Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions
Simple Summary
Abstract
1. Introduction
2. Oncolytic Viruses
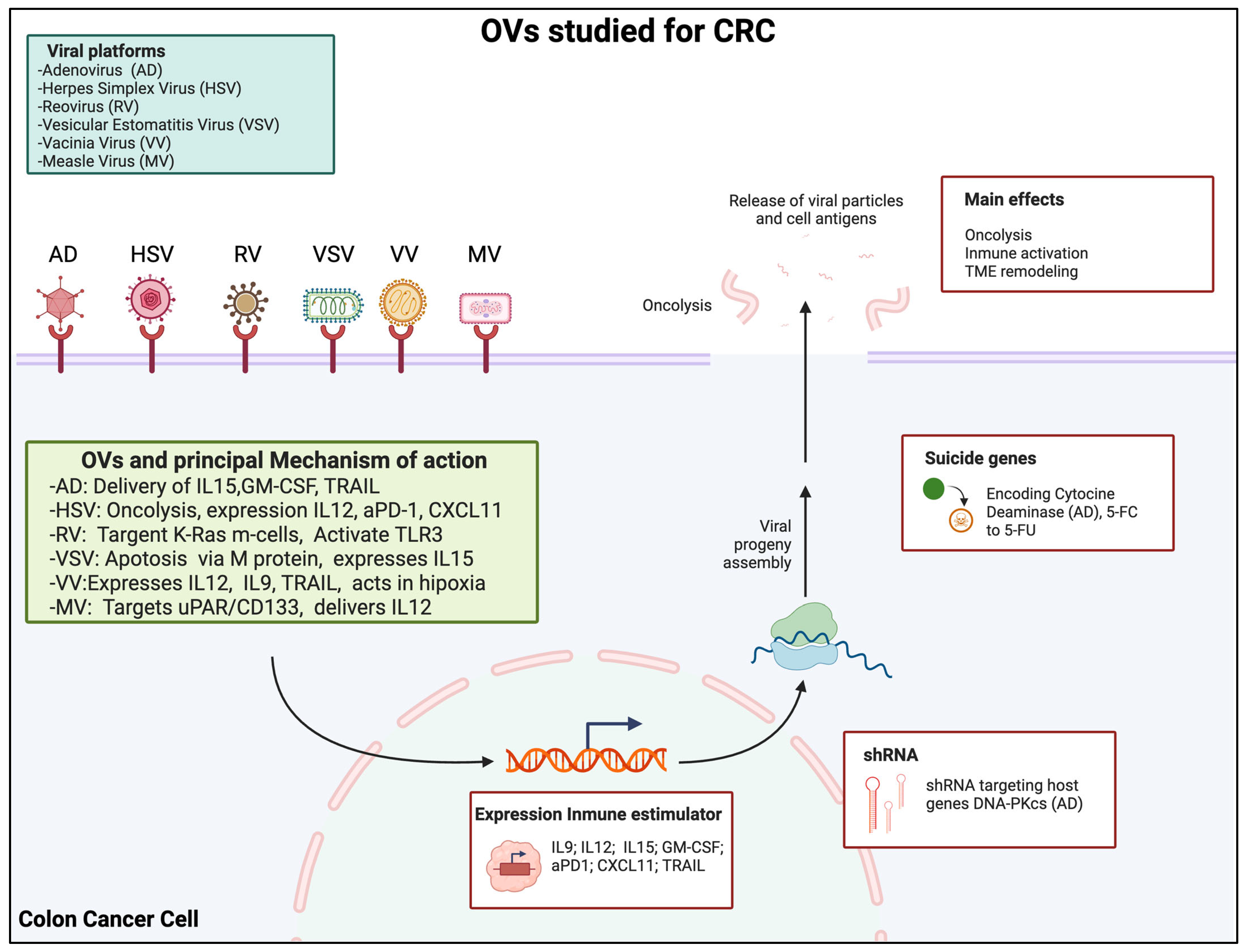
3. Types of OVs Studied for CRC
3.1. Adenovirus
3.1.1. Mechanism of Action
3.1.2. Clinical and Preclinical Evidence
3.2. Herpes Simplex Virus (HSV)
3.2.1. Mechanism of Action
3.2.2. Clinical and Preclinical Evidence
3.3. Reovirus
3.3.1. Mechanism of Action
3.3.2. Clinical and Preclinical Evidence
3.4. Vesicular Stomatitis Virus (VSV)
3.4.1. Mechanism of Action
3.4.2. Clinical and Preclinical Evidence
3.5. Vaccinia Virus (VV)
3.5.1. Mechanism of Action
3.5.2. Clinical and Preclinical Evidence
3.6. Measles Virus (MV)
3.6.1. Mechanism of Action
3.6.2. Clinical and Preclinical Evidence
4. Selection Criteria for CRC Patients for Trials with OVs
5. Limitations, Challenges, and Future Perspectives
5.1. Mechanisms of Resistance
5.2. Personalized Strategies and Combined Therapies
5.3. Virotherapy’s Clinical Limitations in Colon Cancers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| OV | Oncolytic Virus |
| CRC | Colorectal Cancer |
| HSV | Herpes Simplex Virus |
| VSV | Vesicular Stomatitis Virus |
| VV | Vaccinia Virus |
| MV | Measles Virus |
| TME | Tumor Microenvironment |
| IL | Interleukin |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| aPD-1 | Anti-Programmed Death-1 |
| CXCL11 | C-X-C Motif Chemokine Ligand 11 |
| TLR3 | Toll-Like Receptor 3 |
| NK | Natural Killer (cell) |
| MDSC | Myeloid-Derived Suppressor Cell |
| Treg | Regulatory T Cell |
| ICIs | Immune Checkpoint Inhibitors |
| HDAC | Histone Deacetylase |
| CSC | Cancer Stem Cell |
| CAR-T | Chimeric Antigen Receptor T Cell |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2024, 22, e240029. [Google Scholar] [CrossRef]
- Chen, W.; Shi, K.; Yu, Y.; Yang, P.; Bei, Z.; Mo, D.; Yuan, L.; Pan, M.; Chen, Y.; Qian, Z. Drug delivery systems for colorectal cancer chemotherapy. Chin. Chem. Lett. 2024, 35, 109159. [Google Scholar] [CrossRef]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Cremolini, C.; Petrelli, F.; Di Bartolomeo, M.; Loupakis, F.; Maggi, C.; Antoniotti, C.; de Braud, F.; Falcone, A.; Iacovelli, R. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2015, 96, 156–166. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef]
- Al Bitar, S.; El-Sabban, M.; Doughan, S.; Abou-Kheir, W. Molecular mechanisms targeting drug-resistance and metastasis in colorectal cancer: Updates and beyond. World J. Gastroenterol. 2023, 29, 1395–1426. [Google Scholar] [CrossRef]
- Cherri, S.; Oneda, E.; Zanotti, L.; Zaniboni, A. Optimizing the first-line treatment for metastatic colorectal cancer. Front. Oncol. 2023, 13, 1246716. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, Z.; Chen, Y.; Xu, J.; Wang, J.; Wang, Z. Enhancing cancer therapy: The integration of oncolytic virus therapy with diverse treatments. Cancer Cell Int. 2024, 24, 242. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef]
- Ren, Y.; Miao, J.M.; Wang, Y.Y.; Fan, Z.; Kong, X.B.; Yang, L.; Cheng, G. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front. Immunol. 2022, 13, 961796. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Maroun, J.; Miguel, M.-A.; Arun, A.; Autumn, S.; Kah-Whye, P.; Russell, S. Designing and Building Oncolytic Viruses. Future Virol. 2017, 12, 193–213. [Google Scholar] [CrossRef]
- Parato, K.A.; Senger, D.; Forsyth, P.A.; Bell, J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 2005, 5, 965–976. [Google Scholar] [CrossRef]
- Moore, A.E. Effect of inoculation of the viruses of influenza A and herpes simplex on the growth of transplantable tumors in mice. Cancer 1949, 2, 516–524. [Google Scholar] [CrossRef]
- Moore, A.E. The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer 1949, 2, 525–534. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014, 6, 226ra232. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Miao, E.A.; Iwakura, Y.; Murali-Krishna, K.; Aderem, A.; Flavell, R.A.; Papayannopoulou, T.; Shayakhmetov, D.M. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 2009, 31, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Robinson, M.; Han, Z.Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Rojas, J.J.; Sampath, P.; Hou, W.; Thorne, S.H. Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin. Cancer Res. 2015, 21, 5543–5551. [Google Scholar] [CrossRef]
- Ajina, A.; Maher, J. Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer 2017, 5, 90. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hutzen, B.; Wedekind, M.F.; Cripe, T.P. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virother. 2018, 7, 65–77. [Google Scholar] [CrossRef]
- Achard, C.; Surendran, A.; Wedge, M.E.; Ungerechts, G.; Bell, J.; Ilkow, C.S. Lighting a Fire in the Tumor Microenvironment Using Oncolytic Immunotherapy. EBioMedicine 2018, 31, 17–24. [Google Scholar] [CrossRef]
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of oncolytic virotherapy: From genetic modification to combination therapy. Front. Med. 2020, 14, 160–184. [Google Scholar] [CrossRef]
- Stasiak, A.C.; Stehle, T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef]
- Kulanayake, S.; Tikoo, S.K. Adenovirus Core Proteins: Structure and Function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, S.; Luo, Z. Oncolytic Adenovirus, a New Treatment Strategy for Prostate Cancer. Biomedicines 2022, 10, 3262. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, L.; Vitale, M.; Cerullo, V.; Pastore, L. Oncolytic adenoviruses for cancer therapy. Int. J. Mol. Sci. 2021, 22, 2517. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Ehrhardt, A. Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy. Cancers 2020, 12, 1139. [Google Scholar] [CrossRef]
- Fujiwara, T. Multidisciplinary oncolytic virotherapy for gastrointestinal cancer. Ann. Gastroenterol. Surg. 2019, 3, 396–404. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Kuroda, S.; Kanaya, N.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Yagi, C.; Sugimoto, R.; Hamada, Y.; Kikuchi, S.; et al. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol. Ther. 2021, 29, 2920–2930. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Jia, T.; Du, X.; Xu, Y.; Zhao, Y.; Li, L.; Liang, K.; Liang, W.; Sun, H.; et al. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015, 36, 4535–4543. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, Y.; Zhao, Y.; Li, L.; Sun, P.; Liu, H.; Fan, Q.; Liang, K.; Liang, W.; Sun, H.; et al. Combination of E2F-1 promoter-regulated oncolytic adenovirus and cytokine-induced killer cells enhances the antitumor effects in an orthotopic rectal cancer model. Tumour Biol. 2014, 35, 1113–1122. [Google Scholar] [CrossRef]
- Akbulut, H.; Coleri, A.; Sahin, G.; Tang, Y.; Icli, F. A bicistronic adenoviral vector carrying cytosine deaminase and granulocyte-macrophage colony-stimulating factor increases the therapeutic efficacy of cancer gene therapy. Hum. Gene Ther. 2019, 30, 999–1007. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Shi, G.; Yang, Q.; Zhang, Y.; Jiang, Q.; Lin, Y.; Yang, S.; Wang, H.; Cheng, L.; Zhang, X.; Li, Y.; et al. Modulating the Tumor Microenvironment via Oncolytic Viruses and CSF-1R Inhibition Synergistically Enhances Anti-PD-1 Immunotherapy. Mol. Ther. 2019, 27, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Iovine, B.; Kuryk, L.; Capasso, C.; Hirvinen, M.; Vitale, A.; Yliperttula, M.; Bevilacqua, M.A.; Cerullo, V. Oncolytic Adenovirus Loaded with L-carnosine as Novel Strategy to Enhance the Antitumor Activity. Mol. Cancer Ther. 2016, 15, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Song, Y.; Zhang, Y.; Zhang, C.; Yin, J.; Chi, Y.; Zhou, D. A novel oncolytic adenovirus based on simian adenovirus serotype 24. Oncotarget 2017, 8, 26871–26885. [Google Scholar] [CrossRef]
- Bressy, C.; Majhen, D.; Raddi, N.; Jdey, W.; Cornilleau, G.; Zig, L.; Guirouilh-Barbat, J.; Lopez, B.S.; Bawa, O.; Opolon, P.; et al. Combined therapy of colon carcinomas with an oncolytic adenovirus and valproic acid. Oncotarget 2017, 8, 97344–97360. [Google Scholar] [CrossRef][Green Version]
- Kurimasa, A.; Ouyang, H.; Dong, L.J.; Wang, S.; Li, X.; Cordon-Cardo, C.; Chen, D.J.; Li, G.C. Catalytic subunit of DNA-dependent protein kinase: Impact on lymphocyte development and tumorigenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1403–1408. [Google Scholar] [CrossRef]
- Taccioli, G.E.; Amatucci, A.G.; Beamish, H.J.; Gell, D.; Xiang, X.H.; Torres Arzayus, M.I.; Priestley, A.; Jackson, S.P.; Marshak Rothstein, A.; Jeggo, P.A.; et al. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity 1998, 9, 355–366. [Google Scholar] [CrossRef]
- Thompson, L.H.; Brookman, K.W.; Jones, N.J.; Allen, S.A.; Carrano, A.V. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol. Cell Biol. 1990, 10, 6160–6171. [Google Scholar]
- Kon, T.; Zhang, X.; Huang, Q.; Yang, Z.; Liu, S.; Yan, B.; Li, F.; Wang, H.; Li, C.Y. Oncolytic virus-mediated tumor radiosensitization in mice through DNA-PKcs-specific shRNA. Transl. Cancer Res. 2012, 1, 4–14. [Google Scholar]
- Kaneda, A.; Feinberg, A.P. Loss of imprinting of IGF2: A common epigenetic modifier of intestinal tumor risk. Cancer Res. 2005, 65, 11236–11240. [Google Scholar] [CrossRef]
- Sun, H.; Pan, Y.; He, B.; Deng, Q.; Li, R.; Xu, Y.; Chen, J.; Gao, T.; Ying, H.; Wang, F.; et al. Gene therapy for human colorectal cancer cell lines with recombinant adenovirus 5 based on loss of the insulin-like growth factor 2 imprinting. Int. J. Oncol. 2015, 46, 1759–1767. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Zhu, H.; Chen, W.; Hu, X.; Pang, X.; Zhang, J.; Huang, X.; Fang, B.; He, C. Treatment of patient tumor-derived colon cancer xenografts by a TRAIL gene-armed oncolytic adenovirus. Cancer Gene Ther. 2011, 18, 336–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanaya, N.; Kuroda, S.; Kakiuchi, Y.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Morihiro, T.; Kubota, T.; Aoyama, K.; Kikuchi, S.; et al. Immune Modulation by Telomerase-Specific Oncolytic Adenovirus Synergistically Enhances Antitumor Efficacy with Anti-PD1 Antibody. Mol. Ther. 2020, 28, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.J.; Sykelyk, A.; Figueredo, R.; Koropatnick, J. Synergistic cytotoxicity against human tumor cell lines by oncolytic adenovirus dl1520 (ONYX-015) and melphalan. Tumori 2016, 102, 31–39. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef]
- Shen, Y.; Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006, 13, 975–992. [Google Scholar] [CrossRef]
- Yin, J.; Markert, J.M.; Leavenworth, J.W. Modulation of the Intratumoral Immune Landscape by Oncolytic Herpes Simplex Virus Virotherapy. Front. Oncol. 2017, 7, 136. [Google Scholar] [CrossRef]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic herpes simplex virus-based therapies for cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.-M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic herpes simplex virus encoding IL12 controls triple-negative breast cancer growth and metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef]
- Bennett, J.J.; Delman, K.A.; Burt, B.M.; Mariotti, A.; Malhotra, S.; Zager, J.; Petrowsky, H.; Mastorides, S.; Federoff, H.; Fong, Y. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002, 9, 935–945. [Google Scholar] [CrossRef][Green Version]
- McAuliffe, P.F.; Jarnagin, W.R.; Johnson, P.; Delman, K.A.; Federoff, H.; Fong, Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. J. Gastrointest. Surg. 2000, 4, 580–588. [Google Scholar] [CrossRef]
- Gutermann, A.; Mayer, E.; Dehn-Rothfelser, K.V.; Breidenstein, C.; Weber, M.; Muench, M.; Gungor, D.; Suehnel, J.; Moebius, U.; Lechmann, M. Efficacy of oncolytic herpesvirus NV1020 can be enhanced by combination with chemotherapeutics in colon carcinoma cells. Hum. Gene Ther. 2006, 17, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Kooby, D.A.; Carew, J.F.; Halterman, M.W.; Mack, J.E.; Bertino, J.R.; Blumgart, L.H.; Federoff, H.J.; Fong, Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207). FASEB J. 1999, 13, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Warner, S.G.; Haddad, D.; Au, J.; Carson, J.S.; O’Leary, M.P.; Lewis, C.; Monette, S.; Fong, Y. Oncolytic herpes simplex virus kills stem-like tumor-initiating colon cancer cells. Mol. Ther. -Oncolytics 2016, 3, 16013. [Google Scholar] [CrossRef]
- Terai, K.; Bi, D.; Liu, Z.; Kimura, K.; Sanaat, Z.; Dolatkhah, R.; Soleimani, M.; Jones, C.; Bright, A.; Esfandyari, T. A novel oncolytic herpes capable of cell-specific transcriptional targeting of CD133±cancer cells induces significant tumor regression. Stem Cells 2018, 36, 1154–1169. [Google Scholar] [CrossRef]
- Yang, H.; Peng, T.; Li, J.; Wang, Y.; Zhang, W.; Zhang, P.; Peng, S.; Du, T.; Li, Y.; Yan, Q. Treatment of colon cancer with oncolytic herpes simplex virus in preclinical models. Gene Ther. 2016, 23, 450–459. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, W.; Ning, Z.; Zhuang, X.; Lu, H.; Liang, J.; Li, J.; Zhang, Y.; Dong, Y.; Zhang, Y.; et al. A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity. PLoS ONE 2014, 9, e93103. [Google Scholar] [CrossRef]
- Cheng, K.J.; Alshawsh, M.A.; Mejia Mohamed, E.H.; Thavagnanam, S.; Sinniah, A.; Ibrahim, Z.A. HMGB1: An overview of its versatile roles in the pathogenesis of colorectal cancer. Cell Oncol. 2020, 43, 177–193. [Google Scholar] [CrossRef]
- Rapoport, B.L.; Steel, H.C.; Theron, A.J.; Heyman, L.; Smit, T.; Ramdas, Y.; Anderson, R. High Mobility Group Box 1 in Human Cancer. Cells 2020, 9, 1664. [Google Scholar] [CrossRef]
- Cerwenka, A.; Kopitz, J.; Schirmacher, P.; Roth, W.; Gdynia, G. HMGB1: The metabolic weapon in the arsenal of NK cells. Mol. Cell. Oncol. 2016, 3, e1175538. [Google Scholar] [CrossRef]
- Gdynia, G.; Sauer, S.W.; Kopitz, J.; Fuchs, D.; Duglova, K.; Ruppert, T.; Miller, M.; Pahl, J.; Cerwenka, A.; Enders, M.; et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat. Commun. 2016, 7, 10764. [Google Scholar] [CrossRef]
- Shayan, S.; Arashkia, A.; Bahramali, G.; Abdoli, A.; Nosrati, M.S.S.; Azadmanesh, K. Cell type-specific response of colon cancer tumor cell lines to oncolytic HSV-1 virotherapy in hypoxia. Cancer Cell Int. 2022, 22, 164. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Liang, J.; Zhu, Y.; Zeng, B.; Feng, L.; Zhao, C.; Liu, S.; Liu, B.; Zhang, K. oHSV2 can target murine colon carcinoma by altering the immune status of the tumor microenvironment and inducing antitumor immunity. Mol. Ther. Oncolytics 2020, 16, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Goins, W.F.; Hall, B.; Cohen, J.B.; Glorioso, J.C. Retargeting of herpes simplex virus (HSV) vectors. Curr. Opin. Virol. 2016, 21, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 2017, 32, 253–267.e255. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Yang, J.; Liu, J.; Zhou, H.; Sun, C.; Tian, C.; Zhu, C.; Shao, M.; Wang, S. Improved antitumor effectiveness of oncolytic HSV-1 viruses engineered with IL-15/IL-15Rα complex combined with oncolytic HSV-1-aPD1 targets colon cancer. Sci. Rep. 2024, 14, 23671. [Google Scholar] [CrossRef]
- Kolodkin-Gal, D.; Edden, Y.; Hartshtark, Z.; Ilan, L.; Khalaileh, A.; Pikarsky, A.; Pikarsky, E.; Rabkin, S.; Panet, A.; Zamir, G. Herpes simplex virus delivery to orthotopic rectal carcinoma results in an efficient and selective antitumor effect. Gene Ther. 2009, 16, 905–915. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Yu, J.; Wan, Y.; Zhang, C.; Zhang, H.; Cao, Y. Construction of an IL12 and CXCL11 armed oncolytic herpes simplex virus using the CRISPR/Cas9 system for colon cancer treatment. Virus Res. 2023, 323, 198979. [Google Scholar] [CrossRef]
- DeAntoneo, C.; Danthi, P.; Balachandran, S. Reovirus Activated Cell Death Pathways. Cells 2022, 11, 1757. [Google Scholar] [CrossRef]
- Kemp, V.; Hoeben, R.C.; Van den Wollenberg, D.J. Exploring reovirus plasticity for improving its use as oncolytic virus. Viruses 2015, 8, 4. [Google Scholar] [CrossRef]
- Müller, L.; Berkeley, R.; Barr, T.; Ilett, E.; Errington-Mais, F. Past, present and future of oncolytic reovirus. Cancers 2020, 12, 3219. [Google Scholar] [CrossRef]
- Shakoori, A.; Ougolkov, A.; Yu, Z.W.; Zhang, B.; Modarressi, M.H.; Billadeau, D.D.; Mai, M.; Takahashi, Y.; Minamoto, T. Deregulated GSK3beta activity in colorectal cancer: Its association with tumor cell survival and proliferation. Biochem. Biophys. Res. Commun. 2005, 334, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Koh, S.S.; Cho, I.R.; Srisuttee, R.; Park, E.H.; Jhun, B.H.; Kim, Y.G.; Oh, S.; Kwak, J.E.; Johnston, R.N.; et al. Inhibition of GSK-3beta enhances reovirus-induced apoptosis in colon cancer cells. Int. J. Oncol. 2009, 35, 617–624. [Google Scholar] [PubMed]
- Negri, F.; Bottarelli, L.; de’Angelis, G.L.; Gnetti, L. KRAS: A druggable target in colon cancer patients. Int. J. Mol. Sci. 2022, 23, 4120. [Google Scholar] [CrossRef]
- Augustine, T.; John, P.; Friedman, T.; Jiffry, J.; Guzik, H.; Mannan, R.; Gupta, R.; Delano, C.; Mariadason, J.M.; Zang, X.; et al. Potentiating effect of reovirus on immune checkpoint inhibition in microsatellite stable colorectal cancer. Front. Oncol. 2022, 12, 1018767. [Google Scholar] [CrossRef]
- Müller, L.M.E.; Migneco, G.; Scott, G.B.; Down, J.; King, S.; Askar, B.; Jennings, V.; Oyajobi, B.; Scott, K.; West, E.; et al. Reovirus-induced cell-mediated immunity for the treatment of multiple myeloma within the resistant bone marrow niche. J. Immunother. Cancer 2021, 9, e001803. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Wang, F.; Tu, X.; Tong, Z.; Liu, L.; Zheng, Y.; Zhao, P.; Cheng, J.; Li, J. The long-term effectiveness and mechanism of oncolytic virotherapy combined with anti-PD-L1 antibody in colorectal cancer patient. Cancer Gene Ther. 2024, 31, 1412–1426. [Google Scholar] [CrossRef]
- Angioni, R.; Sánchez-Rodríguez, R.; Viola, A.; Molon, B. TGF-β in Cancer: Metabolic Driver of the Tolerogenic Crosstalk in the Tumor Microenvironment. Cancers 2021, 13, 401. [Google Scholar] [CrossRef]
- Groeneveldt, C.; van Ginkel, J.Q.; Kinderman, P.; Sluijter, M.; Griffioen, L.; Labrie, C.; van den Wollenberg, D.J.; Hoeben, R.C.; van der Burg, S.H.; Ten Dijke, P. Intertumoral differences dictate the outcome of TGF-β blockade on the efficacy of viro-immunotherapy. Cancer Res. Commun. 2023, 3, 325–337. [Google Scholar] [CrossRef]
- Maitra, R.; Seetharam, R.; Tesfa, L.; Augustine, T.A.; Klampfer, L.; Coffey, M.C.; Mariadason, J.M.; Goel, S. Oncolytic reovirus preferentially induces apoptosis in KRAS mutant colorectal cancer cells, and synergizes with irinotecan. Oncotarget 2014, 5, 2807. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, S.J.; Lee, H.J.; Yang, H.; Go, E.-J.; Gansukh, E.; Song, K.-H.; Xiang, X.; Park, D.G.; Alain, T. Oral reovirus reshapes the gut microbiome and enhances antitumor immunity in colon cancer. Nat. Commun. 2024, 15, 9092. [Google Scholar] [CrossRef]
- Parakrama, R.; Fogel, E.; Chandy, C.; Augustine, T.; Coffey, M.; Tesfa, L.; Goel, S.; Maitra, R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hastie, E.; Grdzelishvili, V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012, 93, 2529–2545. [Google Scholar] [CrossRef] [PubMed]
- Abdelmageed, A.A.; Dewhurst, S.; Ferran, M.C. Employing the Oncolytic Vesicular Stomatitis Virus in Cancer Virotherapy: Resistance and Clinical Considerations. Viruses 2024, 17, 16. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Okesanya, O.J.; Ukoaka, B.M.; Ibrahim, A.M.; Lucero-Prisno, D.E., III. Vesicular Stomatitis Virus: Insights into Pathogenesis, Immune Evasion, and Technological Innovations in Oncolytic and Vaccine Development. Viruses 2024, 16, 1933. [Google Scholar] [CrossRef]
- Gray, Z.; Tabarraei, A.; Moradi, A.; Kalani, M.R. M51R and Delta-M51 matrix protein of the vesicular stomatitis virus induce apoptosis in colorectal cancer cells. Mol. Biol. Rep. 2019, 46, 3371–3379. [Google Scholar] [CrossRef]
- Day, G.L.; Bryan, M.L.; Northrup, S.A.; Lyles, D.S.; Westcott, M.M.; Stewart, J.H., IV. Immune effects of M51R vesicular stomatitis virus treatment of carcinomatosis from colon cancer. J. Surg. Res. 2020, 245, 127–135. [Google Scholar] [CrossRef]
- Ohteki, T. Critical role for IL-15 in innate immunity. Curr. Mol. Med. 2002, 2, 371–380. [Google Scholar] [CrossRef]
- Stephenson, K.; Barra, N.; Davies, E.; Ashkar, A.; Lichty, B. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012, 19, 238–246. [Google Scholar] [CrossRef]
- Larrieux, A.; Sanjuán, R. Murine colon cancer derived cells exhibit heterogeneous resistance profiles against an oncolytic virus. Sci. Rep. 2024, 14, 27209. [Google Scholar] [CrossRef]
- Selman, M.; Rousso, C.; Bergeron, A.; Son, H.H.; Krishnan, R.; El-Sayes, N.A.; Varette, O.; Chen, A.; Le Boeuf, F.; Tzelepis, F. Multi-modal potentiation of oncolytic virotherapy by vanadium compounds. Mol. Ther. 2018, 26, 56–69. [Google Scholar] [CrossRef]
- Alluqmani, N.; Jirovec, A.; Taha, Z.; Varette, O.; Chen, A.; Serrano, D.; Maznyi, G.; Khan, S.; Forbes, N.E.; Arulanandam, R. Vanadyl sulfate-enhanced oncolytic virus immunotherapy mediates the antitumor immune response by upregulating the secretion of pro-inflammatory cytokines and chemokines. Front. Immunol. 2022, 13, 1032356. [Google Scholar] [CrossRef] [PubMed]
- McAusland, T.M.; van Vloten, J.P.; Santry, L.A.; Guilleman, M.M.; Rghei, A.D.; Ferreira, E.M.; Ingrao, J.C.; Arulanandam, R.; Major, P.P.; Susta, L. Combining vanadyl sulfate with Newcastle disease virus potentiates rapid innate immune-mediated regression with curative potential in murine cancer models. Mol. Ther. Oncolytics 2021, 20, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V.; Sergushichev, A.; Loginicheva, E.; Johnson, K.; Korenfeld, D.; Mathyer, M.E.; Kim, H.; Huang, L.-H. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.; Higgins, M.; Hams, E. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Kurmasheva, N.; Said, A.; Wong, B.; Kinderman, P.; Han, X.; Rahimic, A.H.; Kress, A.; Carter-Timofte, M.E.; Holm, E.; van der Horst, D. Octyl itaconate enhances VSVΔ51 oncolytic virotherapy by multitarget inhibition of antiviral and inflammatory pathways. Nat. Commun. 2024, 15, 4096. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Ye, Q.; Liu, Y.; Qian, W. Preclinical and clinical trials of oncolytic vaccinia virus in cancer immunotherapy: A comprehensive review. Cancer Biol. Med. 2023, 20, 646–661. [Google Scholar] [CrossRef]
- Inoue, T.; Byrne, T.; Inoue, M.; Tait, M.E.; Wall, P.; Wang, A.; Dermyer, M.R.; Laklai, H.; Binder, J.J.; Lees, C.; et al. Oncolytic Vaccinia Virus Gene Modification and Cytokine Expression Effects on Tumor Infection, Immune Response, and Killing. Mol. Cancer Ther. 2021, 20, 1481–1494. [Google Scholar] [CrossRef]
- Kilinc, M.O.; Ehrig, K.; Pessian, M.; Minev, B.R.; Szalay, A.A. Colonization of xenograft tumors by oncolytic vaccinia virus (VACV) results in enhanced tumor killing due to the involvement of myeloid cells. J. Transl. Med. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Foloppe, J.; Kintz, J.; Futin, N.; Findeli, A.; Cordier, P.; Schlesinger, Y.; Hoffmann, C.; Tosch, C.; Balloul, J.; Erbs, P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008, 15, 1361–1371. [Google Scholar] [CrossRef]
- Jeong, S.N.; Yoo, S.Y. Novel Oncolytic Virus Armed with Cancer Suicide Gene and Normal Vasculogenic Gene for Improved Anti-Tumor Activity. Cancers 2020, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.Y.; Mealiea, D.; Okamoto, L.; Stojdl, D.F.; McCart, J.A. Deletion of immunomodulatory genes as a novel approach to oncolytic vaccinia virus development. Mol. Ther. Oncolytics 2021, 22, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Génin, P.; Morin, P.; Civas, A. Impairment of interferon-induced IRF-7 gene expression due to inhibition of ISGF3 formation by trichostatin A. J. Virol. 2003, 77, 7113–7119. [Google Scholar] [CrossRef]
- Suh, H.-S.; Choi, S.; Khattar, P.; Choi, N.; Lee, S.C. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J. Neuroimmune Pharmacol. 2010, 5, 521–532. [Google Scholar] [CrossRef]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef]
- Colamonici, O.R.; Domanski, P.; Sweitzer, S.M.; Larner, A.; Buller, R.M.L. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling (∗). J. Biol. Chem. 1995, 270, 15974–15978. [Google Scholar] [CrossRef]
- Kirn, D.H.; Wang, Y.; Le Boeuf, F.; Bell, J.; Thorne, S.H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007, 4, e353. [Google Scholar] [CrossRef]
- MacTavish, H.; Diallo, J.S.; Huang, B.; Stanford, M.; Le Boeuf, F.; De Silva, N.; Cox, J.; Simmons, J.G.; Guimond, T.; Falls, T.; et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS ONE 2010, 5, e14462. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Yang, L.; Chen, J.; Ye, Q.; Qian, W.; Wang, S. Oncolytic vaccinia virus armed with anti-CD47 nanobody elicit potent antitumor effects on multiple tumor models via enhancing innate and adoptive immunity. J. Immunother. Cancer 2024, 12, e009473. [Google Scholar] [CrossRef]
- Francis, L.; Guo, Z.S.; Liu, Z.; Ravindranathan, R.; Urban, J.A.; Sathaiah, M.; Magge, D.; Kalinski, P.; Bartlett, D.L. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget 2016, 7, 22174. [Google Scholar] [CrossRef]
- Li, J.; O’Malley, M.; Sampath, P.; Kalinski, P.; Bartlett, D.L.; Thorne, S.H. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia 2012, 14, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, H.; Ye, J.; Ge, Y.; Wang, H.; Dai, E.; Ren, J.; Liu, W.; Ma, C.; Ju, S. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics 2021, 11, 6668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, M.; Zhao, H.; Huang, Y.; Li, D.; Mao, D.; Zhang, Z.; Zhu, X.; Dong, X.; Zhao, X. IL-9 exerts antitumor effects in colon cancer and transforms the tumor microenvironment in vivo. Technol. Cancer Res. Treat. 2019, 18, 1533033819857737. [Google Scholar] [CrossRef]
- Ye, J.; Chen, L.; Waltermire, J.; Zhao, J.; Ren, J.; Guo, Z.; Bartlett, D.L.; Liu, Z. Intratumoral Delivery of Interleukin 9 via Oncolytic Vaccinia Virus Elicits Potent Antitumor Effects in Tumor Models. Cancers 2024, 16, 1021. [Google Scholar] [CrossRef]
- Wu, R.; Tong, S.; Yin, J.; Zhu, Z.; Mao, Z.; Xu, L. Oncolytic vaccinia virus acts synergistically with anti-PD-L1 antibody to enhance the killing of colon cancer cells by CD8(+) T cells. Pathol. Res. Pr. 2023, 247, 154535. [Google Scholar] [CrossRef]
- Shakiba, Y.; Vorobyev, P.O.; Yusubalieva, G.M.; Kochetkov, D.V.; Zajtseva, K.V.; Valikhov, M.P.; Kalsin, V.A.; Zabozlaev, F.G.; Semkina, A.S.; Troitskiy, A.V.; et al. Oncolytic therapy with recombinant vaccinia viruses targeting the interleukin-15 pathway elicits a synergistic response. Mol. Ther. Oncolytics 2023, 29, 158–168. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, H.; Ren, J.; Liu, W.; Chen, L.; Chen, H.; Ye, J.; Dai, E.; Ma, C.; Ju, S.; et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J. Immunother. Cancer 2020, 8, e000710. [Google Scholar] [CrossRef]
- Engeland, C.E.; Ungerechts, G. Measles virus as an oncolytic immunotherapy. Cancers 2021, 13, 544. [Google Scholar] [CrossRef]
- Kleinlützum, D.; Hanauer, J.D.; Muik, A.; Hanschmann, K.-M.; Kays, S.-K.; Ayala-Breton, C.; Peng, K.-W.; Mühlebach, M.D.; Abel, T.; Buchholz, C.J. Enhancing the oncolytic activity of CD133-targeted measles virus: Receptor extension or chimerism with vesicular stomatitis virus are most effective. Front. Oncol. 2017, 7, 127. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Chen, J. Oncolytic Measles Virus Encoding Interleukin-12 Mediated Antitumor Activity and Immunologic Control of Colon Cancer In Vivo and Ex Vivo. Cancer Biother. Radiopharm. 2021, 36, 774–782. [Google Scholar]
- Jing, Y.; Tong, C.; Zhang, J.; Nakamura, T.; Iankov, I.; Russell, S.J.; Merchan, J.R. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009, 69, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zaias, J.; Duncan, R.; Russell, S.J.; Merchan, J.R. In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models. Gene Ther. 2014, 21, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.D.; Lauer, U.M.; Herold-Mende, C.; et al. Specific Elimination of CD133+ Tumor Cells with Targeted Oncolytic Measles Virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Mechanism of Action Trial of ColoAd1 (MOA). 2013. Available online: https://www.clinicaltrials.gov/study/NCT02053220 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Clinical Study of BioTTT001 in Combination with Toripalimab and Regorafenib in Patients with Colorectal Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT06283134 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Pilot Study of GVAX in Colorectal Cancer Cells. 2023. Available online: https://clinicaltrials.gov/study/NCT01952730 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Standard of Care Alone or in Combination with Ad-CEA Vaccine and Avelumab in People with Previously Untreated Metastatic Colorectal Cancer QUILT-2.004. 2021. Available online: https://clinicaltrials.gov/study/NCT03050814 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Multi-Targeted Recombinant Ad5 (CEA/MUC1/Brachyury) Based Immunotherapy Vaccine Regimen in People with Advanced Cancer. 2020. Available online: https://clinicaltrials.gov/study/NCT03384316 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Interleukin-12 Gene in Treating Patients with Liver Metastases Secondary to Colorectal Cancer. 2017. Available online: https://clinicaltrials.gov/study/NCT00072098 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Phase I Study of Ad5-hGCC (Human Guanylyl Cyclase C)-PADRE in Stage I/II Colon Cancer. 2025. Available online: https://clinicaltrials.gov/study/NCT01972737 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Vaccine (Ad5.F35-hGCC-PADRE) for the Treatment of Gastrointestinal Adenocarcinoma. 2025. Available online: https://clinicaltrials.gov/study/NCT04111172 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Safety Study of Human MUC-1 (Mucin-1) Adenoviral Vector Vaccine for Immunotherapy of Epithelial Cancers (MUC-1). 2016. Available online: https://clinicaltrials.gov/study/NCT02140996 (accessed on 13 May 2025).
- U.S. National Library of Medicine. VB-111 in Combination with Nivolumab in People with Metastatic Colorectal Cancer (mCRC). 2023. Available online: https://clinicaltrials.gov/study/NCT04166383 (accessed on 13 May 2025).
- U.S. National Library of Medicine. RP2/RP3 in Combination with Atezolizumab and Bevacizumab for the Treatment of Patients with CRC. 2025. Available online: https://clinicaltrials.gov/study/NCT05733611 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Clinical Study of T3011 in Combination with Toripalimab and Regorafenib in Patients with Colorectal Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT06283303 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Gene Therapy in Treating Patients with Colon Cancer That Has Spread to the Liver. 2013. Available online: https://clinicaltrials.gov/study/NCT00012155 (accessed on 13 May 2025).
- U.S. National Library of Medicine. First-line Maintenance of OH2 Injection for Advanced Colorectal Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT05648006 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Study of ONCR-177 Alone and in Combination with PD-1 Blockade in Adult Subjects with Advanced and/or Refractory Cutaneous, Subcutaneous or Metastatic Nodal Solid Tumors or with Liver Metastases of Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT04348916 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Phase I Clinical Study of Intratumoral Injection Oncolytic Vaccinia Virus GC001 in Patient with Advanced Solid Tumors. 2024. Available online: https://clinicaltrials.gov/study/NCT06508307 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Trial of Perioperative CV301 Vaccination in Combination with Nivolumab and Systemic Chemotherapy for Metastatic CRC. 2024. Available online: https://clinicaltrials.gov/study/NCT03547999 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Vaccine Therapy in Treating Patients with Colorectal, Stomach, or Pancreatic Cancer. 2017. Available online: https://clinicaltrials.gov/study/NCT01191684 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Vaccine Therapy and Pembrolizumab in Treating Patients with Solid Tumors That Have Failed Prior Therapy. 2025. Available online: https://clinicaltrials.gov/study/NCT02432963 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Safety Study of Recombinant Vaccinia Virus Administered Intravenously in Patients with Metastatic, Refractory Colorectal Carcinoma. 2016. Available online: https://clinicaltrials.gov/study/NCT01380600 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Recombinant Vaccinia Virus Administered Intravenously in Patients with Metastatic, Refractory Colorectal Carcinoma. 2021. Available online: https://clinicaltrials.gov/study/NCT01394939 (accessed on 13 May 2025).
- U.S. National Library of Medicine. A Phase I/II Study of Pexa-Vec Oncolytic Virus in Combination with Immune Checkpoint Inhibition in Refractory Colorectal Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT03206073 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Vaccine Therapy and Sargramostim with or Without Docetaxel in Treating Patients with Metastatic Lung Cancer or Metastatic Colorectal Cancer. 2014. Available online: https://clinicaltrials.gov/study/NCT00088933 (accessed on 13 May 2025).
- U.S. National Library of Medicine. Vaccine Therapy in Treating Patients with Liver or Lung Metastases From Colorectal Cancer. 2015. Available online: https://clinicaltrials.gov/study/NCT00103142 (accessed on 13 May 2025).
- Flatt, J.W.; Kim, R.; Smith, J.G.; Nemerow, G.R.; Stewart, P.L. An intrinsically disordered region of the adenovirus capsid is implicated in neutralization by human alpha defensin 5. PLoS ONE 2013, 8, e61571. [Google Scholar] [CrossRef]
- Smith, J.G.; Silvestry, M.; Lindert, S.; Lu, W.; Nemerow, G.R.; Stewart, P.L. Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog. 2010, 6, e1000959. [Google Scholar] [CrossRef]
- Vragniau, C.; Hübner, J.-M.; Beidler, P.; Gil, S.; Saydaminova, K.; Lu, Z.-Z.; Yumul, R.; Wang, H.; Richter, M.; Sova, P. Studies on the interaction of tumor-derived HD5 alpha defensins with adenoviruses and implications for oncolytic adenovirus therapy. J. Virol. 2017, 91, e02185-18. [Google Scholar] [CrossRef]
- Kulu, Y.; Kawasaki, H.; Donahue, J.M.; Kasuya, H.; Cusack, J.C.; Choi, E.W.; Kuruppu, D.K.; Fuchs, B.C.; Tanabe, K.K. Concurrent chemotherapy inhibits herpes simplex virus-1 replication and oncolysis. Cancer Gene Ther. 2013, 20, 133–140. [Google Scholar] [CrossRef]
- Song, T.; Haddad, D.; Adusumilli, P.; Kim, T.; Stiles, B.; Hezel, M.; Socci, N.; Gönen, M.; Fong, Y. Molecular network pathways and functional analysis of tumor signatures associated with development of resistance to viral gene therapy. Cancer Gene Ther. 2012, 19, 38–48. [Google Scholar] [CrossRef]
- Van Houdt, W.; Smakman, N.; van Den Wollenberg, D.; Emmink, B.; Veenendaal, L.; Van Diest, P.; Hoeben, R.; Borel Rinkes, I.; Kranenburg, O. Transient infection of freshly isolated human colorectal tumor cells by reovirus T3D intermediate subviral particles. Cancer Gene Ther. 2008, 15, 284–292. [Google Scholar] [CrossRef]
- Larrieux, A.; Sanjuán, R. Cellular resistance to an oncolytic virus is driven by chronic activation of innate immunity. Iscience 2023, 26, 105749. [Google Scholar] [CrossRef]
- Clarke, P.; Meintzer, S.M.; Wang, Y.; Moffitt, L.A.; Richardson-Burns, S.M.; Johnson, G.L.; Tyler, K.L. JNK regulates the release of proapoptotic mitochondrial factors in reovirus-infected cells. J. Virol. 2004, 78, 13132–13138. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.; Kim, A.; Han, H.-S.; Han, J.; Jun, H.-S.; Yoon, J.-W. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 2003, 77, 5649–5656. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.; Coulson, B.S. Rotavirus activates JNK and p38 signaling pathways in intestinal cells, leading to AP-1-driven transcriptional responses and enhanced virus replication. J. Virol. 2006, 80, 10624–10633. [Google Scholar] [CrossRef]
- Rahaus, M.; Desloges, N.; Wolff, M.H. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 2004, 85, 3529–3540. [Google Scholar] [CrossRef]
- Hu, W.; Hofstetter, W.; Guo, W.; Li, H.; Pataer, A.; Peng, H.H.; Guo, Z.S.; Bartlett, D.L.; Lin, A.; Swisher, S.G. JNK-deficiency enhanced oncolytic vaccinia virus replication and blocked activation of double-stranded RNA-dependent protein kinase. Cancer Gene Ther. 2008, 15, 616–624. [Google Scholar] [CrossRef]
- Feola, S.; Chiaro, J.; Martins, B.; Russo, S.; Fusciello, M.; Ylösmäki, E.; Bonini, C.; Ruggiero, E.; Hamdan, F.; Feodoroff, M. A novel immunopeptidomic-based pipeline for the generation of personalized oncolytic cancer vaccines. Elife 2022, 11, e71156. [Google Scholar] [CrossRef]
- Feola, S.; Hamdan, F.; Russo, S.; Chiaro, J.; Fusciello, M.; Feodoroff, M.; Antignani, G.; D’Alessio, F.; Mölsä, R.; Stigzelius, V. Novel peptide-based oncolytic vaccine for enhancement of adaptive antitumor immune response via co-engagement of innate Fcγ and Fcα receptors. J. Immunother. Cancer 2024, 12, e008342. [Google Scholar] [CrossRef]
- Le Boeuf, F.; Gebremeskel, S.; McMullen, N.; He, H.; Greenshields, A.L.; Hoskin, D.W.; Bell, J.C.; Johnston, B.; Pan, C.; Duncan, R. Reovirus FAST protein enhances vesicular stomatitis virus oncolytic virotherapy in primary and metastatic tumor models. Mol. Ther. Oncolytics 2017, 6, 80–89. [Google Scholar] [CrossRef]
- Malilas, W.; Koh, S.S.; Srisuttee, R.; Boonying, W.; Cho, I.; Jeong, C.; Johnston, R.; Chung, Y. Cancer upregulated gene 2, a novel oncogene, confers resistance to oncolytic vesicular stomatitis virus through STAT1-OASL2 signaling. Cancer Gene Ther. 2013, 20, 125–132. [Google Scholar] [CrossRef]
- Nakatake, M.; Kurosaki, H.; Kuwano, N.; Horita, K.; Ito, M.; Kono, H.; Okamura, T.; Hasegawa, K.; Yasutomi, Y.; Nakamura, T. Partial deletion of glycoprotein B5R enhances vaccinia virus neutralization escape while preserving oncolytic function. Mol. Ther. Oncolytics 2019, 14, 159–171. [Google Scholar] [CrossRef]
- Burton, C.; Bartee, E. Syncytia formation in oncolytic virotherapy. Mol. Ther. Oncolytics 2019, 15, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nakatake, M.; Kuwano, N.; Kaitsurumaru, E.; Kurosaki, H.; Nakamura, T. Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment. Mol. Ther. 2021, 29, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, H.; Zhu, Z.; Liu, Z.; Ma, C.; Lee, Y.J.; Bartlett, D.L.; Guo, Z.-S. Ferroptosis inducer improves the efficacy of oncolytic virus-mediated cancer immunotherapy. Biomedicines 2022, 10, 1425. [Google Scholar] [CrossRef]
- Yamaki, M.; Shinozaki, K.; Sakaguchi, T.; Meseck, M.; Ebert, O.; Ohdan, H.; Woo, S.L. The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer. Int. J. Mol. Med. 2013, 31, 299–306. [Google Scholar] [CrossRef]
- Zeh, H.J.; Downs-Canner, S.; McCart, J.A.; Guo, Z.S.; Rao, U.N.; Ramalingam, L.; Thorne, S.H.; Jones, H.L.; Kalinski, P.; Wieckowski, E.; et al. First-in-man study of western reserve strain oncolytic vaccinia virus: Safety, systemic spread, and antitumor activity. Mol. Ther. 2015, 23, 202–214. [Google Scholar] [CrossRef]
- Goossens, M.; Pauwels, K.; Willemarck, N.; Breyer, D. Environmental risk assessment of clinical trials involving modified vaccinia virus Ankara (MVA)-based vectors. Curr. Gene Ther. 2013, 13, 413–420. [Google Scholar] [CrossRef]
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses—Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef]
- Badrinath, N.; Jeong, Y.I.; Woo, H.Y.; Bang, S.Y.; Kim, C.; Heo, J.; Kang, D.H.; Yoo, S.Y. Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes. Int. J. Pharm. 2018, 552, 437–442. [Google Scholar] [CrossRef]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017, 8, 14754. [Google Scholar] [CrossRef]
| Virus Type | Therapy | Cancer Status | Study Status | Phase | Participants | Clinical Trial ID |
|---|---|---|---|---|---|---|
| Adenovirus | ColoAd1 | Resectable | Completed | 1 | 17 | NCT02053220 |
| BioTTT001 + Toraplizumab and Regorafenib | Liver Metastasis | Not yet recruiting | 1 | 40 | NCT06283134 | |
| GVAX | Liver Metastasis | Terminated | 1 (Pilot study) | 1 | NCT01952730 † | |
| Ad-CEA + avelumab | Metastatic | Terminated | 2 | 30 | NCT03050814 † | |
| ETBX-011, ETBX-061, ETBX-051 | Advanced | Completed | 1 | 11 | NCT03384316 † | |
| IL-12 | Metastatic | Terminated | 1 | 22 | NCT00072098 | |
| Ad5-hGCC-PADRE | Stage I/II | Completed | 1 | 1 | NCT01972737 | |
| Ad5.F35-hGCC-PADRE | Stage III/IV | Active, not recruiting | 2 | 81 | NCT04111172 | |
| Ad-sig-hMUC-1/ecdCD40L | Recurrent or Metastatic | Unknown status | 1 | 24 | NCT02140996 | |
| VB-111 + Nivolumab | Metastatic | Completed | 2 | 14 | NCT04166383 | |
| Herpes Simplex Virus | RP2/RP3 + Atezolizumab and Bevacizumab | Advanced | Active, not recruiting | 2 | 4 | NCT05733611 |
| T3011 + Toripalimab and Regorafenib | Liver Metastasis | Not yet recruiting | 1 | 8 | NCT06283303 | |
| NV1020 | Liver Metastasis | Completed | 1 | No data | NCT00012155 | |
| OH2 + Capecitabine | Advanced | Terminated | 2 | 7 | NCT05648006 | |
| ONCR-177 | Refractory, Metastatic | Terminated | 1 | 66 | NCT04348916 | |
| Reovirus | REOLYSIN + FOLFIRI and bevacizumab | Metastatic | Completed | 1 | 36 | NCT01274624 |
| Vaccinia Virus | GC001 | Advanced | Recruiting | 1 | 21 | NCT06508307 |
| CV301 + Nivolumab and Systemic Chemotherapy | Metastatic | Active, not recruiting | 2 | 78 | NCT03547999 | |
| p53MVA | Unresectable and chemotherapy resistant | Completed | 1 | 12 | NCT01191684 | |
| p53MVA + pembrolizumab | Advanced | Active, not recruiting | 1 | 11 | NCT02432963 | |
| JX-594 | Refractory | Completed | 1 | 15 | NCT01469611 | |
| JX-594 | Liver Metastasis | Terminated | 2a | 2 | NCT01329809 | |
| JX-594 | Metastatic, Refractory | Completed | 1b | 15 | NCT01380600 | |
| JX-594 | Metastatic, Refractory | Completed | 1/2a | 52 | NCT01394939 | |
| JX-594 + Tremelimumab/Durvalumab | Refractory | Completed | 1/2 | 34 | NCT03206073 † | |
| vaccinia-CEA-TRICOM + docetaxel | Metastatic | Terminated | 1 | 60 | NCT00088933 † | |
| vaccinia-CEA-MUC-1-TRICOM | Metastatic | Completed | 2 | 74 | NCT00103142 † | |
| Measles Virus | MVF-HER-2 (266–296/597–626) | Metastatic | Completed | 1 | 65 | NCT01376505 |
| PD1-Vaxx | Operable high MSI a | Not yet recruiting | 2 | 44 | NCT06692959 |
| Virus | Genome/Type | Tumor Selectivity | Immunostimulatory Potential | Clinical Safety | Clinical Maturity * | Key Advantages | Critical Limitations |
|---|---|---|---|---|---|---|---|
| Adenovirus | dsDNA, non-enveloped | High (e.g., LOI, p53 loss) | High (e.g., IL-15, GM-CSF) | Well characterized | Phase I–II in CRC | Genetically tractable, scalable production | Pre-existing immunity, blocked by HD5, limited systemic delivery |
| Herpes Simplex Virus (HSV) | dsDNA, enveloped | High (solid tumors) | Very high | High (e.g., T-VEC approved) | Phase I–II in CRC | Large transgene capacity, antiviral control options | Complex manufacturing, latency potential |
| Reovirus | dsRNA, non-enveloped | Moderate (RAS-mutant preference) | Moderate | Excellent | Phase I in CRC | Oral delivery, native tropism, minimal engineering | JAM1 receptor localization limits infectivity in primary tumors |
| Vesicular Stomatitis Virus (VSV) | ssRNA, enveloped | High (IFN-defective cells) | High | Moderate | Preclinical/Phase I | Rapid replication, fusogenic potential, and low seroprevalence | Systemic toxicity, fast immune clearance |
| Vaccinia Virus (VV) | dsDNA, enveloped | High (hypoxia adapted) | High | High (extensive safety data) | Phase I–II in CRC | Cytoplasmic replication, large genome for transgenes | Manufacturing burden, proinflammatory, and immune clearance |
| Measles Virus (MV) | ssRNA, enveloped | High (via uPAR/CD133 targeting) | High | High (attenuated strains) | Phase I in CRC | Tumor tropism, engineered targeting, systemic administration | Risk of neutralizing antibodies, less clinical experience in CRC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Domínguez, F.; Quezada-Monrás, C.; Cárcamo, L.; Muñoz, J.P.; Carrillo-Beltrán, D. Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions. Cancers 2025, 17, 1854. https://doi.org/10.3390/cancers17111854
Pérez-Domínguez F, Quezada-Monrás C, Cárcamo L, Muñoz JP, Carrillo-Beltrán D. Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions. Cancers. 2025; 17(11):1854. https://doi.org/10.3390/cancers17111854
Chicago/Turabian StylePérez-Domínguez, Francisco, Claudia Quezada-Monrás, Leonardo Cárcamo, Juan P. Muñoz, and Diego Carrillo-Beltrán. 2025. "Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions" Cancers 17, no. 11: 1854. https://doi.org/10.3390/cancers17111854
APA StylePérez-Domínguez, F., Quezada-Monrás, C., Cárcamo, L., Muñoz, J. P., & Carrillo-Beltrán, D. (2025). Oncolytic Viruses as a Novel Therapeutic Approach for Colorectal Cancer: Mechanisms, Current Advances, and Future Directions. Cancers, 17(11), 1854. https://doi.org/10.3390/cancers17111854




