1. Introduction
Oral cancer, a subset of head and neck cancer, remains a significant global health challenge. Surgical intervention is often required, but perioperative complications can negatively affect outcomes. One critical aspect of perioperative care is ensuring optimal nutritional status, which can significantly influence recovery and complication rates [
1]. Previous research has demonstrated that nutritional interventions, particularly those that modulate immune function, may reduce postoperative complications in various surgical fields [
2,
3]. Immunoenhancing nutritional agents—such as arginine, omega-3 fatty acids, and glutamine—have been shown to support immune responses, reduce inflammation, and promote wound healing [
4,
5]. These agents are collectively referred to as immunonutrition. While immunonutrition has shown beneficial effects in gastrointestinal and other surgical fields, its specific application in oral cancer surgery has not been thoroughly investigated. A previous systematic review on head and neck cancer patients [
6] included some oral cancer cases but did not provide focused analysis or subgroup evaluation. Moreover, the evidence was drawn from heterogeneous populations and interventions. Therefore, our review aims to clarify whether immunoenhancing nutritional agents provide perioperative benefits specifically in oral cancer surgery, a population with unique anatomical and nutritional challenges.
However, despite promising results observed in various surgical disciplines, the specific role of immunoenhancing nutritional interventions in the perioperative care of patients with oral cancer remains uncertain [
7]. While some previous studies have suggested a potential benefit in terms of reduced postoperative complications and shorter hospital stays [
8,
9], others have reported mixed results. This inconsistency necessitates a more comprehensive understanding of this topic, especially given the unique challenges associated with oral cancer surgery, such as the potential for postoperative impaired oral intake.
This systematic review (SR) was conducted to collate and analyze the available evidence on the efficacy of immunoenhancing nutritional interventions in the perioperative care of patients with oral cancer. By comparing outcomes between patients receiving immunonutrition and those receiving conventional nutritional therapy, we aim to clarify its clinical value and inform perioperative management strategies in oral oncology.
2. Materials and Methods
2.1. Study Details
This systematic review and meta-analysis was conducted following the PRISMA 2020 guidelines [
10,
11] (PRISMA-2020; (
Supplementary Materials Table S1)). The protocol was registered with PROSPERO (CRD 42024558163).
The results are expressed as risk ratios or mean differences. Data synthesis was performed using Review Manager (RevMan) Version 5.4 (The Cochrane Collaboration).
2.2. Search Strategy and Database Selection
We conducted a comprehensive literature search across three major databases: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and Ichushi-Web (a Japanese medical database). These databases were selected to ensure coverage of both international and Japanese clinical research, considering that a significant number of relevant studies in oral cancer are published in Japan. Although databases such as SCOPUS, EMBASE, and LILACS were not included in this review, we acknowledge this as a limitation and address it in the Discussion. While no language restrictions were applied during the search, we also recognize the potential for publication bias due to the exclusion of certain grey literature and non-indexed sources. The search period ranged from 15 February 2018 to 28 March 2022. This time frame was chosen to supplement the existing Cochrane systematic review [
6], which covered literature up to early 2018. Our study thus aims to provide an updated and focused synthesis on oral cancer, expanding upon the previous broader head and neck cancer reviews. The detailed search strategy is provided in
Supplementary Table S2.
2.3. Study Selection Criteria and Literature Search
Two authors (SH and MW) independently screened all identified studies in a two-phase process. Titles and abstracts were first screened based on eligibility, followed by full-text review. Disagreements were resolved through discussion with a third reviewer (TA or DT).
Eligibility was assessed using the PICO framework, as detailed below:
Population (P): Adult patients (≥18 years) undergoing surgery for oral cancer.
Intervention (I): Perioperative immunonutrition administered either preoperatively, postoperatively, or both. Immunonutrition included formulas containing arginine, glutamine, omega-3 fatty acids, and/or nucleotides.
Comparison (C): Standard nutritional care (e.g., intravenous fluids or polymeric enteral formulas without immunoenhancing components).
Outcomes (O): Studies were included if they reported at least one of the following:
- -
Surgical site infection (primary outcome);
- -
Suture or healing complications;
- -
Length of hospital stay;
- -
Adverse events related to nutrition;
- -
Mortality (within 30 days).
Studies with missing or unclear data were resolved by contacting the original authors.
2.4. Inclusion and Exclusion Criteria
Inclusion criteria:
Study design: Randomized controlled trials (RCTs).
Participants: Adults aged 18 years and older.
Population: Studies in which ≥20% of participants had oral cancer, or subgroup data specific to oral cancer could be extracted.
Intervention: Immunonutrition administered orally or enterally in the perioperative period (pre-, post-, or both). Immunonutrition was defined as formulas containing immune-modulating components such as arginine, glutamine, omega-3 fatty acids, and nucleotides.
Comparator: Standard nutritional care (e.g., IV fluids or non-immunoenhanced polymeric formulas).
Reported at least one outcome of interest (see
Section 2.5).
Exclusion criteria:
- -
Non-randomized or quasi-randomized studies;
- -
Studies involving patients under 18 years;
- -
Head and neck cancer studies lacking oral cancer-specific data;
- -
Interventions not meeting the definition of immunonutrition;
- -
Duplicated reports, abstracts only, or protocol-only studies.
Oral cancer was defined by ICD-10 anatomical categories: oral tongue, floor of mouth, buccal mucosa, hard palate, upper and lower gingiva, and retromolar trigone.
2.5. Study Selection Process
Eligible studies were selected in two phases. In the first phase, two authors (SH and MW) independently screened the titles and abstracts. In the second phase, the same authors independently reviewed the full texts of potentially eligible studies based on the inclusion and exclusion criteria described in
Section 2.4. Irrelevant studies were excluded. Disagreements were resolved through discussion with a third author (TA or DT). In cases of missing or unclear data, the original study authors were contacted for clarification.
2.6. Quality Assessment of Included Studies
The risk of bias for the included RCTs was evaluated using the Cochrane Risk of Bias 2.0 (RoB 2) tool [
12,
13]. The following domains were assessed:
Each domain and the overall risk were rated as low risk, high risk, or unclear. Two independent reviewers (SH and MW) conducted the assessments, with discrepancies resolved by discussion.
2.7. Summary of Findings
A “Summary of Findings” table (
Table 1) was created to compare immunonutritional therapy with standard nutritional care for each outcome. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach was used to assess the overall certainty of evidence, which was rated as very low, low, moderate, or high [
14]. This helps in interpreting the reliability and clinical applicability of the results.
2.8. Statistical Analysis and Data Synthesis
Meta-analyses were conducted to compare immunonutritional therapy with standard nutritional care. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for all relevant outcomes. A fixed-effects model was applied using Review Manager (RevMan) Version 5.4 (The Cochrane Collaboration). Heterogeneity was assessed using chi-square tests and the **I2 statistic. Values of I2 < 40% were considered to indicate low or no heterogeneity, 50–75% mild heterogeneity, and >75% substantial heterogeneity.
3. Results
3.1. Literature Search and Selection
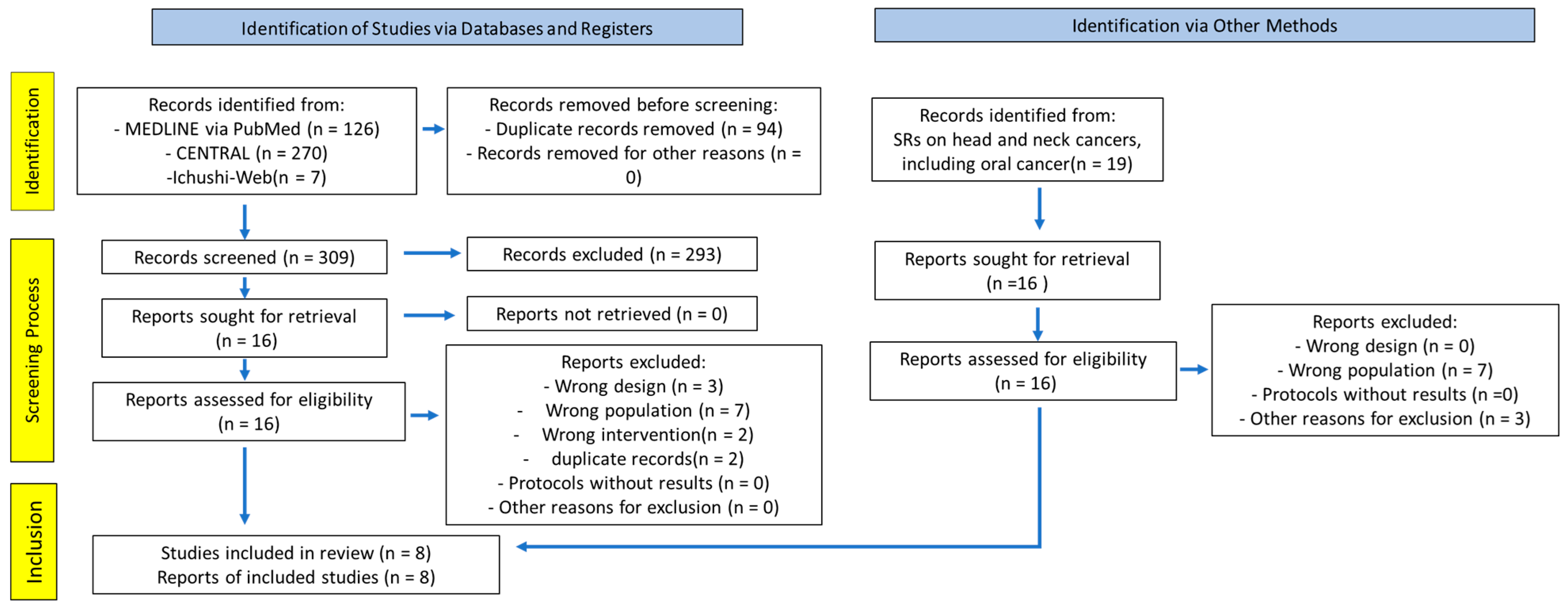
Figure 1 illustrates the PRISMA flow diagram. SRs on head and neck cancers, including oral cancer, were identified as key SRs, and 16 RCTs obtained from these SRs were included [
6]. The combined search of Cochrane CENTRAL, PubMed, and Ichushi-Web yielded 309 articles (PubMed, 126; CENTRAL, 270; Ichushi-Web, 7; duplicates, 94). All titles and abstracts were screened, and 293 articles were excluded. Subsequently, 32 studies were eligible for full-text screening. After full-text screening, 24 articles were excluded primarily due to incompatible study designs and domains (
Supplementary Materials Table S3). We reviewed the criteria and outcomes of each study, as well as the references. Eight articles were finally included in the analysis [
15,
16,
17,
18,
19,
20,
21,
22].
3.2. Data Extraction
The eight identified RCTs compared perioperative immunonutrition with standard nutritional therapy in adult patients undergoing scheduled surgery for oral cancer. The outcomes and corresponding numbers of eligible patients were as follows: suture and healing failure, 493 (eight RCTs) patients; SSI, 513 (eight RCTs) patients; length of hospital stay, 539 (seven RCTs) patients; and adverse events related to immunonutrition, 369 (five RCTs) patients.
Table 1 summarizes the findings and presents the key characteristics and results of the included studies.
Table 2 provides a summary of the included RCTs, outlining their characteristics, timing of intervention, reported outcomes, and notable findings.
Table 1.
Summary of findings.
Table 1.
Summary of findings.
| Certainty Assessment | Sample size | Effectiveness | Certainty of the Evidence (Grade) |
|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Non-Directivity | Imprecision | Immunonutrition | Standard | Relative Effect | Absolute Effect | |
|---|
| (95% CI) | (95% CI) |
|---|
| Surgical site infection |
| 8 | RCT | Serious a | Not serious | Not serious | Serious b | 21/265 (7.9%) | 32/248 (12.9%) | RR 0.61 | 50 fewer per 1000 | ⊕⊕⊝⊝ |
| (0.37–1.01) | (81 fewer to 1 more) | Low |
| 10.00% | | 39 fewer per 1000 | |
| | (63 fewer to 1 more) | |
| 40.00% | | 156 fewer per 1000 | |
| | (252 fewer to 4 more) | |
| Incidence of suture and healing anomalies |
| 8 | RCT | Serious a | Not serious | Serious d | Not serious | 19/257 (7.4%) | 41/236 (17.4%) | RR 0.46 | 94 fewer per 1000 | ⊕⊕⊝⊝ |
| (0.27–0.80) | (127 fewer to 35 fewer) | Low |
| Nutritional adverse events |
| 5 | RCT | Not serious | Not serious | Not serious | Serious b | 42/192 (21.9%) | 27/177 (15.3%) | RR 1.47 | 72 more per 1000 | ⊕⊕⊕⊝ |
| (0.87–2.48) | (20 fewer to 226 more) | Moderate |
| Number of hospital days |
| 7 | RCT | Serious a | Not serious | Not serious | Not serious | 294 | 245 | - | Median < 3.06 | ⊕⊕⊕⊝ |
| | Moderate |
| Mortality |
| a, b, c | | | | | | | | Not estimable | | - |
3.3. Outcome Evaluation of Meta-Analysis
We analyzed five key outcomes across the included randomized controlled trials (RCTs). A summary of the findings is provided below, organized by outcome type. The GRADE approach was used to assess the certainty of evidence.
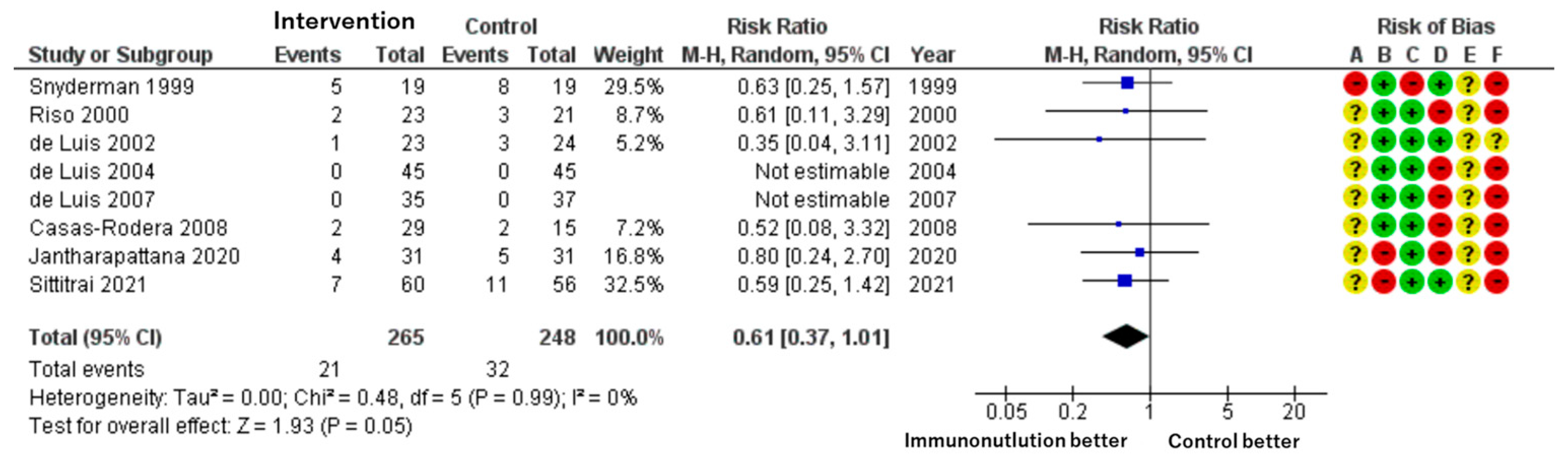
Patients included: 513 across eight RCTs.
Effect estimate: RR = 0.61; 95% CI: 0.37 to 1.01.
Certainty: low.
Immunonutrition was associated with a lower risk of SSI compared to standard care. The absolute reduction was approximately 50 fewer infections per 1000 patients. However, the confidence interval includes the possibility of no effect. The baseline risk in control groups ranged from 3% to 41%, as reported by Cannon et al. [
23] (
Figure 2).
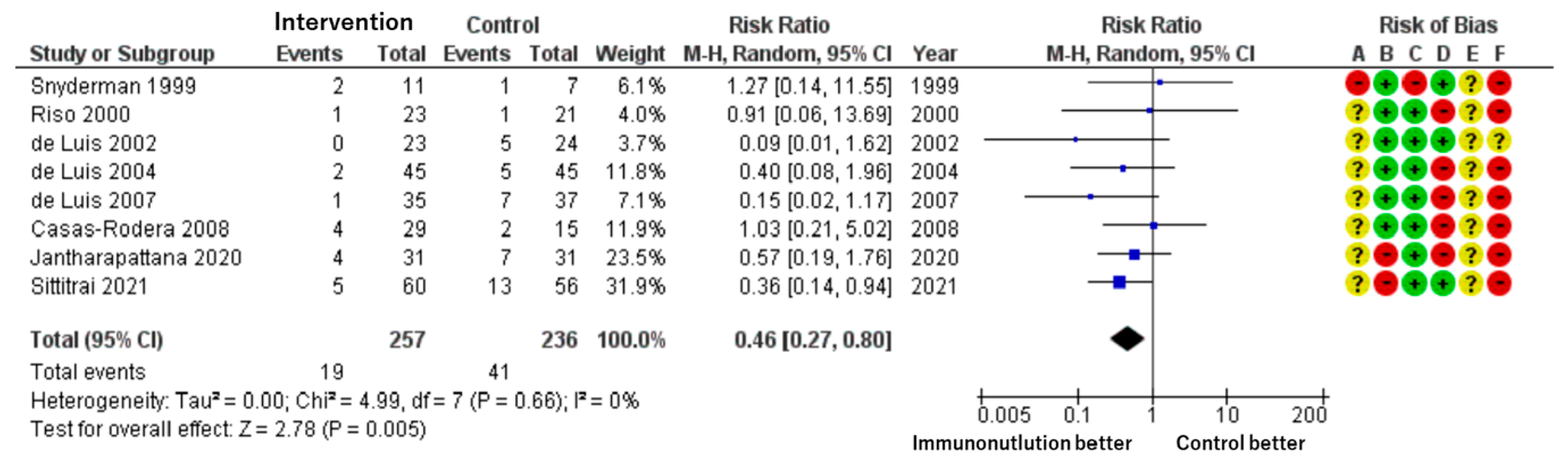
Patients included: 493 across eight RCTs.
Effect estimate: RR = 0.46; 95% CI: 0.27 to 0.80.
Certainty: low.
A significantly lower rate of suture or wound healing complications (e.g., fistula formation) was observed in the immunonutrition group. The incidence was reduced by 94 per 1000 patients, from 17.4% in the control group to 7.4% in the intervention group (
Figure 3).
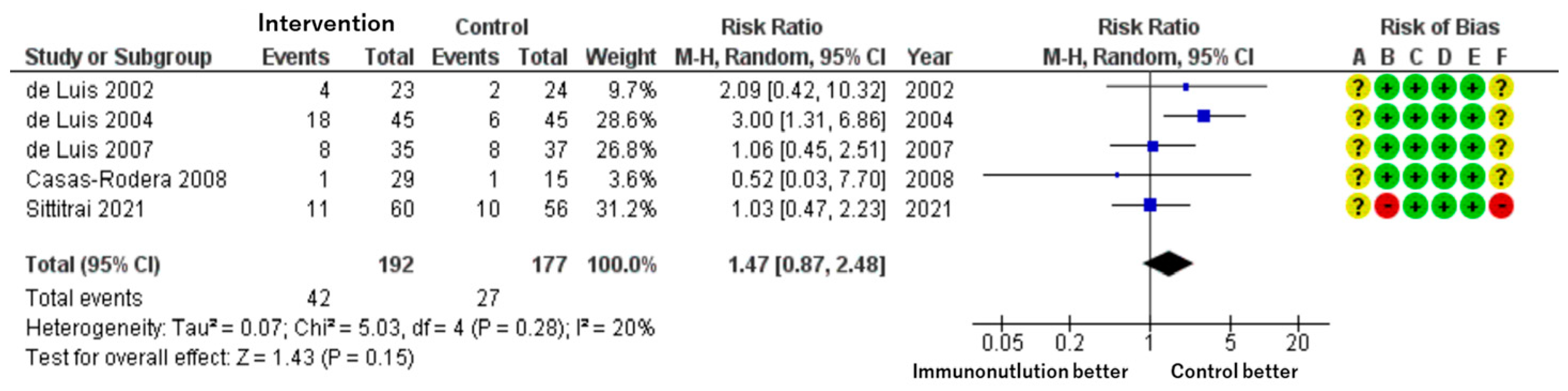
Patients included: 369 across five RCTs.
Effect estimate: RR = 1.47; 95% CI: 0.87 to 2.48.
Certainty: moderate.
Although adverse events such as nausea, diarrhea, and abdominal discomfort were more frequently reported in the immunonutrition group, the difference was not statistically significant. The estimate suggests a possible 72 more events per 1000 patients, but the wide confidence interval indicates imprecision (
Figure 4).
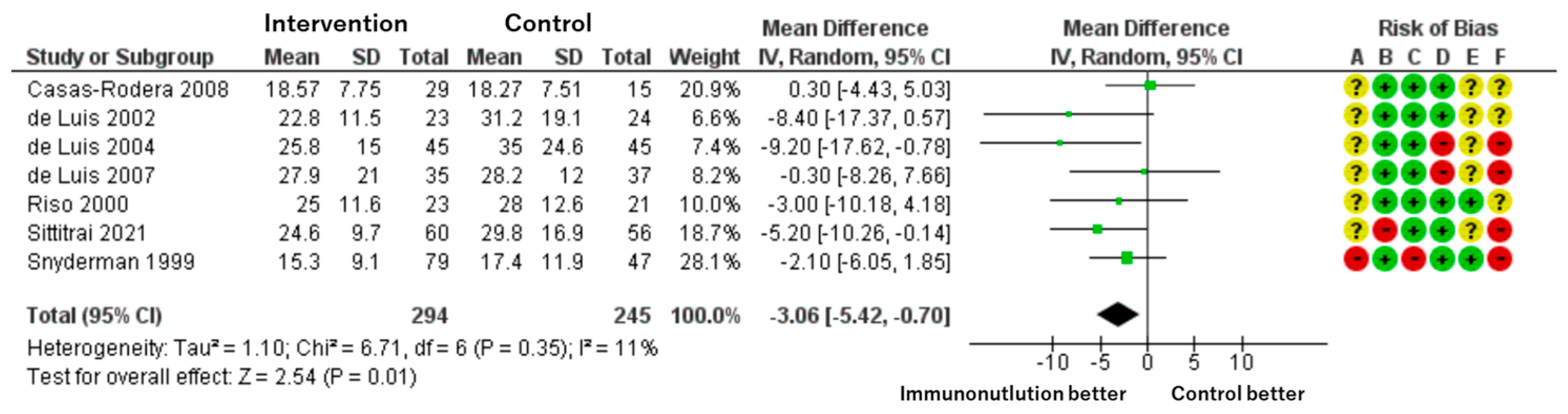
Patients included: 539 across seven RCTs.
Effect estimate: mean difference = −3.06 days; 95% CI: −5.42 to −0.70.
Certainty: moderate.
Immunonutrition shortened the length of hospital stay by an average of 3 days compared to standard nutrition. The effect size was statistically significant and consistent across studies, though some risk of bias remains (
Figure 5).
Data on mortality were insufficient for meta-analysis. None of the included studies reported mortality as a primary endpoint, and data were inconsistently presented across trials. Therefore, no pooled estimate was calculated.
3.4. Risk of Bias in Included Studies
We assessed each included study for risk of bias using the Cochrane Risk of Bias assessment tool [
24] with the five domains and overall bias, as described in the methods section. Among the identified studies, overall bias showed some concerns related to the whole outcome, and there was at least one concern in several domains (
Figure 2,
Figure 3,
Figure 4 and
Figure 5).
3.5. Certainty of Cumulative Evidence
The certainty of the evidence for each outcome was assessed using the GRADE approach (
Table 1).
For the outcomes suture/healing failure and SSI, the evidence was of low certainty owing to the serious risk of bias in several domains, imprecision of effect estimates (wide CIs), and indirectness (one study with a predominantly female population).
The evidence for the outcomes adverse events related to nutritional supplements and length of hospital stay was of moderate certainty. There was serious imprecision in the effect estimate for adverse events, but no other factors affected the certainty. For the length of hospital stay, there was a serious risk of bias in several domains, but no other factors affected the certainty.
Overall, the certainty of the evidence ranged from low to moderate, suggesting that further research might have impacted the confidence in the effect estimates and may, therefore, change the estimates. The outcomes mortality, suture/healing failure, and SSI were considered critical for decision-making, whereas adverse events and length of hospital stay were considered important.
There were insufficient studies to assess publication bias using funnel plots since the number of included studies for each outcome was below 10.
In conclusion, while the evidence suggests the potential benefits of perioperative immunonutrition in reducing suture/healing failure, SSI, and length of hospital stay, the certainty of the evidence was limited by the risk of bias, imprecision, and indirectness. More high-quality RCTs remain warranted to improve the level of confidence in the effect estimates and assess the impact on mortality.
4. Discussion
This SR and meta-analysis comprehensively evaluated the potential benefits of immunonutrition in the perioperative management of patients undergoing surgery for oral cancer. The results suggest that administering immunonutrients, such as arginine, omega-3 fatty acids, and nucleotides, significantly affects surgical outcomes, reducing the incidence of complications, such as suture and healing failure and SSIs, while facilitating faster recovery and shorter hospital stays. Although the meta-analysis demonstrated a statistically significant reduction in hospital stay, the relatively wide confidence interval (−5.42 to −0.70 days) suggests variability across studies and limits the precision of this finding. This indicates that while immunonutrition may reduce hospital stay, the exact magnitude of benefit remains uncertain and should be interpreted with caution. These findings are consistent with the evidence supporting the use of immunonutrition in various surgical contexts, particularly in gastrointestinal surgery [
25,
26].
In gastrointestinal surgery, numerous studies have demonstrated the efficacy of immunonutrition in reducing postoperative complications and improving clinical outcomes. In a meta-analysis, Drover et al. [
27] found that immunonutrition in patients undergoing elective gastrointestinal surgery significantly reduced infectious complications and length of hospital stay compared with standard nutrition. Similarly, an SR by Marimuthu et al. [
28] reported that immunonutrition was associated with a lower incidence of wound infections and shorter hospital stays in patients undergoing surgery for gastrointestinal cancers.
The consistency of these findings across different surgical specialties underscores the potential of immunonutrition as a valuable adjunct in perioperative care. However, it is essential to note that the evidence supporting immunonutrition in patients undergoing oral cancer surgery is less robust than that in those undergoing gastrointestinal surgery. This disparity highlights the need for further research to consolidate the findings of the present meta-analysis and explore the specific nuances of immunonutrition in the context of oral cancer.
Although anatomical and functional differences exist between oral cancer and gastrointestinal surgeries, the consistent benefits of immunonutrition observed in various surgical domains suggest its potential applicability in oral oncology. Our findings offer preliminary support for this concept.
One key challenge in the perioperative management of patients with head and neck cancer is the high prevalence of malnutrition, which can reach 50% in this population [
29]. Malnutrition compromises immune function, impairs wound healing, and increases the risk of postoperative complications [
30]. Therefore, incorporating immunonutrition into perioperative care protocols may help address the unique nutritional challenges faced by patients with oral cancer and improve surgical outcomes.
However, successful implementation of immunonutrition in the case of oral cancer surgery requires a multidisciplinary approach that considers the specific needs and challenges faced by this patient population. For instance, anatomical and functional alterations resulting from oral cancer surgery may necessitate the use of alternative feeding routes, such as enteral tube feeding or parenteral nutrition [
31].
Integrating immunonutrition into these feeding strategies requires careful planning and coordination among healthcare teams to ensure optimal delivery and patient compliance [
32].
Moreover, the potential economic implications of incorporating immunonutrition into perioperative care protocols must be considered. The possibly higher upfront costs of immunonutrition formulas compared to those of standard nutrition will be compensated for by the potential cost savings associated with reduced complications and shorter hospital stays [
33]. Future studies should include robust cost-effectiveness analyses to provide a more comprehensive understanding of the financial impact of immunonutrition in the perioperative care of oral cancer patients.
Further high-quality, large-scale randomized controlled trials (RCTs) are needed to validate the effectiveness of immunonutrition in patients undergoing oral cancer surgery. Future studies should employ well-defined inclusion criteria, clearly delineate primary outcomes such as surgical site infection or healing failure, and explore long-term endpoints such as survival, quality of life, and cost-effectiveness. Additionally, protocol standardization across institutions and inclusion of diverse populations will be critical to strengthen the generalizability and clinical impact of future findings.
5. Limitations
This systematic review has several limitations. First, although the number of randomized controlled trials focusing specifically on oral cancer was limited, we included studies where oral cancer patients constituted ≥20% of the population or could be extracted through subgroup analysis. As such, some clinical heterogeneity remains. Second, while no language restrictions were applied and Japanese literature was included via Ichushi-Web, the search did not encompass databases such as SCOPUS, EMBASE, LILACS, or grey literature, which may have led to publication bias. Third, our search period was set from 2018 to 2022, not to merely update or supplement a previous Cochrane review, but because that review—although comprehensive—focused on head and neck cancers broadly and did not specifically target oral cancer. However, since it included relevant trials involving oral cancer patients, we considered it a key systematic review and used it to define the starting point for our literature search. Lastly, one of the included studies had a predominantly female sample, which may limit the generalizability of some findings.
6. Conclusions
The results of this SR and meta-analysis provide compelling evidence of the potential benefits of immunonutrition in the perioperative management of patients with oral cancer. Although the evidence base may not be as extensive as that for gastrointestinal surgery, the consistency of findings across different surgical specialties underscores immunonutrition as a valuable adjunct to perioperative care. Moving forward, it is crucial to refine protocols, elucidate the underlying mechanisms, and optimize the delivery of immunonutrition in the context of oral cancer surgery. By fostering interdisciplinary collaboration, promoting patient-centered care, and leveraging lessons learned from other surgical specialties, we can unlock the full potential of immunonutrition and revolutionize the perioperative management of oral cancer, ultimately improving the lives of patients.
Supplementary Materials
The following supporting information can be downloaded at
https://www.mdpi.com/article/10.3390/cancers17111855/s1: Table S1: PRISMA 2020 Checklist; Table S2: The detailed search strategy; Table S3: Characteristics of studies excluded from the qualitative and quantitative synthesis. Reference [
34] is cited in the supplementary materials.
Author Contributions
Conceptualization, S.-i.H. and T.A.; methodology, D.T., H.Y. and M.A.; validation; S.-i.H.; formal analysis, S.-i.H. and H.Y.; investigation, S.-i.H., M.W. and T.A.; data curation, S.-i.H.; writing—original draft preparation, S.-i.H.; writing—review and editing, S.-i.H.; visualization, S.-i.H.; supervision, N.U. and H.K.; project administration, S.-i.H.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by the Japanese Society of Oral and Maxillofacial Surgeons.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this systematic review and meta-analysis are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the members of the Japanese Society of Oral Surgeons Guideline Development Committee and the Japanese Society of Oral Oncology Guideline Development Committee as well as the staff of the Secretariat for their invaluable assistance with this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hiraoka, S.I.; Shimada, Y.; Kawasaki, Y.; Akutagawa, M.; Tanaka, S. Preoperative nutritional evaluation, surgical site infection, and prognosis in patients with oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 168–175. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, M.B.; August, D.A. Perioperative nutrition support in cancer patients. Nutr. Clin. Pract. 2012, 27, 586–592. [Google Scholar] [CrossRef]
- Chow, O.; Barbul, A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv. Wound Care 2014, 3, 46–53. [Google Scholar] [CrossRef]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Howes, N.; Lewis, S.J.; Thomas, S. Immunonutrition for patients undergoing surgery for head and neck cancer. Cochrane Database Syst. Rev. 2018, 8, Cd010954. [Google Scholar] [CrossRef]
- Bertrand, P.C.; Piquet, M.-A.; Bordier, I.; Monnier, P.; Roulet, M. Preoperative nutritional support at home in head and neck cancer patients: From nutritional benefits to the prevention of the alcohol withdrawal syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 435–440. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.A.; Mayer, C.; Bojaxhiu, B.; Aeberhard, C.; Schuetz, P.; Stanga, Z.; Giger, R. Effect of preoperative immunonutrition on complications after salvage surgery in head and neck cancer. J. Otolaryngol. Head. Neck Surg. 2019, 48, 25. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Schnemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. The GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; GRADE Working Group: Hamilton, ON, Canada, 2013. [Google Scholar]
- Casas-Rodera, P.; Gómez-Candela, C.; Benítez, S.; Mateo, R.; Armero, M.; Castillo, R.; Culebras, J.M. Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: A prospective, randomized clinical trial. Nutr. Hosp. 2008, 23, 105–110. [Google Scholar]
- de Luis, D.; Aller, R.; Izaola, O.; Cuellar, L.; Terroba, M. Postsurgery enteral nutrition in head and neck cancer patients. Eur. J. Clin. Nutr. 2002, 56, 1126–1129. [Google Scholar] [CrossRef]
- de Luis, D.A.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Aller, R. Randomized clinical trial with an enteral arginine-enhanced formula in early postsurgical head and neck cancer patients. Eur. J. Clin. Nutr. 2004, 58, 1505–1508. [Google Scholar] [CrossRef]
- de Luis, D.A.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Martin, T.; Aller, R. Clinical and biochemical outcomes after a randomized trial with a high dose of enteral arginine formula in postsurgical head and neck cancer patients. Eur. J. Clin. Nutr. 2007, 61, 200–204. [Google Scholar] [CrossRef]
- Riso, S.; Aluffi, P.; Brugnani, M.; Farinetti, F.; Pia, F.; D’Andrea, F. Postoperative enteral immunonutrition in head and neck cancer patients. Clin. Nutr. 2000, 19, 407–412. [Google Scholar] [CrossRef]
- Snyderman, C.H.; Kachman, K.; Rd, L.M.; Wagner, R.; D’Amico, F.; Bumpous, J.; Rueger, R. Reduced postoperative infections with an immune-enhancing nutritional supplement. Laryngoscope 1999, 109, 915–921. [Google Scholar] [CrossRef]
- Jantharapattana, K.; Orapipatpong, O. Efficacy of EPA-enriched supplement compared with standard formula on body weight changes in malnourished patients with head and neck cancer undergone surgery: A randomized study. Head Neck 2020, 42, 188–197. [Google Scholar] [CrossRef]
- Sittitrai, P.; Ruenmarkkaew, D.; Booyaprapa, S.; Kasempitakpong, B. Effect of a perioperative immune-enhancing diet in clean-contaminated head and neck cancer surgery: A randomized controlled trial. Int. J. Surg. 2021, 93, 106051. [Google Scholar] [CrossRef]
- Cannon, R.B.; Houlton, J.J.; Mendez, E.; Futran, N.D. Methods to reduce postoperative surgical site infections after head and neck oncology surgery. Lancet Oncol. 2017, 18, e405–e413. [Google Scholar] [CrossRef]
- Review Manager (RevMan), Version 5.4. Computer program. The Cochrane Collaboration: London, UK, 2020.
- Matsui, R.; Sagawa, M.; Sano, A.; Sakai, M.; Hiraoka, S.; Tabei, I.; Imai, T.; Matsumoto, H.; Onogawa, S.; Sonoi, N.; et al. Impact of Perioperative Immunonutrition on Postoperative Outcomes for Patients Undergoing Head and Neck or Gastrointestinal Cancer Surgeries: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2024, 279, 419–428. [Google Scholar] [CrossRef]
- Mingliang, W.; Zhangyan, K.; Fangfang, F.; Huizhen, W.; Yongxiang, L. Perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy: The first meta-analysis of randomized clinical trials. Dis. Esophagus 2020, 33, doz111. [Google Scholar] [CrossRef] [PubMed]
- Drover, J.W.; Dhaliwal, R.; Weitzel, L.; Wischmeyer, P.E.; Ochoa, J.B.; Heyland, D.K. Perioperative use of arginine-supplemented diets: A systematic review of the evidence. J. Am. Coll. Surg. 2011, 212, 385–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, K.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann. Surg. 2012, 255, 1060–1068. [Google Scholar] [CrossRef]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional considerations for head and neck cancer patients: A review of the literature. J. Oral. Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Leandro-Merhi, V.A.; Costa, C.L.; Saragiotto, L.; de Aquino, J.L.B. Nutritional Indicators Of Malnutrition In Hospitalized Patients. Arq. Gastroenterol. 2019, 56, 447–450. [Google Scholar] [CrossRef]
- Rabinovitch, R.; Grant, B.; Berkey, B.A.; Raben, D.; Ang, K.K.; Fu, K.K.; Cooper, J.S. Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: A secondary analysis of RTOG trial 90-03. Head Neck 2006, 28, 287–296. [Google Scholar] [CrossRef]
- Wan, G.; Yuan, H.; Xue, H.; Li, H.; Hu, H.; Zhang, X. Assessment of compliance with oral nutritional supplementation and exploration of barriers and facilitators for patients after gastrectomy: A mixed-methods study. J. Adv. Nurs. 2021, 77, 2845–2859. [Google Scholar] [CrossRef]
- Reis, A.M.D.; Kabke, G.B.; Fruchtenicht, A.V.G.; Barreiro, T.D.; Moreira, L.F. Cost-Effectiveness of Perioperative Immunonutrition in Gastrointestinal Oncologic Surgery: A Systematic Review. Arq. Bras. Cir. Dig. 2016, 29, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).