Bridging Immune Evasion and Vascular Dynamics for Novel Therapeutic Frontiers in Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Global Epidemiological Landscape
| Parameter | United States (US) | Global (WHO Data) |
|---|---|---|
| Incidence Rate | Approximately 6–8 cases per 100,000 people annually | Approximately 110–20 cases per 100,000 people globally (varies widely by region) |
| Annual New Cases | ≈12.5 per 100,000 population new cases (projected for 2024) | ≈11.2 per 100,000 population new cases globally |
| Leading Risk Factors | Hepatitis C infection, alcohol-related liver disease, metabolic-associated fatty liver disease (MAFLD)/MASH | Hepatitis B and C infections, aflatoxin exposure, metabolic-associated liver disease, MAFLD |
| Most Affected Age Group | 50–70 years | Varies by region; generally, affects individuals aged 40–70 years |
| Gender Distribution | Male—Female ≈ 2–3:1 | Male—Female ≈ 2–4:1 (higher male predominance in HBV-endemic regions) |
| Five-Year Survival Rate | ~20% for localized HCC, ~11% overall | ~18% globally (varies widely by region and stage at diagnosis); <10% in high-burden regions (due to late diagnosis, limited treatment access) |
| Primary Etiological Agents | Hepatitis C (historically the most common), but MAFLD and metabolic syndrome-related HCC rates are rising | Hepatitis B is the leading cause globally, especially in Asia and Africa; rising cases from MAFLD in Western countries |
| Mortality Rate | ≈9.3 per 100,000 population deaths annually | ≈10.2 per 100,000 population deaths globally annually |
| Regional Hotspots | Highest incidence rates in Southern and Southwestern states | High-incidence regions include East Asia (China), Southeast Asia, sub-Saharan Africa, and parts of the Middle East |
| Trends | Increasing due to MAFLD and obesity, especially in younger populations, historically driven by hepatitis C | Rising in Western countries due to MAFLD; stabilized or decreasing in regions with effective hepatitis B vaccination |
| Screening and Early Detection | Routine screening for high-risk individuals (e.g., those with cirrhosis and hepatitis B/C) is increasing | Varies by region; established in some high-risk areas (e.g., Asia); less common in low-resource settings |
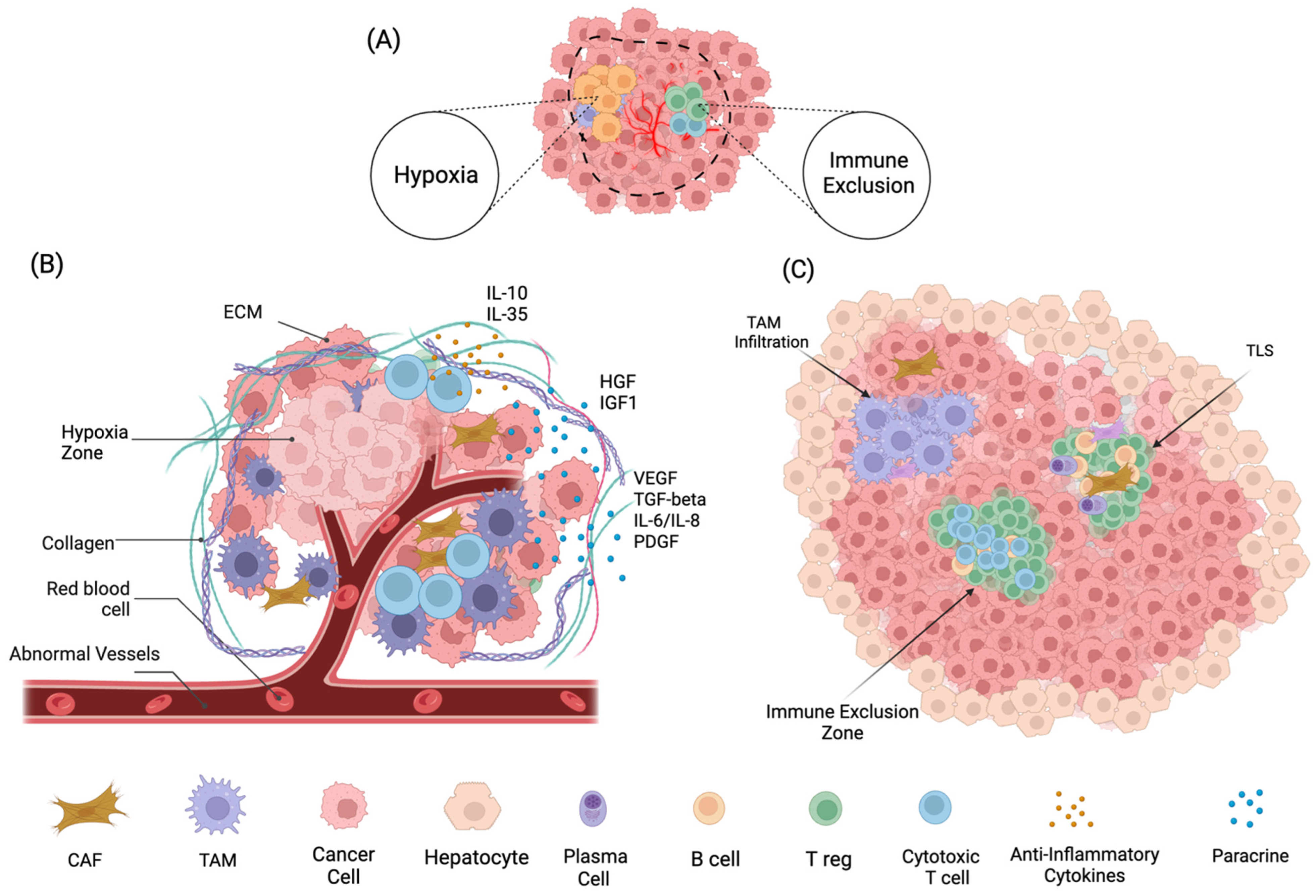
3. Tumor Microenvironment Landscape in HCC
Emerging Therapeutic Implications in Remodeling HCC TME
4. Angiogenesis Signaling in HCC
5. Clinical Trials and Treatment Approaches Targeting TME and Angiogenesis
| Category | Trial Name | Therapy Combination | Phase | PFS | OS | Key Findings |
|---|---|---|---|---|---|---|
| IO | CheckMate-040 [126] | Nivolumab (PD-1) + Ipilimumab (CTLA-4) | I/II | 5.1 months vs. 4.3 months (nivolumab/ ipilimumab plus cabozantinib) | 20.2 months vs. 22.1 months for the triplet arm | Doublet demonstrates encouraging antitumor activity and consistent safety profiles for the combination therapies in patients with advanced HCC. |
| KEYNOTE-224 [127,128,129] | Pembrolizumab (PD-1) | II | 4 months (95% CI, 2–8) | 13.9 months | Pembrolizumab monotherapy provides durable antitumor activity, promising overall survival, and has a manageable safety profile in patients with advanced HCC who have not received prior systemic therapy. | |
| KEYNOTE-240 [130,131,132] | Pembrolizumab (PD-1) | III | 3.0 months vs. 2.8 months (placebo) HR 0.72 | 13.9 months vs. 10.6 months (placebo) HR of 0.78 | Pembrolizumab provides clinical benefits in terms of antitumor activity and safety, supporting its use as a second-line therapy in advanced HCC despite not achieving statistical significance in primary endpoints. | |
| Combination | KN046 [133] | KN046 (PD-L1/CTLA-4 bispecific antibody) monotherapy | II | 11.0 months (95% CI, 8.2–15.2) | 16.4 months (95% CI, 11.20-not estimable), 12-month OS rate of 60.0% (95% CI, 45.9–71.6) | KN046 combined with lenvatinib showed promising efficacy and a manageable safety profile in patients with advanced unresectable or metastatic HCC. The objective response rate (ORR) was 45.5% (95% CI, 31.97–59.45). |
| INSPIRE [134] | Atezolizumab (anti-PD-L1) + Bevacizumab (anti-VEGF) | II | 4.8 months | 12.9 months | The study highlighted the potential of pembrolizumab as a therapeutic option for HCC, particularly in patients who have progressed on or are intolerant to sorafenib. | |
| HIMALAYA [135,136] | Durvalumab (PD-L1) + Tremelimumab (CTLA-4) | III | 3.8 months (STRIDE) vs. 4.3 months (sorafenib) | 16.4 months vs. 13.7 months (sorafenib) | These findings support the use of STRIDE as a novel first-line systemic therapy for unresectable HCC, demonstrating sustained long-term survival benefits and a favorable safety profile. | |
| IMbrave150 [119,137] | Atezolizumab (PD-L1) + Bevacizumab (VEGF) | III | 6.8 months vs. 4.3 months (sorafenib) HR 0.59 (95% CI, 0.47 to 0.76) | 19.2 months vs. 13.4 months (sorafenib) HR 0.66 (95% CI, 0.52 to 0.85) | Significant improvement in both PFS and OS, establishing a new first-line treatment standard in HCC. The combination therapy also delayed the deterioration of quality of life and functioning compared to sorafenib. | |
| CARES-310 [120,138] | Camrelizumab (PD-1) + Rivoceranib (TKI) | III | 5.6 months vs. 3.7 months (sorafenib) HR 0.52 (95% CI, 0.41–0.65; one-sided p < 0.0001) | 22.1 months vs. 15.2 months (sorafenib) HR of 0.62 (95% CI, 0.49–0.80; one-sided p < 0.0001). | Demonstrated strong efficacy in PFS and OS, supporting IO-TKI combination as a new and effective first-line treatment option for unresectable HCC. | |
| CheckMate-9DW | Nivolumab (PD-1) + Cabozantinib (TKI) | III | Not Available | Not Available | Detailed results regarding these endpoints are not available in the current medical literature. | |
| LEAP-002 [121,135,139] | Pembrolizumab (PD-1) + Lenvatinib (TKI) | III | 8.2 months vs. 8.0 months (lenvatinib plus placebo) HR 0.87 (95% CI, 0.73–1.02) | 21.2 months vs. 19.0 months (lenvatinib plus placebo) HR 0.84 (95% CI, 0.71–1.00) | While the combination did not significantly improve survival outcomes, it highlighted the potential activity of lenvatinib plus pembrolizumab in advanced HCC with an objective response rate (ORR) of 26.3% for lenvatinib plus pembrolizumab versus 17.5% for lenvatinib plus placebo. |
6. Spatial Omics and Biomarker Discovery for Precision Medicine in HCC
6.1. Spatial Prognostic and Predictive Biomarkers
6.2. Emerging Biomarkers for Dynamic TME Monitoring
7. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Wang, J.J.; Luu, M.; Noureddin, M.; Kosari, K.; Agopian, V.G.; Rich, N.E.; Lu, S.C.; Tseng, H.R.; Nissen, N.N.; et al. The Mortality and Overall Survival Trends of Primary Liver Cancer in the United States. J. Natl. Cancer Inst. 2021, 113, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, B.; Liu, W.; Wang, S.; Chen, R.; Chen, M.; Fu, Z. Declining disease burden of HCC in the United States, 1992–2017: A population-based analysis. Hepatology 2022, 76, 576–588. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. An updated overview on hepatocellular carcinoma in patients with Metabolic dysfunction-Associated Steatotic Liver Disease: Trends, pathophysiology and risk-based surveillance. Metabolism 2025, 162, 156080. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Suparan, K.; Sukphutanan, B.; Kaeosri, C.; Tothanarungroj, P.; Sirimangklanurak, S.; Kalligeros, M.; Polpichai, N.; Pang, Y.; Wijarnpreecha, K.; et al. Changes in the epidemiological trends of primary liver cancer in the Asia-Pacific region. Sci. Rep. 2024, 14, 19544. [Google Scholar] [CrossRef]
- Yip, T.C.; Lee, H.W.; Chan, W.K.; Wong, G.L.; Wong, V.W. Asian perspective on NAFLD-associated HCC. J. Hepatol. 2022, 76, 726–734. [Google Scholar] [CrossRef]
- Golabi, P.; Paik, J.M.; AlQahtani, S.; Younossi, Y.; Tuncer, G.; Younossi, Z.M. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009–2019. J. Hepatol. 2021, 75, 795–809. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, J.; Yang, Z.; He, X.; Xing, Z.; Zu, J.; Xie, E.; Henry, L.; Chong, C.R.; John, E.M.; et al. Trends in Hepatocellular Carcinoma Mortality Rates in the US and Projections Through 2040. JAMA Netw. Open 2024, 7, e2445525. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Alvarez, C.S.; Petrick, J.L.; Parisi, D.; McMahon, B.J.; Graubard, B.I.; McGlynn, K.A. Racial/ethnic disparities in hepatocellular carcinoma incidence and mortality rates in the United States, 1992–2018. Hepatology 2022, 76, 589–598. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Pineros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Filliol, A.; Saito, Y.; Nair, A.; Dapito, D.H.; Yu, L.X.; Ravichandra, A.; Bhattacharjee, S.; Affo, S.; Fujiwara, N.; Su, H.; et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 2022, 610, 356–365. [Google Scholar] [CrossRef]
- Matsuda, M.; Seki, E. Hepatic Stellate Cell-Macrophage Crosstalk in Liver Fibrosis and Carcinogenesis. Semin. Liver Dis. 2020, 40, 307–320. [Google Scholar] [CrossRef]
- Yahoo, N.; Dudek, M.; Knolle, P.; Heikenwalder, M. Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J. Hepatol. 2023, 79, 538–551. [Google Scholar] [CrossRef]
- Galasso, L.; Cerrito, L.; Maccauro, V.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Ainora, M.E.; Gasbarrini, A.; Zocco, M.A. Inflammatory Response in the Pathogenesis and Treatment of Hepatocellular Carcinoma: A Double-Edged Weapon. Int. J. Mol. Sci. 2024, 25, 7191. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.N.; Khammash, H.A.; Miranda, A.M.; Rutt, L.N.; Twardy, S.M.; Anton, P.E.; Campbell, M.L.; Garza-Ortiz, C.; Orlicky, D.J.; Pelanda, R.; et al. Liver sinusoidal endothelial cells regulate the balance between hepatic immunosuppression and immunosurveillance. Front. Immunol. 2024, 15, 1497788. [Google Scholar] [CrossRef]
- Song, M.; He, J.; Pan, Q.Z.; Yang, J.; Zhao, J.; Zhang, Y.J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Liu, Z.; Zhang, R.; Chao, J.; Wang, M.; Liu, M.; Qiao, L.; Xuan, Z.; Zhao, H.; et al. POSTN(+) cancer-associated fibroblasts determine the efficacy of immunotherapy in hepatocellular carcinoma. J. Immunother. Cancer 2024, 12, e008721. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, M.; Jin, Z.; Sun, D.; Zhu, T.; Liu, X.; Tan, X.; Shi, G. FNDC5 induces M2 macrophage polarization and promotes hepatocellular carcinoma cell growth by affecting the PPARgamma/NF-kappaB/NLRP3 pathway. Biochem. Biophys. Res. Commun. 2021, 582, 77–85. [Google Scholar] [CrossRef]
- Chi, H.C.; Lin, Y.H.; Wu, Y.H.; Chang, C.C.; Wu, C.H.; Yeh, C.T.; Hsieh, C.C.; Lin, K.H. CCL16 is a pro-tumor chemokine that recruits monocytes and macrophages to promote hepatocellular carcinoma progression. Am. J. Cancer Res. 2024, 14, 3600–3613. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, L.; Hu, X.; Feng, R.; Zhao, N.; Zhang, L.; Hu, W.; Zhang, J.; Huang, S.; Liu, L.; et al. Matrix metalloproteinase-21 promotes metastasis via increasing the recruitment and M2 polarization of macrophages in HCC. Cancer Sci. 2023, 114, 423–435. [Google Scholar] [CrossRef]
- Sattiraju, A.; Kang, S.; Giotti, B.; Chen, Z.; Marallano, V.J.; Brusco, C.; Ramakrishnan, A.; Shen, L.; Tsankov, A.M.; Hambardzumyan, D.; et al. Hypoxic niches attract and sequester tumor-associated macrophages and cytotoxic T cells and reprogram them for immunosuppression. Immunity 2023, 56, 1825–1843.e6. [Google Scholar] [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Shao, Z. Extracellular vesicles derived from cancer-associated fibroblasts carry tumor-promotive microRNA-1228-3p to enhance the resistance of hepatocellular carcinoma cells to sorafenib. Hum. Cell 2023, 36, 296–311. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Zhang, Q.; Liu, Z.; Wang, T.; Wu, Z.; Wu, W. CAF-Released Exosomal miR-20a-5p Facilitates HCC Progression via the LIMA1-Mediated beta-Catenin Pathway. Cells 2022, 11, 3857. [Google Scholar] [CrossRef]
- Eun, J.W.; Yoon, J.H.; Ahn, H.R.; Kim, S.; Kim, Y.B.; Lim, S.B.; Park, W.; Kang, T.W.; Baek, G.O.; Yoon, M.G.; et al. Cancer-associated fibroblast-derived secreted phosphoprotein 1 contributes to resistance of hepatocellular carcinoma to sorafenib and lenvatinib. Cancer Commun. 2023, 43, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, E.; Bai, Z.; Jia, Y.; Wang, B.; Dai, Y.; Zhuo, W.; Zeng, G.; Liu, X.; Cai, C.; et al. Cancer-associated fibroblasts induce sorafenib resistance of hepatocellular carcinoma cells through CXCL12/FOLR1. BMC Cancer 2023, 23, 1198. [Google Scholar] [CrossRef]
- Calitz, C.; Rosenquist, J.; Degerstedt, O.; Khaled, J.; Kopsida, M.; Fryknas, M.; Lennernas, H.; Samanta, A.; Heindryckx, F. Influence of extracellular matrix composition on tumour cell behaviour in a biomimetic in vitro model for hepatocellular carcinoma. Sci. Rep. 2023, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Hu, N.; Li, H.; Tao, C.; Xiao, T.; Rong, W. The Role of Metabolic Reprogramming in the Tumor Immune Microenvironment: Mechanisms and Opportunities for Immunotherapy in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 5584. [Google Scholar] [CrossRef]
- Desert, R.; Chen, W.; Ge, X.; Viel, R.; Han, H.; Athavale, D.; Das, S.; Song, Z.; Lantvit, D.; Cano, L.; et al. Hepatocellular carcinomas, exhibiting intratumor fibrosis, express cancer-specific extracellular matrix remodeling and WNT/TGFB signatures, associated with poor outcome. Hepatology 2023, 78, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef]
- Siddhartha, R.; Garg, M. Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem. Mol. Cancer Ther. 2023, 22, 291–305. [Google Scholar] [CrossRef]
- Ge, J.; Jiang, H.; Chen, J.; Chen, X.; Zhang, Y.; Shi, L.; Zheng, X.; Jiang, J.; Chen, L. TGF-beta signaling orchestrates cancer-associated fibroblasts in the tumor microenvironment of human hepatocellular carcinoma: Unveiling insights and clinical significance. BMC Cancer 2025, 25, 113. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef]
- You, M.; Gao, Y.; Fu, J.; Xie, R.; Zhu, Z.; Hong, Z.; Meng, L.; Du, S.; Liu, J.; Wang, F.S.; et al. Epigenetic regulation of HBV-specific tumor-infiltrating T cells in HBV-related HCC. Hepatology 2023, 78, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.J.M.; Chuah, S.; Shuen, T.; Prawira, A.; Ba, R.; Lim, M.C.; Chua, J.; Nguyen, P.H.D.; Lim, C.J.; Wasser, M.; et al. GATA4 downregulation enhances CCL20-mediated immunosuppression in hepatocellular carcinoma. Hepatol. Commun. 2024, 8, e0508. [Google Scholar] [CrossRef]
- Luo, X.; Huang, W.; Li, S.; Sun, M.; Hu, D.; Jiang, J.; Zhang, Z.; Wang, Y.; Wang, Y.; Zhang, J.; et al. SOX12 Facilitates Hepatocellular Carcinoma Progression and Metastasis through Promoting Regulatory T-Cells Infiltration and Immunosuppression. Adv. Sci. 2024, 11, e2310304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Jeffery, H.C.; Hunter, S.; Bhogal, R.; Birtwistle, J.; Braitch, M.K.; Roberts, S.; Ming, M.; Hannah, J.; Thomas, C.; et al. Human intrahepatic regulatory T cells are functional, require IL-2 from effector cells for survival, and are susceptible to Fas ligand-mediated apoptosis. Hepatology 2016, 64, 138–150. [Google Scholar] [CrossRef]
- Guo, C.L.; Yang, X.H.; Cheng, W.; Xu, Y.; Li, J.B.; Sun, Y.X.; Bi, Y.M.; Zhang, L.; Wang, Q.C. Expression of Fas/FasL in CD8+ T and CD3+ Foxp3+ Treg cells--relationship with apoptosis of circulating CD8+ T cells in hepatocellular carcinoma patients. Asian Pac. J. Cancer Prev. 2014, 15, 2613–2618. [Google Scholar] [CrossRef]
- Zhuo, B.; Zhang, Q.; Xie, T.; Wang, Y.; Chen, Z.; Zuo, D.; Guo, B. Integrative epigenetic analysis reveals AP-1 promotes activation of tumor-infiltrating regulatory T cells in HCC. Cell. Mol. Life Sci. 2023, 80, 103. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Hou, J.; Li, W.; Wang, X.; Xiang, L.; Tan, D.; Wang, W.; Jiang, L.; Claret, F.X.; et al. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS ONE 2020, 15, e0231003. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Ma, Z.; Hao, M.; Wang, S.; Li, J.; Fang, Y.; Yu, L.; Huang, Y.; Wang, C.; et al. Sorafenib Promotes Treg Cell Differentiation To Compromise Its Efficacy via VEGFR/AKT/Foxo1 Signaling in Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101454. [Google Scholar] [CrossRef]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Hong, Z.; Zhu, Z.; Liu, J.; Markowitz, G.J.; et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2022, 76, 148–159. [Google Scholar] [CrossRef]
- Khanam, A.; Ghosh, A.; Chua, J.V.; Kottilil, S. Blockade of CCR4 breaks immune tolerance in chronic hepatitis B patients by modulating regulatory pathways. J. Transl. Med. 2023, 21, 271. [Google Scholar] [CrossRef]
- Liang, Y.; Qiao, L.; Qian, Q.; Zhang, R.; Li, Y.; Xu, X.; Xu, Z.; Bu, Q.; Wang, H.; Li, X.; et al. Integrated single-cell and spatial transcriptomic profiling reveals that CD177(+) Tregs enhance immunosuppression through apoptosis and resistance to immunotherapy in hepatocellular carcinoma. Oncogene 2025, 44, 1578–1591. [Google Scholar] [CrossRef]
- Shu, D.H.; Ho, W.J.; Kagohara, L.T.; Girgis, A.; Shin, S.M.; Danilova, L.; Lee, J.W.; Sidiropoulos, D.N.; Mitchell, S.; Munjal, K.; et al. Immunotherapy response induces divergent tertiary lymphoid structure morphologies in hepatocellular carcinoma. Nat. Immunol. 2024, 25, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Teillaud, J.L.; Houel, A.; Panouillot, M.; Riffard, C.; Dieu-Nosjean, M.C. Tertiary lymphoid structures in anticancer immunity. Nat. Rev. Cancer 2024, 24, 629–646. [Google Scholar] [CrossRef]
- Li, J.; Nie, Y.; Jia, W.; Wu, W.; Song, W.; Li, Y. Effect of Tertiary Lymphoid Structures on Prognosis of Patients with Hepatocellular Carcinoma and Preliminary Exploration of Its Formation Mechanism. Cancers 2022, 14, 5157. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Gao, R.; Xie, T.; Li, L.; Tang, L.; Han, X.; Shi, Y. DKK1+ tumor cells inhibited the infiltration of CCL19+ fibroblasts and plasma cells contributing to worse immunotherapy response in hepatocellular carcinoma. Cell Death Dis. 2024, 15, 797. [Google Scholar] [CrossRef]
- Wei, Y.; Lao, X.M.; Xiao, X.; Wang, X.Y.; Wu, Z.J.; Zeng, Q.H.; Wu, C.Y.; Wu, R.Q.; Chen, Z.X.; Zheng, L.; et al. Plasma Cell Polarization to the Immunoglobulin G Phenotype in Hepatocellular Carcinomas Involves Epigenetic Alterations and Promotes Hepatoma Progression in Mice. Gastroenterology 2019, 156, 1890–1904.e16. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Antiangiogenesis: Vessel Regression, Vessel Normalization, or Both? Cancer Res. 2022, 82, 15–17. [Google Scholar] [CrossRef]
- Vucur, M.; Ghallab, A.; Schneider, A.T.; Adili, A.; Cheng, M.; Castoldi, M.; Singer, M.T.; Buttner, V.; Keysberg, L.S.; Kusgens, L.; et al. Sublethal necroptosis signaling promotes inflammation and liver cancer. Immunity 2023, 56, 1578–1595.e8. [Google Scholar] [CrossRef]
- Mohammed, S.; Thadathil, N.; Selvarani, R.; Nicklas, E.H.; Wang, D.; Miller, B.F.; Richardson, A.; Deepa, S.S. Necroptosis contributes to chronic inflammation and fibrosis in aging liver. Aging Cell 2021, 20, e13512. [Google Scholar] [CrossRef]
- Sin, S.Q.; Mohan, C.D.; Goh, R.M.W.; You, M.; Nayak, S.C.; Chen, L.; Sethi, G.; Rangappa, K.S.; Wang, L. Hypoxia signaling in hepatocellular carcinoma: Challenges and therapeutic opportunities. Cancer Metastasis Rev. 2023, 42, 741–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Morine, Y.; Tokuda, K.; Yamada, S.; Saito, Y.; Nishi, M.; Ikemoto, T.; Shimada, M. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway. Int. J. Oncol. 2021, 59, 59. [Google Scholar] [CrossRef]
- Cheng, S.L.; Wu, C.H.; Tsai, Y.J.; Song, J.S.; Chen, H.M.; Yeh, T.K.; Shen, C.T.; Chiang, J.C.; Lee, H.M.; Huang, K.W.; et al. CXCR4 antagonist-loaded nanoparticles reprogram the tumor microenvironment and enhance immunotherapy in hepatocellular carcinoma. J. Control. Release 2025, 379, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Pratama, M.Y.; Vo, N.V.T.; Masadah, R.; Tiribelli, C. The Crosstalk between Tumor Cells and the Microenvironment in Hepatocellular Carcinoma: The Role of Exosomal microRNAs and their Clinical Implications. Cancers 2020, 12, 823. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Targeting tumor associated macrophages in hepatocellular carcinoma. Biochem. Pharmacol. 2022, 199, 114990. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Guo, Y.; Bi, C.; Liu, M.; Wang, G. Biological impact and therapeutic implication of tumor-associated macrophages in hepatocellular carcinoma. Cell Death Dis. 2024, 15, 498. [Google Scholar] [CrossRef]
- Rohrle, N.; Knott, M.M.L.; Anz, D. CCL22 Signaling in the Tumor Environment. Adv. Exp. Med. Biol. 2020, 1231, 79–96. [Google Scholar] [CrossRef]
- Weiss, J.M.; Subleski, J.J.; Back, T.; Chen, X.; Watkins, S.K.; Yagita, H.; Sayers, T.J.; Murphy, W.J.; Wiltrout, R.H. Regulatory T cells and myeloid-derived suppressor cells in the tumor microenvironment undergo Fas-dependent cell death during IL-2/alphaCD40 therapy. J. Immunol. 2014, 192, 5821–5829. [Google Scholar] [CrossRef]
- Yao, C.; Wu, S.; Kong, J.; Sun, Y.; Bai, Y.; Zhu, R.; Li, Z.; Sun, W.; Zheng, L. Angiogenesis in hepatocellular carcinoma: Mechanisms and anti-angiogenic therapies. Cancer Biol. Med. 2023, 20, 25–43. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhou, X.; Lu, M.; He, M.; Tian, Y.; Liu, L.; Wang, M.; Tan, W.; Deng, Y.; Yang, X.; et al. Bclaf1 promotes angiogenesis by regulating HIF-1alpha transcription in hepatocellular carcinoma. Oncogene 2019, 38, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- von Marschall, Z.; Cramer, T.; Hocker, M.; Finkenzeller, G.; Wiedenmann, B.; Rosewicz, S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 2001, 48, 87–96. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Zhou, S.; Yao, F.; Zhang, R.; You, W.; Dai, J.; Yu, K.; Zhang, Y.; Baheti, T.; et al. Endothelial DGKG promotes tumor angiogenesis and immune evasion in hepatocellular carcinoma. J. Hepatol. 2024, 80, 82–98. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Z.; Yang, L.; Gao, Y.; Zhu, Q.; Hu, L.; Huang, D.; Xu, Q. Hypoxia-inducible factors in hepatocellular carcinoma (Review). Oncol. Rep. 2020, 43, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, Z.; Wu, J.; Jiang, C.; Wu, J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. BioMed. Res. Int. 2014, 2014, 409272. [Google Scholar] [CrossRef]
- Chiu, D.K.; Tse, A.P.; Xu, I.M.; Di Cui, J.; Lai, R.K.; Li, L.L.; Koh, H.Y.; Tsang, F.H.; Wei, L.L.; Wong, C.M.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8, 517. [Google Scholar] [CrossRef]
- Chiu, D.K.; Xu, I.M.; Lai, R.K.; Tse, A.P.; Wei, L.L.; Koh, H.Y.; Li, L.L.; Lee, D.; Lo, R.C.; Wong, C.M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016, 64, 797–813. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Bangoura, G.; Liu, Z.S.; Qian, Q.; Jiang, C.Q.; Yang, G.F.; Jing, S. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 3176–3182. [Google Scholar] [CrossRef]
- Geis, T.; Doring, C.; Popp, R.; Grossmann, N.; Fleming, I.; Hansmann, M.L.; Dehne, N.; Brune, B. HIF-2alpha-dependent PAI-1 induction contributes to angiogenesis in hepatocellular carcinoma. Exp. Cell Res. 2015, 331, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Xu, Y.; Yang, X.R.; Wang, W.M.; Bai, H.; Shi, R.Y.; Nayar, S.K.; Devbhandari, R.P.; He, Y.Z.; Zhu, Q.F.; et al. Hypoxia inducible factor 2 alpha inhibits hepatocellular carcinoma growth through the transcription factor dimerization partner 3/E2F transcription factor 1-dependent apoptotic pathway. Hepatology 2013, 57, 1088–1097. [Google Scholar] [CrossRef]
- Yang, S.L.; Liu, L.P.; Niu, L.; Sun, Y.F.; Yang, X.R.; Fan, J.; Ren, J.W.; Chen, G.G.; Lai, P.B. Downregulation and pro-apoptotic effect of hypoxia-inducible factor 2 alpha in hepatocellular carcinoma. Oncotarget 2016, 7, 34571–34581. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Mai, W.; Gao, Y. HIF-2alpha regulates proliferation, invasion, and metastasis of hepatocellular carcinoma cells via VEGF/Notch1 signaling axis after insufficient radiofrequency ablation. Front. Oncol. 2022, 12, 998295. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.; Chen, J.; Sun, Y.; Lin, H.; Jin, R.A.; Tang, M.; Liang, X.; Cai, X. Increasing AR by HIF-2alpha inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017, 8, e3095. [Google Scholar] [CrossRef] [PubMed]
- Vanderborght, B.; De Muynck, K.; Lefere, S.; Geerts, A.; Degroote, H.; Verhelst, X.; Van Vlierberghe, H.; Devisscher, L. Effect of isoform-specific HIF-1alpha and HIF-2alpha antisense oligonucleotides on tumorigenesis, inflammation and fibrosis in a hepatocellular carcinoma mouse model. Oncotarget 2020, 11, 4504–4520. [Google Scholar] [CrossRef]
- Shi, Y.; Gilkes, D.M. HIF-1 and HIF-2 in cancer: Structure, regulation, and therapeutic prospects. Cell. Mol. Life Sci. 2025, 82, 44. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H. Emerging Role of Cancer-Associated Fibroblasts in Progression and Treatment of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3941. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, D.; Li, J.; Huang, D.; Zhao, Y.; Gao, T.; Zhuang, Z.; Cui, Y.; Zheng, D.Y.; Tang, Y. Mechanisms of tumor-associated macrophages affecting the progression of hepatocellular carcinoma. Front. Pharmacol. 2023, 14, 1217400. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, Y. CAF-mediated tumor vascularization: From mechanistic insights to targeted therapies. Cell Signal 2025, 132, 111827. [Google Scholar] [CrossRef]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages. BioMed. Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627. [Google Scholar] [CrossRef]
- Pasquier, J.; Ghiabi, P.; Chouchane, L.; Razzouk, K.; Rafii, S.; Rafii, A. Angiocrine endothelium: From physiology to cancer. J. Transl. Med. 2020, 18, 52. [Google Scholar] [CrossRef]
- Giannelli, G.; Villa, E.; Lahn, M. Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014, 74, 1890–1894. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Diaz, D. Transforming Growth Factor-beta-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Soukupova, J.; Malfettone, A.; Bertran, E.; Hernandez-Alvarez, M.I.; Penuelas-Haro, I.; Dituri, F.; Giannelli, G.; Zorzano, A.; Fabregat, I. Epithelial-Mesenchymal Transition (EMT) Induced by TGF-beta in Hepatocellular Carcinoma Cells Reprograms Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 5543. [Google Scholar] [CrossRef]
- Malfettone, A.; Soukupova, J.; Bertran, E.; Crosas-Molist, E.; Lastra, R.; Fernando, J.; Koudelkova, P.; Rani, B.; Fabra, A.; Serrano, T.; et al. Transforming growth factor-beta-induced plasticity causes a migratory stemness phenotype in hepatocellular carcinoma. Cancer Lett. 2017, 392, 39–50. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Ghadyani, F.; Hashemi, M.; Abbaspour, A.; Zabolian, A.; Javanshir, S.; Razzazan, M.; Mirzaei, S.; Entezari, M.; Goharrizi, M.; et al. Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: Promises and Challenges. Pharmacol. Res. 2023, 187, 106553. [Google Scholar] [CrossRef]
- Ngo, M.T.; Jeng, H.Y.; Kuo, Y.C.; Diony Nanda, J.; Brahmadhi, A.; Ling, T.Y.; Chang, T.S.; Huang, Y.H. The Role of IGF/IGF-1R Signaling in Hepatocellular Carcinomas: Stemness-Related Properties and Drug Resistance. Int. J. Mol. Sci. 2021, 22, 1931. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Assenzio, B.; Deregibus, M.C.; Camussi, G. The proangiogenic phenotype of human tumor-derived endothelial cells depends on thrombospondin-1 downregulation via phosphatidylinositol 3-kinase/Akt pathway. J. Mol. Med. 2006, 84, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chi, H.; Zhao, X.; Pan, R.; Wei, Y.; Han, Y. Role of Exosomes in Immune Microenvironment of Hepatocellular Carcinoma. J. Oncol. 2022, 2022, 2521025. [Google Scholar] [CrossRef]
- Xu, H.Z.; Lin, X.Y.; Xu, Y.X.; Xue, H.B.; Lin, S.; Xu, T.W. An emerging research: The role of hepatocellular carcinoma-derived exosomal circRNAs in the immune microenvironment. Front. Immunol. 2023, 14, 1227150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wu, N.; Feng, Y.; Wang, J.; Ma, L.; Chen, Y. The role of exosomes in liver cancer: Comprehensive insights from biological function to therapeutic applications. Front. Immunol. 2024, 15, 1473030. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Machairas, N.; Stergiou, I.E.; Arvanitakis, K.; Germanidis, G.; Frampton, A.E.; Theocharis, S. Unveiling the Yin-Yang Balance of M1 and M2 Macrophages in Hepatocellular Carcinoma: Role of Exosomes in Tumor Microenvironment and Immune Modulation. Cells 2023, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ahmed, A.T.; Rab, S.O.; Uthirapathy, S.; Ballal, S.; Kalia, R.; Arya, R.; Nathiya, D.; Kariem, M.; Kadhim, A.J. Cancer-associated fibroblast-derived exosomes in cancer progression: A focus on hepatocellular carcinoma. Exp. Cell Res. 2025, 445, 114424. [Google Scholar] [CrossRef]
- Ge, Y.; Jiang, L.; Dong, Q.; Xu, Y.; Yam, J.W.P.; Zhong, X. Exosome-mediated Crosstalk in the Tumor Immune Microenvironment: Critical Drivers of Hepatocellular Carcinoma Progression. J. Clin. Transl. Hepatol. 2025, 13, 143–161. [Google Scholar] [CrossRef]
- Hu, R.; Jahan, M.S.; Tang, L. ExoPD-L1: An assistant for tumor progression and potential diagnostic marker. Front. Oncol. 2023, 13, 1194180. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Z.; Liu, L.; Zhang, R.; Geng, Y.; Fan, M.; Zhu, W.; Lu, M.; Lu, L.; Jia, H.; et al. GOLM1 exacerbates CD8(+) T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct. Target. Ther. 2021, 6, 397. [Google Scholar] [CrossRef]
- Lin, X.; Shao, H.; Tang, Y.; Wang, Q.; Yang, Z.; Wu, H.; Xing, T. High expression of circulating exosomal PD-L1 contributes to immune escape of hepatocellular carcinoma and immune clearance of chronic hepatitis B. Aging 2024, 16, 11373–11384. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Yin, W.X.; Jiang, M.; Han, J.Y.; Kuai, X.W.; Sun, R.; Sun, Y.F.; Ji, J.L. Function and biomedical implications of exosomal microRNAs delivered by parenchymal and nonparenchymal cells in hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 5435–5451. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gong, W.S.; Xing, X.S.; Zhou, H.; Wang, X.L.; Xu, Y.; Zhou, X.L.; Xue, W.L. miR-183-5p-enriched extracellular vesicles promote the crosstalk between hepatocellular carcinoma cell and endothelial cell via SIK1/PI3K/AKT and CCL20/CCR6 signaling pathways. Front. Oncol. 2025, 15, 1532239. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Feng, X.; Liu, H.; Tong, R.; Wu, J.; Li, C.; Yu, H.; Chen, Y.; Cheng, Q.; Chen, J.; et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39, 6529–6543. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Zhu, A.X.; Baron, A.D.; Malfertheiner, P.; Kudo, M.; Kawazoe, S.; Pezet, D.; Weissinger, F.; Brandi, G.; Barone, C.A.; Okusaka, T.; et al. Ramucirumab as Second-Line Treatment in Patients With Advanced Hepatocellular Carcinoma: Analysis of REACH Trial Results by Child-Pugh Score. JAMA Oncol. 2017, 3, 235–243. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Kikuchi, H.; Birch, G.; Matsui, A.; Morita, A.; Kobayashi, T.; Ruan, Z.; Huang, P.; Hernandez, A.; Coyne, E.M.; et al. Preventing NK cell activation in the damaged liver induced by cabozantinib/PD-1 blockade increases survival in hepatocellular carcinoma models. bioRxiv 2023. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Cubillo Gracian, A.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab Plus Cabozantinib With or Without Ipilimumab for Advanced Hepatocellular Carcinoma: Results From Cohort 6 of the CheckMate 040 Trial. J. Clin. Oncol. 2023, 41, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Borbath, I.; Van Laethem, J.-L.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Bergamo, F.; Stål, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma (aHCC): 3-year follow-up of the phase 2 KEYNOTE-224 study. J. Clin. Oncol. 2022, 40, 4109. [Google Scholar] [CrossRef]
- Van Laethem, J.-L.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab (pembro) monotherapy for previously untreated advanced hepatocellular carcinoma (HCC): Phase II KEYNOTE-224 study. J. Clin. Oncol. 2021, 39, 297. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Merle, P.; Kudo, M.; Edeline, J.; Bouattour, M.; Cheng, A.L.; Chan, S.L.; Yau, T.; Garrido, M.; Knox, J.; Daniele, B.; et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: Longer Term Follow-Up from the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2023, 12, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; Edeline, J.; Bouattour, M.; Cheng, A.-L.; Chan, S.L.; Yau, T.; Garrido, M.; Knox, J.J.; Daniele, B.; Zhu, A.X.; et al. Pembrolizumab (pembro) vs placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: Updated data from the randomized, phase III KEYNOTE-240 study. J. Clin. Oncol. 2021, 39, 268. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Bao, Q.; Jin, K.; Liu, M.; Liu, W.; Yan, X.; Wang, L.; Zhang, Y.; Wang, G.; et al. The anti-PD-L1/CTLA-4 bispecific antibody KN046 plus lenvatinib in advanced unresectable or metastatic hepatocellular carcinoma: A phase II trial. Nat. Commun. 2025, 16, 1443. [Google Scholar] [CrossRef]
- Clouthier, D.L.; Lien, S.C.; Yang, S.Y.C.; Nguyen, L.T.; Manem, V.S.K.; Gray, D.; Ryczko, M.; Razak, A.R.A.; Lewin, J.; Lheureux, S.; et al. An interim report on the investigator-initiated phase 2 study of pembrolizumab immunological response evaluation (INSPIRE). J. Immunother. Cancer 2019, 7, 72. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 1830–1850. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Ren, Z.; Bai, Y.; Gu, S.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib vs sorafenib as first-line therapy for unresectable hepatocellular carcinoma (uHCC): Final overall survival analysis of the phase 3 CARES-310 study. J. Clin. Oncol. 2024, 42, 4110. [Google Scholar] [CrossRef]
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib alone as first-line therapy for advanced hepatocellular carcinoma: Longer-term efficacy and safety results from the phase 3 LEAP-002 study. J. Clin. Oncol. 2024, 42, 482. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef]
- Palla, G.; Fischer, D.S.; Regev, A.; Theis, F.J. Spatial components of molecular tissue biology. Nat. Biotechnol. 2022, 40, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, S.; Cheng, Z.; Liu, Z.; Zhang, L.; Jiang, K.; Geng, H.; Qian, R.; Wang, J.; Huang, X.; et al. Multi-region sequencing with spatial information enables accurate heterogeneity estimation and risk stratification in liver cancer. Genome Med. 2022, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.D.; Ma, S.; Phua, C.Z.J.; Kaya, N.A.; Lai, H.L.H.; Lim, C.J.; Lim, J.Q.; Wasser, M.; Lai, L.; Tam, W.L.; et al. Intratumoural immune heterogeneity as a hallmark of tumour evolution and progression in hepatocellular carcinoma. Nat. Commun. 2021, 12, 227. [Google Scholar] [CrossRef]
- Jing, S.Y.; Liu, D.; Feng, N.; Dong, H.; Wang, H.Q.; Yan, X.; Chen, X.F.; Qu, M.C.; Lin, P.; Yi, B.; et al. Spatial multiomics reveals a subpopulation of fibroblasts associated with cancer stemness in human hepatocellular carcinoma. Genome Med. 2024, 16, 98. [Google Scholar] [CrossRef]
- Fan, G.; Xie, T.; Li, L.; Tang, L.; Han, X.; Shi, Y. Single-cell and spatial analyses revealed the co-location of cancer stem cells and SPP1+ macrophage in hypoxic region that determines the poor prognosis in hepatocellular carcinoma. NPJ Precis. Oncol. 2024, 8, 75. [Google Scholar] [CrossRef]
- Virtanen, A.S.J.; Huopaniemi, T.; Narhi, M.V.O.; Pertovaara, A.; Wallgren, K. The effect of temporal parameters on subjective sensations evoked by electrical tooth stimulation. Pain 1987, 30, 361–371. [Google Scholar] [CrossRef]
- Liu, Y.; Xun, Z.; Ma, K.; Liang, S.; Li, X.; Zhou, S.; Sun, L.; Liu, Y.; Du, Y.; Guo, X.; et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 2023, 78, 770–782. [Google Scholar] [CrossRef]
- Jin, H.; Kim, W.; Yuan, M.; Li, X.; Yang, H.; Li, M.; Shi, M.; Turkez, H.; Uhlen, M.; Zhang, C.; et al. Identification of SPP1 (+) macrophages as an immune suppressor in hepatocellular carcinoma using single-cell and bulk transcriptomics. Front. Immunol. 2024, 15, 1446453. [Google Scholar] [CrossRef]
- Long, F.; Zhong, W.; Zhao, F.; Xu, Y.; Hu, X.; Jia, G.; Huang, L.; Yi, K.; Wang, N.; Si, H.; et al. DAB2 (+) macrophages support FAP (+) fibroblasts in shaping tumor barrier and inducing poor clinical outcomes in liver cancer. Theranostics 2024, 14, 4822–4843. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Xu, L.; Shi, X.; Xue, H.; Liu, J.; Bai, S.; Wu, Y.; Yang, Z.; Xue, F.; et al. Single cell analyses reveal the PD-1 blockade response-related immune features in hepatocellular carcinoma. Theranostics 2024, 14, 3526–3547. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Ye, Y.; Shen, H.; Zhang, R.; Li, H.; Song, T.; Zhang, R.; Liu, P.; Chen, G.; Wang, H.; et al. Macrophage-coated tumor cluster aggravates hepatoma invasion and immunotherapy resistance via generating local immune deprivation. Cell Rep. Med. 2024, 5, 101505. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhou, T.; Li, S.; Wu, J.; Tang, J.; Ma, G.; Yang, S.; Hu, J.; Wang, K.; Shen, S.; et al. Spatial single-cell protein landscape reveals vimentin(high) macrophages as immune-suppressive in the microenvironment of hepatocellular carcinoma. Nat. Cancer 2024, 5, 1557–1578. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.A.; Pai, R.; Bleriot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef]
- Li, Z.; Pai, R.; Gupta, S.; Currenti, J.; Guo, W.; Di Bartolomeo, A.; Feng, H.; Zhang, Z.; Li, Z.; Liu, L.; et al. Presence of onco-fetal neighborhoods in hepatocellular carcinoma is associated with relapse and response to immunotherapy. Nat. Cancer 2024, 5, 167–186. [Google Scholar] [CrossRef]
- Murai, H.; Kodama, T.; Maesaka, K.; Tange, S.; Motooka, D.; Suzuki, Y.; Shigematsu, Y.; Inamura, K.; Mise, Y.; Saiura, A.; et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 2023, 77, 77–91. [Google Scholar] [CrossRef]
- Salie, H.; Wischer, L.; D’Alessio, A.; Godbole, I.; Suo, Y.; Otto-Mora, P.; Beck, J.; Neumann, O.; Stenzinger, A.; Schirmacher, P.; et al. Spatial single-cell profiling and neighbourhood analysis reveal the determinants of immune architecture connected to checkpoint inhibitor therapy outcome in hepatocellular carcinoma. Gut 2025, 74, 451–466. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Sugimoto, K.; Tsujikawa, H.; Matsuda, K.; Nomura, R.; Ueno, A.; Masugi, Y.; Yamazaki, K.; Effendi, K.; Takeuchi, H.; et al. Spatial Dynamics of T- and B-Cell Responses Predicts Clinical Outcome of Resectable and Unresectable Hepatocellular Carcinoma. Clin. Cancer Res. 2024, 30, 5666–5680. [Google Scholar] [CrossRef]
- Yang, M.; Song, X.; Zhang, F.; Li, M.; Chang, W.; Wang, Z.; Li, M.; Shan, H.; Li, D. Spatial proteomic landscape of primary and relapsed hepatocellular carcinoma reveals immune escape characteristics in early relapse. Hepatology 2025, 81, 1452–1467. [Google Scholar] [CrossRef]
- Lemaitre, L.; Adeniji, N.; Suresh, A.; Reguram, R.; Zhang, J.; Park, J.; Reddy, A.; Trevino, A.E.; Mayer, A.T.; Deutzmann, A.; et al. Spatial analysis reveals targetable macrophage-mediated mechanisms of immune evasion in hepatocellular carcinoma minimal residual disease. Nat. Cancer 2024, 5, 1534–1556. [Google Scholar] [CrossRef]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, L.; Danilova, L.; Mo, G.; Zhu, Q.; Deshpande, A.; Bell, A.T.F.; Elisseeff, J.; Popel, A.S.; Anders, R.A.; et al. Spatial transcriptomics analysis of neoadjuvant cabozantinib and nivolumab in advanced hepatocellular carcinoma identifies independent mechanisms of resistance and recurrence. Genome Med. 2023, 15, 72. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, X.; Li, Y.; Han, Y.; Peng, Y.; Wu, W.; Wang, X.; Ma, J.; Hu, E.; Zhou, X.; et al. Comprehensive molecular classification predicted microenvironment profiles and therapy response for HCC. Hepatology 2024, 80, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, E.; Huang, Z. The predictive value of next generation sequencing for matching advanced hepatocellular carcinoma patients to targeted and immunotherapy. Front. Immunol. 2024, 15, 1358306. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Suzuki, H.; Lemaitre, L.; Kubota, N.; Hoshida, Y. Molecular and immune landscape of hepatocellular carcinoma to guide therapeutic decision-making. Hepatology 2025, 81, 1038–1057. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, A.; Nogami, W.; Nashiki, K.; Haruna, M.; Miwa, H.; Hagiwara, M.; Nagira, M.; Wada, H.; Nagira, Y. Immunotherapy Targeting CCR8+ Regulatory T Cells Induces Antitumor Effects via Dramatic Changes to the Intratumor CD8+ T Cell Profile. J. Immunol. 2023, 211, 673–682. [Google Scholar] [CrossRef]
- Ding, L.; Qian, J.; Yu, X.; Wu, Q.; Mao, J.; Liu, X.; Wang, Y.; Guo, D.; Su, R.; Xie, H.; et al. Blocking MARCO(+) tumor-associated macrophages improves anti-PD-L1 therapy of hepatocellular carcinoma by promoting the activation of STING-IFN type I pathway. Cancer Lett. 2024, 582, 216568. [Google Scholar] [CrossRef]
- Zeng, Q.; Klein, C.; Caruso, S.; Maille, P.; Allende, D.S.; Minguez, B.; Iavarone, M.; Ningarhari, M.; Casadei-Gardini, A.; Pedica, F.; et al. Artificial intelligence-based pathology as a biomarker of sensitivity to atezolizumab-bevacizumab in patients with hepatocellular carcinoma: A multicentre retrospective study. Lancet Oncol. 2023, 24, 1411–1422. [Google Scholar] [CrossRef]
- Chen, B.; Garmire, L.; Calvisi, D.F.; Chua, M.S.; Kelley, R.K.; Chen, X. Harnessing big ‘omics’ data and AI for drug discovery in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 238–251. [Google Scholar] [CrossRef]

| Cell Type | HCC TME Role | Key Differences in Other Tumors |
|---|---|---|
| Cancer-Associated Fibroblasts (CAFs) | Drive fibrosis, immunosuppression, and angiogenesis via TGF-β and HGF; contribute to a dense, fibrotic microenvironment. | Fibrosis is less extensive; CAFs less central in shaping TME. |
| Tumor-Associated Macrophages (TAMs) | Predominantly M2-polarized; secrete IL-10, VEGF, and TGF-β; promotes immune evasion and angiogenesis. | TAMs are less M2-polarized and not as strongly shaped by liver-induced immune suppression. |
| Endothelial Cells | Form abnormal, leaky vasculature due to VEGF and hypoxia; enhance hypoxia and limit immune infiltration. | Vascular remodeling is less hypoxia-driven and more structured angiogenesis. |
| Regulatory T-cells (Tregs) | Abundant due to liver’s immune tolerance; suppress CD8+ T cells and promote immune evasion. | Lower baseline Treg levels and less impact on TME. |
| CD8+ T Cells | Exhausted or dysfunctional; impaired cytotoxicity due to inflammation and high PD-1 expression. | More functional CD8+ T cells; less exhaustion. |
| Natural Killer (NK) Cells | Reduced activity due to tolerogenic liver environment and hypoxia limits cytotoxic potential. | NK cells are more active; less suppression by hypoxia or TGF-β. |
| Myeloid-Derived Suppressor Cells (MDSCs) | Abundant due to inflammation; suppress T-cell activity and enhance immune suppression. | Lower frequency and suppressive capacity of MDSCs. |
| Extracellular Matrix (ECM) | Densely fibrotic due to liver disease, it forms a physical barrier to immune cells and promotes tumor spread. | ECM remodeling is driven by tumor cells, not pre-existing fibrosis. |
| Factor | Source | Mechanism | |
|---|---|---|---|
| Paracrine | VEGF | Tumor cells, CAFs | Binds VEGFR2 to induce endothelial proliferation and vascular permeability. |
| IL-6/IL-8 | TAMs, HCC cells | Activates STAT3 in endothelial cells, enhancing survival and MMP expression. | |
| PDGF | CAFs | Recruits pericytes to stabilize nascent vessels, albeit abnormally, fostering leaky vasculature that facilitates metastasis. | |
| HGF | Stromal cells, plasma | Triggers c-MET signaling to promote vascular mimicry and metastasis. | |
| TGF-β | CAFs, Tregs | Induces EndMT, enabling vessel co-option and immune evasion. | |
| IGF-1 | Tumor cells | promotes endothelial proliferation via PI3K/AKT signaling, while suppressing antiangiogenic thrombospondin-1 (TSP-1). | |
| Exosomal Cargo | PD-L1 | Tumor cells | Suppresses T-cell activation to promote immune evasion. Potential biomarker for immunosuppression. |
| miR-210 | Tumor cells | Suppresses Ephrin-A3, destabilizing endothelial junctions to aid intravasation. Associated with increased vascularization. | |
| miR-122 | Tumor cells | Regulates cell proliferation and modulates drug sensitivity. Investigated as a diagnostic/therapeutic response marker. | |
| miR-21 | Tumor cells, TAMs | Enhances cell proliferation and invasion. Considered a prognostic marker for HCC progression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Anand, N.; Guo, Z.; Li, M.; Santiago Figueroa, M.; Jung, L.; Kelly, S.; Franses, J.W. Bridging Immune Evasion and Vascular Dynamics for Novel Therapeutic Frontiers in Hepatocellular Carcinoma. Cancers 2025, 17, 1860. https://doi.org/10.3390/cancers17111860
Wu S, Anand N, Guo Z, Li M, Santiago Figueroa M, Jung L, Kelly S, Franses JW. Bridging Immune Evasion and Vascular Dynamics for Novel Therapeutic Frontiers in Hepatocellular Carcinoma. Cancers. 2025; 17(11):1860. https://doi.org/10.3390/cancers17111860
Chicago/Turabian StyleWu, Sulin, Namrata Anand, Zhoubo Guo, Mingyang Li, Marcos Santiago Figueroa, Lauren Jung, Sarah Kelly, and Joseph W. Franses. 2025. "Bridging Immune Evasion and Vascular Dynamics for Novel Therapeutic Frontiers in Hepatocellular Carcinoma" Cancers 17, no. 11: 1860. https://doi.org/10.3390/cancers17111860
APA StyleWu, S., Anand, N., Guo, Z., Li, M., Santiago Figueroa, M., Jung, L., Kelly, S., & Franses, J. W. (2025). Bridging Immune Evasion and Vascular Dynamics for Novel Therapeutic Frontiers in Hepatocellular Carcinoma. Cancers, 17(11), 1860. https://doi.org/10.3390/cancers17111860





