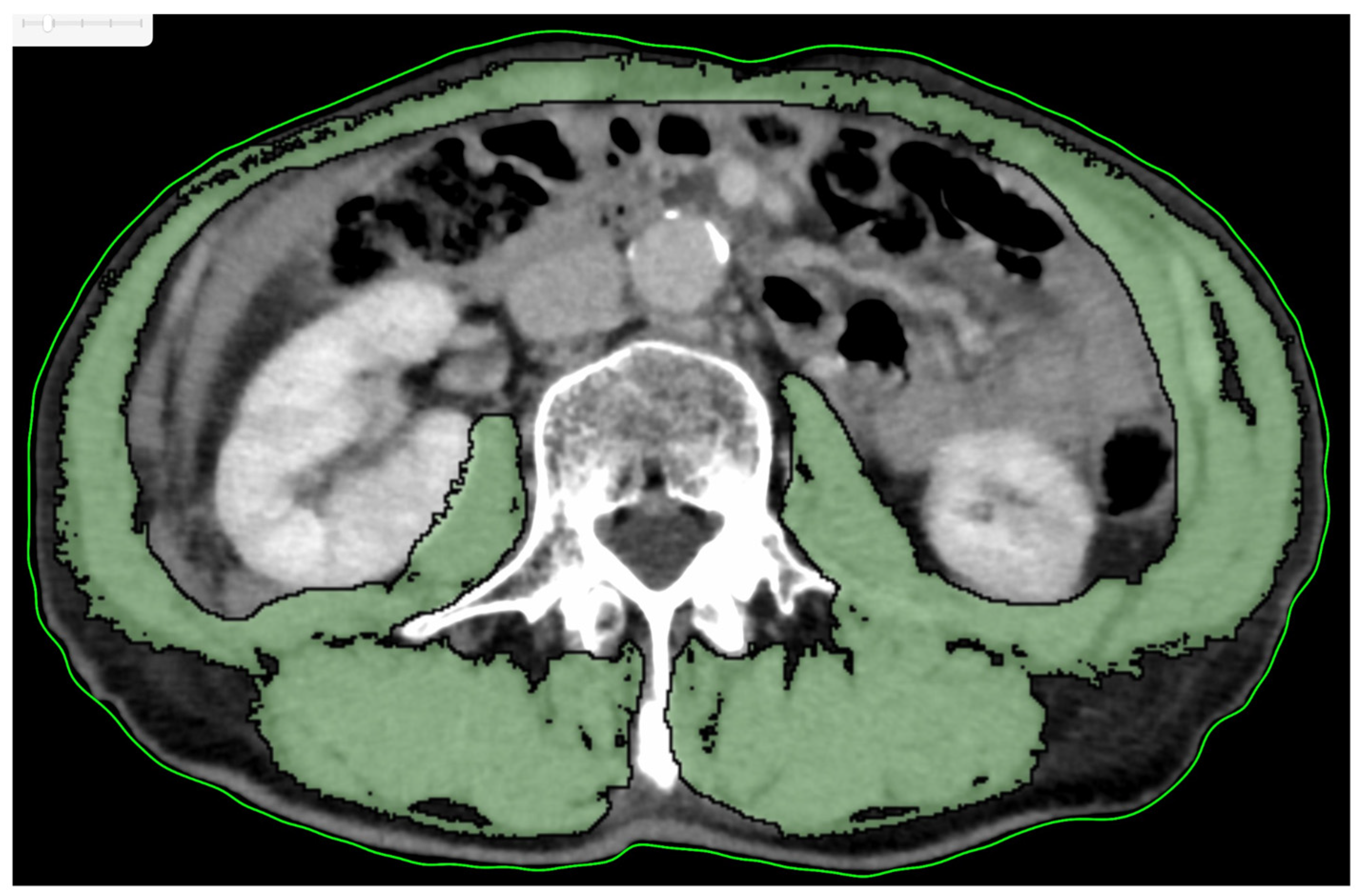





| Variables | Total No. of Patients | Low SMI No. of Patients (%) n = 47 | High SMI No. of Patients (%) n = 45 | p-Value † | |
|---|---|---|---|---|---|
| Sex | Male | 59 | 30 (63.8) | 29 (64.4) | 0.951 |
| Female | 33 | 17 (36.2) | 16 (35.6) | ||
| Age (years) | <65 | 37 | 14 (29.8) | 23 (51.1) | 0.037 * |
| ≥65 | 55 | 33 (70.2) | 22 (48.9) | ||
| BMI (kg/m2) | <18.5 | 11 | 8 (17.0) | 3 (6.7) | 0.199 |
| ≥18.5 | 81 | 39 (83.0) | 42 (93.3) | ||
| Tabaco consumption | Ever | 45 | 25 (53.2) | 20 (44.4) | 0.401 |
| Never | 47 | 22 (46.8) | 25 (55.6) | ||
| Alcohol consumption | Present | 44 | 24 (51.1) | 20 (44.4) | 0.525 |
| Absent | 48 | 23 (48.9) | 25 (55.6) | ||
| Primary site | Tongue | 41 | 25 (53.2) | 16 (35.6) | 0.368 |
| Lower gingiva | 35 | 15 (31.9) | 20 (44.4) | ||
| Buccal mucosa | 8 | 3 (6.4) | 5 (11.1) | ||
| Floor of the mouth | 7 | 4 (8.5) | 3 (6.7) | ||
| Upper gingiva | 1 | 0 (0) | 1 (2.2) | ||
| Clinical T classification | T2 | 17 | 9 (19.1) | 8 (17.8) | 0.722 |
| T3 | 15 | 9 (19.1) | 6 (13.3) | ||
| T4a | 47 | 24 (51.1) | 23 (51.1) | ||
| T4b | 13 | 5 (10.6) | 8 (17.8) | ||
| Clinical N classification | N0 | 41 | 25 (53.2) | 16 (35.6) | 0.140 |
| N1 | 11 | 3 (6.4) | 8 (17.8) | ||
| N2b | 35 | 15 (31.9) | 20 (44.4) | ||
| N2c | 4 | 3 (6.4) | 1 (2.2) | ||
| N3b | 1 | 1 (2.1) | 0 (0) | ||
| Stage classification | II | 9 | 5 (10.6) | 4 (8.9) | 0.760 |
| III | 11 | 7 (14.9) | 4 (8.9) | ||
| IVa | 58 | 29 (61.7) | 29 (64.4) | ||
| IVb | 14 | 6 (12.8) | 8 (17.8) | ||
| Histological grade | G1 | 49 | 21 (44.7) | 28 (62.2) | 0.122 |
| G2 | 34 | 19 (40.4) | 15 (33.3) | ||
| G3 | 9 | 7 (14.9) | 2 (4.4) | ||
| Preoperative radiotherapy | Present | 6 | 3 (6.4) | 3 (6.7) | 1.000 |
| Absent | 86 | 44 (93.6) | 42 (93.3) | ||
| Pathological N classification | N0 | 51 | 27 (57.4) | 24 (53.3) | 0.657 |
| N1 | 13 | 7 (14.9) | 6 (13.3) | ||
| N2b | 18 | 10 (21.3) | 8 (17.8) | ||
| N2c | 1 | 0 (0) | 1 (2.2) | ||
| N3b | 9 | 3 (6.4) | 6 (13.3) | ||
| Surgical site infection | Present | 11 | 5 (10.6) | 6 (13.3) | 0.690 |
| Absent | 81 | 42 (89.4) | 39 (86.7) | ||
| Delirium | Present | 27 | 15 (31.9) | 12 (26.7) | 0.581 |
| Absent | 65 | 32 (68.1) | 33 (73.3) | ||
| Postoperative pneumonia | Present | 21 | 8 (17.0) | 13 (28.9) | 0.175 |
| Absent | 71 | 39 (83.0) | 32 (71.1) | ||
| Primary recurrence | Present | 14 | 9 (19.1) | 5 (11.1) | 0.283 |
| Absent | 78 | 38 (80.9) | 40 (88.9) | ||
| Neck recurrence | Present | 11 | 8 (17.0) | 3 (6.7) | 0.199 |
| Absent | 81 | 39 (83.0) | 42 (93.3) | ||
| Distant metastasis | Present | 20 | 13 (27.7) | 7 (15.6) | 0.159 |
| Absent | 72 | 34 (72.3) | 38 (84.4) | ||
| Free flap type | ALT | 37 | 23 (48.9) | 14 (31.1) | 0.210 |
| FF | 31 | 13 (27.7) | 18 (40.0) | ||
| RAMF | 24 | 11 (23.4) | 13 (28.9) | ||
| Variables (Continuous) | Low SMI Median (Range) | High SMI Median (Range) | p-Value †† | ||
| HCU duration | (days) | 7 (4–10) | 7 (5–12) | 0.411 | |
| Hospital stay duration | (days) | 38 (22–152) | 36 (19–98) | 0.537 | |
| PS | 0 (0–1) | 0 (0–1) | 0.692 | ||
| ASA | 2 (1–3) | 2 (1–3) | 0.540 | ||
| Bleeding count | (mL) | 374 (126–1031) | 510 (70–1278) | 0.004 ** | |
| Operative time | (h:min) | 11:05 (8:24–15:15) | 11:47 (8:20–15:31) | 0.133 | |
| Albumin | (mg/dL) | 4.2 (3.3–5.1) | 4.2 (3.2–4.8) | 0.820 | |
| NLR | 2.74 (1.09–11.70) | 2.39 (0.93–15.23) | 0.246 | ||
| LMR | 4.38 (1.14–11.15) | 5.43 (1.81–8.80) | 0.060 | ||
| PLR | 149.54 (73.03–529.79) | 134.81 (34.52–291.06) | 0.193 | ||
| PNI | 116.90 (63.01–228.00) | 127.45 (89.50–222.00) | 0.119 | ||
| MAR | 88.64 (0–200.00) | 90.24 (34.55–160.98) | 0.919 | ||
| CAR | 0.0200 (0.0063–0.1921) | 0.0136 (0.0063–0.6641) | 0.379 |
| Variables | No. of Patients (%) | OS (%) | p † | DFS (%) | p † | |
|---|---|---|---|---|---|---|
| Age (years) | ≥65 | 55 (59.8) | 60.1 | 0.013 * | 58.0 | 0.057 |
| <65 | 37 (40.2) | 86.1 | 78.4 | |||
| Sex | Male | 59 (64.1) | 68.4 | 0.658 | 66.1 | 0.665 |
| Female | 33 (35.9) | 74.8 | 67.3 | |||
| BMI (kg/m2) | <18.5 | 11 (12.0) | 54.5 | 0.051 | 54.5 | 0.231 |
| ≥18.5 | 81 (88.0) | 73.2 | 68.1 | |||
| SMI | High | 45 (48.9) | 81.1 | 0.042 * | 81.4 | 0.070 |
| Low | 47 (51.1) | 60.2 | 65.4 | |||
| Tobacco consumption | Ever | 45 (48.9) | 74.3 | 0.535 | 64.4 | 0.601 |
| Never | 47 (51.1) | 68.4 | 68.7 | |||
| Alcohol consumption | Ever | 44 (47.8) | 76.3 | 0.442 | 68.2 | 0.804 |
| Never | 48 (52.2) | 66.5 | 65.2 | |||
| Diabetes mellitus | Present | 21 (22.8) | 71.4 | 0.929 | 66.7 | 0.965 |
| None | 71 (77.2) | 70.2 | 66.5 | |||
| Cardiovascular disease | Present | 7 (7.6) | 47.6 | 0.284 | 57.1 | 0.442 |
| None | 85 (92.4) | 73.3 | 67.3 | |||
| Cerebrovascular disease | Present | 8 (8.7) | 87.5 | 0.320 | 100 | 0.062 |
| None | 84 (91.3) | 69.0 | 63.3 | |||
| History of cancer | Present | 16 (17.4) | 80.2 | 0.400 | 80.8 | 0.241 |
| None | 76 (82.6) | 69.0 | 63.6 | |||
| Preoperative radiotherapy | Present | 6 (6.5) | 66.7 | 0.829 | 83.3 | 0.381 |
| Absent | 86 (93.5) | 70.8 | 65.3 | |||
| Primary site | Tongue | 41 (44.6) | 67.5 | <0.001 ** | 63.4 | <0.001 ** |
| Lower gingiva | 35 (38.1) | 100 | 100 | |||
| Buccal mucosa | 8 (8.7) | 75.0 | 0 | |||
| Floor of the mouth | 7 (7.6) | 0 | 42.9 | |||
| Upper gingiva | 1 (1.1) | 100 | 100 | |||
| Clinical T classification | T2 | 17 (18.5) | 74.9 | 0.871 | 64.7 | 0.904 |
| T3 | 15 (16.3) | 80.0 | 64.0 | |||
| T4a | 47 (51.1) | 67.8 | 68.0 | |||
| T4b | 13 (14.1) | 68.4 | 61.5 | |||
| Clinical N classification | N0 | 41 (44.6) | 77.4 | 0.014 * | 78.0 | 0.018 * |
| N1 | 11 (12.0) | 77.9 | 53.0 | |||
| N2b | 35 (38.0) | 70.4 | 65.7 | |||
| N2c | 4 (4.3) | 25.0 | 25.0 | |||
| N3b | 1 (1.1) | 0 | 0 | |||
| Clinical stage classification | II | 9 (9.8) | 77.8 | 0.803 | 66.7 | 0.830 |
| III | 11 (12.0) | 72.7 | 54.5 | |||
| IVA | 58 (63.0) | 71.6 | 70.6 | |||
| IVB | 14 (15.2) | 63.5 | 57.1 | |||
| Pathological surgical margin (mm) | ≥5 | 51 | 73.3 | 0.059 | 69.0 | 0.076 |
| <5 | 34 | 73.5 | 67.6 | |||
| Positive | 5 | 0 | 20.0 | |||
| pN classification | N0 | 51 (55.4) | 83.4 | <0.001 ** | 86.2 | <0.001 ** |
| N1 | 13 (14.1) | 57.1 | 61.5 | |||
| N2b | 18 (19.6) | 72.2 | 42.9 | |||
| N2c | 1 (1.1) | 0 | 0 | |||
| N3b | 9 (9.8) | 22.2 | 22.2 | |||
| pN | N1-3b | 41 (44.6) | 55.8 | 0.007 ** | 42.5 | <0.001 ** |
| N0 | 51 (55.4) | 83.4 | 86.2 | |||
| ENE | Present | 9 (9.8) | 22.2 | <0.001 ** | 22.2 | <0.001 ** |
| None | 83 (90.2) | 76.1 | 71.3 | |||
| Histological grade | G1 | 49 (53.3) | 74.9 | 0.829 | 69.3 | 0.974 |
| G2 | 34 (37.0) | 67.1 | 63.0 | |||
| G3 | 9 (9.8) | 66.7 | 66.7 | |||
| Albumin | <4.0 | 18 (19.6) | 43.2 | 0.001 ** | 44.4 | 0.018 * |
| (mg/dL) | ≥4.0 | 74(80.4) | 77.7 | 72.0 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Values † | HR (95% CI) | p-Values † |
| Age (years) | 3.225 (1.209–8.602) | 0.019 * | ||
| ≥65 vs. <65 | ||||
| SMI | ||||
| Low vs. High | 2.339 (1.008–5.429) | 0.048 * | 2.900(1.226–6.862) | 0.015 ** |
| pN | ||||
| N0 vs. N1-3b | 3.008 (1.297–6.976) | 0.010 * | ||
| ENE | ||||
| Present vs. Absent | 6.147 (2.527–14.949) | <0.001 ** | 7.727 (3.083–19.368) | <0.001 ** |
| Albumin (mg/dL) | ||||
| <4.0 vs. ≥4.0 | 3.429 (1.532–7.676) | 0.003 ** |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Values † | HR (95% CI) | p-Value † |
| pN | ||||
| N0 vs. N1-3b | 4.445 (1.903–10.379) | <0.001 * | 4.248 (1.813–9.953) | <0.001 ** |
| ENE | ||||
| Present vs. Absent | 3.384 (1.435–7.980) | 0.005 ** | ||
| Albumin (mg/dL) | ||||
| <4.0 vs. ≥4.0 | 2.294 (1.069–4.921) | 0.033 * | 2.039 (0.944–4.406) | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, A.; Yamagata, K.; Fukuzawa, S.; Sasaki, K.; Takaoka, S.; Uchida, F.; Ishibashi-Kanno, N.; Sekido, M.; Bukawa, H. The Association Between Skeletal Muscle Mass and Surgical Site Infection and Prognosis in Patients Undergoing Free Flap Reconstructive Surgery for Oral Squamous Cell Carcinoma: A Single-Center, Retrospective Study. Cancers 2025, 17, 1729. https://doi.org/10.3390/cancers17101729
Noguchi A, Yamagata K, Fukuzawa S, Sasaki K, Takaoka S, Uchida F, Ishibashi-Kanno N, Sekido M, Bukawa H. The Association Between Skeletal Muscle Mass and Surgical Site Infection and Prognosis in Patients Undergoing Free Flap Reconstructive Surgery for Oral Squamous Cell Carcinoma: A Single-Center, Retrospective Study. Cancers. 2025; 17(10):1729. https://doi.org/10.3390/cancers17101729
Chicago/Turabian StyleNoguchi, Atsuro, Kenji Yamagata, Satoshi Fukuzawa, Kaoru Sasaki, Shohei Takaoka, Fumihiko Uchida, Naomi Ishibashi-Kanno, Mitsuru Sekido, and Hiroki Bukawa. 2025. "The Association Between Skeletal Muscle Mass and Surgical Site Infection and Prognosis in Patients Undergoing Free Flap Reconstructive Surgery for Oral Squamous Cell Carcinoma: A Single-Center, Retrospective Study" Cancers 17, no. 10: 1729. https://doi.org/10.3390/cancers17101729
APA StyleNoguchi, A., Yamagata, K., Fukuzawa, S., Sasaki, K., Takaoka, S., Uchida, F., Ishibashi-Kanno, N., Sekido, M., & Bukawa, H. (2025). The Association Between Skeletal Muscle Mass and Surgical Site Infection and Prognosis in Patients Undergoing Free Flap Reconstructive Surgery for Oral Squamous Cell Carcinoma: A Single-Center, Retrospective Study. Cancers, 17(10), 1729. https://doi.org/10.3390/cancers17101729








