Single-Port Versus Multi-Port Robotic Radical Prostatectomy in Elderly Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Evaluation
2.2. Intraoperative and Postoperative Evaluation
2.3. Surgical Procedure
2.4. Endpoint
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SP | Single port |
| MP | Multi-port |
| RARP | Robot-assisted radical prostatectomy |
| PCa | Prostate cancer |
| CCI | Charlson Comorbidity Index |
| ASA | American Society of Anesthesiologists |
| BMI | Body mass index |
| ISUP | International Society of Urological Pathology |
| EBL | Estimated intraoperative blood loss |
| LOS | Length of stay |
| SDD | Same-day discharge |
| PONV | Postoperative nausea and vomiting |
References
- Graham, L.S.; Lin, J.K.; Lage, D.E.; Kessler, E.R.; Parikh, R.B.; Morgans, A.K. Management of Prostate Cancer in Older Adults. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390396. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Hatakeyama, S.; Sakamoto, S.; Kato, T.; Inokuchi, J.; Matsui, Y.; Kitamura, H.; Nishiyama, H.; Habuchi, T. Management of prostate cancer in older patients. Jpn. J. Clin. Oncol. 2022, 52, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Burdenski, K.; Tselis, N.; Rödel, C.; Brandts, C.; Ahrens, M.; Koellermann, J.; Graefen, M.; Heinzer, H.; Haese, A.; et al. Radical Prostatectomy vs. Radiation Therapy for Locally Advanced and Clinically Nodal Positive Prostate Cancer. Clin. Genitourin. Cancer 2025, 23, 102370. [Google Scholar] [CrossRef]
- Esperto, F.; Cacciatore, L.; Tedesco, F.; Testa, A.; Callè, P.; Ragusa, A.; Deanesi, N.; Minore, A.; Prata, F.; Brassetti, A.; et al. Impact of Robotic Technologies on Prostate Cancer Patients’ Choice for Radical Treatment. J. Pers. Med. 2023, 13, 794. [Google Scholar] [CrossRef]
- Kesavan, A.; Vickneson, K.; Esuvaranathan, K. Hospital readmissions for patients with prostate cancer are higher after radiotherapy than after prostatectomy. Investig. Clin. Urol. 2022, 63, 34–41. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Paris 2024; European Association of Urology: Arnhem, The Netherlands, 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Stankovic, M.; Weber, C.; Koser, M.; Weidner, N. Frailty as Predictor for Early Functional Outcomes After Radical Prostatectomy. J. Frailty Sarcopenia Falls 2025, 10, 28–36. [Google Scholar]
- Hamilton, A.S.; Albertsen, P.C.; Johnson, T.K.; Hoffman, R.; Morrell, D.; Deapen, D.; Penson, D.F. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011, 107, 576–584. [Google Scholar] [CrossRef]
- Sletten, R.; Christiansen, O.B.; Oldervoll, L.M.; Åstrøm, L.; Skjellegrind, H.K.; Benth, J.Š.; Kirkevold, Ø.; Bergh, S.; Grønberg, B.H.; Rostoft, S.; et al. The association between age and long-term quality of life after curative treatment for prostate cancer: A cross-sectional study. Scand. J. Urol. 2024, 59, 31–38. [Google Scholar] [CrossRef]
- Preisser, F.; Mazzone, E.; Nazzani, S.; Knipper, S.; Tian, Z.; Mandel, P.; Pompe, R.; Saad, F.; Montorsi, F.; Shariat, S.F.; et al. Impact of Age on Perioperative Outcomes at Radical Prostatectomy: A Population-Based Study. Eur. Urol. Focus 2020, 6, 1213–1219. [Google Scholar] [CrossRef]
- Togashi, K.; Hatakeyama, S.; Okamoto, T.; Kojima, Y.; Iwamura, H.; Fujita, N.; Narita, T.; Hamano, I.; Hamaya, T.; Yoneyama, T.; et al. Oncologic and patient-reported outcomes after robot-assisted radical prostatectomy in men aged ≥75 years. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 729.e17–729.e25. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Prata, F.; Iannuzzi, A.; Tedesco, F.; Cacciatore, L.; Rocca, A.; Caccia, P.; Bogea, C.; Marelli, M.; Civitella, A.; et al. Safety and feasibility of ‘three arms settings’ robot-assisted radical prostatectomy using the Hugo RAS system: Surgical set-up in a double-center large case series. World J. Urol. 2024, 42, 517. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Moreno, Y.; Echevarria, S.; Vidal-Valderrama, C.; Stefano-Pianetti, L.; Cordova-Guilarte, J.; Navarro-Gonzalez, J.; Acevedo-Rodríguez, J.; Dorado-Avila, G.; Osorio-Romero, L.; Chavez-Campos, C.; et al. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus 2023, 15, e42370. [Google Scholar] [CrossRef]
- Covas Moschovas, M.; Bhat, S.; Rogers, T.; Thiel, D.; Onol, F.; Roof, S.; Sighinolfi, M.C.; Rocco, B.; Patel, V. Applications of the da Vinci single port (SP) robotic platform in urology: A systematic literature review. Minerva Urol. Nephrol. 2021, 73, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Haberal, H.B.; Lambertini, L.; Avesani, G.; Pettenuzzo, G.; Pacini, M.; Valenzi, F.M.; Aljoulani, M.; Sauer, R.C.; Torres-Anguiano, J.R.; Crivellaro, S. Optimizing Prostate Cancer Surgery for Seniors: Single-Port Robotic-Assisted Platform. J. Laparoendosc. Adv. Surg. Tech. 2025, 35, 240–246. [Google Scholar] [CrossRef]
- Valenzi, F.M.; Fuschi, A.; Al Salhi, Y.; Sequi, M.B.; Suraci, P.P.; Pacini, M.; Scalzo, S.; Rera, O.A.; Antonioni, A.; Graziani, D.; et al. Is early continence recovery related to the length of spared urethra? A prospective multicenter study comparing preoperative MRI and histologic specimen measurements after robotic radical prostatectomy. Eur. J. Surg. Oncol. 2024, 50, 108319. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Wakamiya, T.; Kawabata, H.; Deguchi, R.; Muraoka, S.; Inagaki, T.; Noda, Y.; Yamashita, S.; Kohjimoto, Y.; Sonomura, T.; et al. Comparison of Prognosis and Health-Related Quality of Life Between Robot-Assisted Radical Prostatectomy Versus High-Dose-Rate Brachytherapy Combined With External Beam Radiation Therapy and Hormone Therapy for High-Risk Prostate Cancer. Prostate 2025, 85, 327–336. [Google Scholar] [CrossRef]
- Anceschi, U.; Morelli, M.; Flammia, R.S.; Brassetti, A.; Dell’Oglio, P.; Galfano, A.; Tappero, S.; Vecchio, E.; Martiriggiano, M.; Luciani, L.G.; et al. Predictors of trainees’ proficiency during the learning curve of robot-assisted radical prostatectomy at high- -volume institutions: Results from a multicentric series. Cent. Eur. J. Urol. 2023, 76, 38–43. [Google Scholar]
- Kostakopoulos, N.; Bellos, T.; Malovrouvas, E.; Katsimperis, S.; Kostakopoulos, A. Robot-Assisted Urological Oncology Procedures, Outcomes, and Safety in Frail Patients: A Narrative Review of Available Studies. Urol. Res. Pract. 2024, 50, 36–41. [Google Scholar] [CrossRef]
- Hassanipour-Azgomi, S.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Towhidi, F.; Jamehshorani, S.; Salehiniya, H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 2016, 4, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.C.; Hirai, H.W.; Ho, J.M.W.; Chan, F.C.H.; Tsoi, K.K.F.; Ng, C.F. Global incidence of prostate cancer in developing and developed countries with changing age structures. PLoS ONE 2019, 14, e0221775. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, V.; Carino, D.; Corvino, R.; Salciccia, S.; De Berardinis, E.; Krajewski, W.; Nowak, Ł.; Łaszkiewicz, J.; Szydełko, T.; Nair, R.; et al. Surgical Technique and Perioperative Outcomes of the “Sapienza” Urology Residency Program’s Trocar Placement Configuration During Robotic-Assisted Radical Prostatectomy (RARP): A Retrospective, Single-Centre Observational Study Comparing Experienced Attendings vs. Post-Graduate Year I–III Residents as Bedside Assistants. Cancers 2024, 17, 20. [Google Scholar]
- Mandel, P.; Chandrasekar, T.; Chun, F.; Huland, H.; Tilki, D. Radical prostatectomy in patients aged 75 years or older: Review of the literature. Asian J. Androl. 2019, 21, 32–36. [Google Scholar]
- Ditonno, F.; Franco, A.; Licari, L.C.; Bologna, E.; Manfredi, C.; Katz, D.O.; Huang, J.H.; Latchamsetty, K.C.; Coogan, C.L.; Cherullo, E.E.; et al. Implementation of single-port robotic urologic surgery: Experience at a large academic center. J. Robot. Surg. 2024, 18, 119. [Google Scholar] [CrossRef]
- Picozzi, P.; Nocco, U.; Labate, C.; Gambini, I.; Puleo, G.; Silvi, F.; Pezzillo, A.; Mantione, R.; Cimolin, V. Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics 2024, 13, 4675. [Google Scholar] [CrossRef]
- Rosendal, C.; Markin, S.; Hien, M.D.; Motsch, J.; Roggenbach, J. Cardiac and hemodynamic consequences during capnoperitoneum and steep Trendelenburg positioning: Lessons learned from robot-assisted laparoscopic prostatectomy. J. Clin. Anesth. 2014, 26, 383–389. [Google Scholar] [CrossRef]
- Chavali, J.S.; Pedraza, A.M.; Soputro, N.A.; Ramos-Carpinteyro, R.; Mikesell, C.D.; Kaouk, J. Single-Port Extraperitoneal vs. Multiport Transperitoneal Robot-Assisted Radical Prostatectomy: A Propensity Score-Matched Analysis. Cancers 2024, 16, 2994. [Google Scholar] [CrossRef]
- Pellegrino, A.A.; Pellegrino, F.; Cannoletta, D.; Calvo, R.S.; Anguiano, J.T.; Morgantini, L.; Briganti, A.; Montorsi, F.; Crivellaro, S. Learning Curve for Single-port Robot-assisted Urological Surgery: Single-center Experience and Implications for Adoption. Eur. Urol. Focus, 2024; in press. [Google Scholar] [CrossRef]
| Variable | GROUP A Age < 65 | GROUP B Age ≥ 65 | p Value Within the MP Group According to Age Group | p Value Within the SP Group According to Age Group | ||||
|---|---|---|---|---|---|---|---|---|
| MP | SP | p Value | MP | SP | p Value | |||
| N° of cases, n (%) | 99 (49.0) | 103 (51.0) | 54 (39.7) | 82 (60.3) | ||||
| Age yy, median (IQR) | 60 (6) | 60 (6) | 0.219 | 69 (4) | 69 (5) | 0.504 | <0.001 | <0.001 |
| BMI, Kg/m2 | 29.9 (7.54) | 28.1 (8.13) | 0.930 | 27.9 (5.58) | 28.1 (6.70) | 0.361 | 0.025 | 0.194 |
| ASA, n (%) | 0.254 | 0.127 | 0.128 | 0.647 | ||||
| 1 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| 2 | 44 (44.4) | 49 (47.6) | 17 (31.5) | 38 (46.3) | ||||
| 3 | 51 (51.5) | 54 (52.4) | 37 (68.5) | 44 (53.7) | ||||
| 4 | 3 (3.1) | 0 (0.0) | 0 (0.0) | 1 (1.2) | ||||
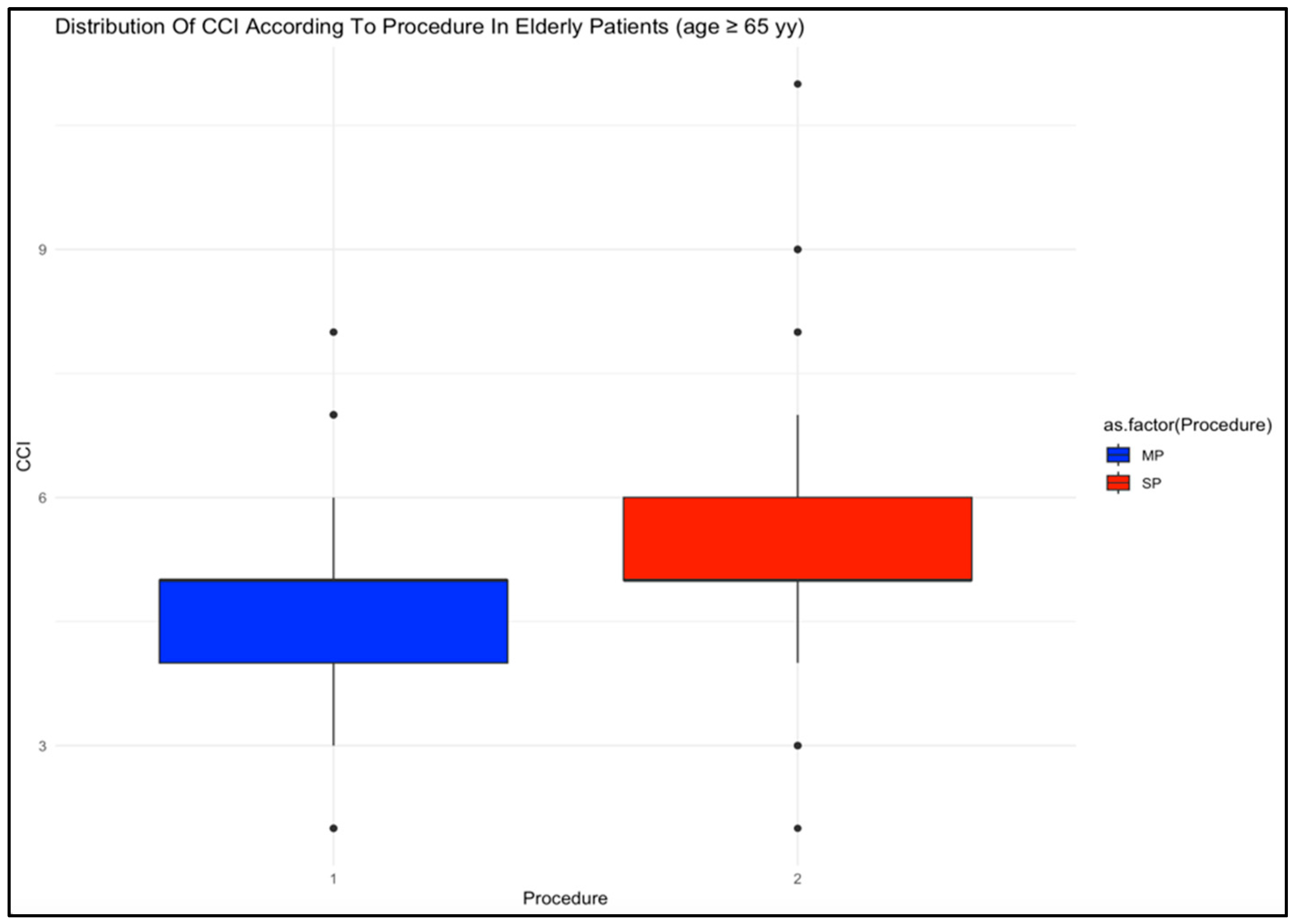
| CCI n, median (IQR) | 3 (2) | 4 (2) | <0.001 | 5 (1) | 5 (1) | 0.001 | <0.001 | <0.001 |
| D’Amico risk classification, n (%) | 0.612 | 0.055 | 0.076 | 0.840 | ||||
| Low | 8 (8.1) | 13 (12.6) | 0 (0.0) | 8 (9.8) | ||||
| Intermediate | 80 (80.8) | 79 (76.7) | 46 (85.2) | 65 (79.2) | ||||
| High | 11 (11.1) | 11 (10.7) | 8 (14.8) | 9 (11.0) | ||||
| Race, n (%) | 0.178 | 0.151 | 0.571 | 0.132 | ||||
| African American | 65 (65.6) | 64 (62.1) | 31 (57.4) | 41 (50.0) | ||||
| Caucasian | 19 (19.2) | 15 (14.6) | 12 (22.2) | 16 (19.5) | ||||
| Hispanic | 8 (8.2) | 20 (19.4) | 5 (9.3) | 15 (18.3) | ||||
| Asian | 1 (1.0) | 1 (1.0) | 2 (3.6) | 2 (2.4) | ||||
| Other | 6 (6.0) | 3 (2.9) | 4 (7.5) | 8 (9.8) | ||||
| Smoking, n (%) | <0.001 | 0.107 | 0.510 | 0.205 | ||||
| No | 35 (35.4) | 66 (64.1) | 22 (40.7) | 45 (54.9) | ||||
| Yes | 64 (64.6) | 37 (35.9) | 32 (59.3) | 37 (45.1) | ||||
| Substance Abuse, n (%) | 0.730 | 0.573 | 0.492 | 0.657 | ||||
| no | 66 (66.7) | 71 (68.9) | 33 (61.1) | 54 (65.9) | ||||
| yes | 33 (33.3) | 32 (31.1) | 21 (38.9) | 28 (34.1) | ||||
| Hypertension, n (%) | 0.743 | 0.973 | 0.227 | 0.258 | ||||
| No | 27 (27.3) | 26 (25.2) | 10 (18.5) | 15 (18.3) | ||||
| Yes | 72 (72.2) | 77 (74.8) | 44 (81.5) | 67 (81.7) | ||||
| Hypertension therapy, n (%) | 0.862 | 0.959 | 0.283 | 0.310 | ||||
| No | 28 (28.3) | 28 (27.2) | 11 (20.4) | 17 (20.7) | ||||
| Yes | 71 (71.7) | 75 (72.8) | 43 (79.6) | 65 (79.3) | ||||
| Anticoagulation or Antiplatelet therapy, n (%) | 0.266 | 0.362 | 0.005 | 0.002 | ||||
| No | 67 (67.7) | 77 (74.8) | 24 (44.4) | 43 (52.4) | ||||
| Yes | 32 (32.3) | 26 (25.2) | 30 (55.6) | 39 (47.6) | ||||
| PSA ng/mL, median (IQR) | 7.5 (6.02) | 7.5 (7.43) | 0.350 | 7.5 (7.43) | 7.4 (7.72) | 0.179 | 0.997 | 0.639 |
| ISUP, n (%) | 0.770 | 0.072 | 0.061 | 0.851 | ||||
| 1 | 19 (19.2) | 15 (14.6) | 1 (1.9) | 12 (14.6) | ||||
| 2 | 41 (41.4) | 48 (46.6) | 21 (38.8) | 33 (40.2) | ||||
| 3 | 14 (14.1) | 17 (16.5) | 13 (24.1) | 18 (22.0) | ||||
| 4 | 19 (19.2) | 16 (15.5) | 13 (24.1) | 11 (13.4) | ||||
| 5 | 6 (6.1) | 7 (6.8) | 6 (11.1) | 8 (9.8) | ||||
| cT stage, n (%) | 0.547 | 0.649 | 0.246 | 0.094 | ||||
| T1 | 78 (78.8) | 86 (83.5) | 37 (68.5) | 63 (76.8) | ||||
| T2 | 13 (13.1) | 13 (12.6) | 8 (14.8) | 8 (9.8) | ||||
| T3 | 8 (8.1) | 4 (3.9) | 9 (16.7) | 11 (13.4) | ||||
| cN stage, n (%) | 0.326 | 0.412 | 0.898 | 0.864 | ||||
| N0/Nx | 99 (100) | 102 (99) | 54 (100) | 81 (98.8) | ||||
| N1 | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.2) | ||||
| Variable | Group A Age < 65 | Group B Age ≥ 65 | p Value Within the MP Group According to Age Group | p Value Within the SP Group According to Age Group | ||||
|---|---|---|---|---|---|---|---|---|
| MP | SP | p Value | MP | SP | p Value | |||
| N° of cases, n (%) | 99 (49.0) | 103 (51.0) | 54 (39.7) | 82 (60.3) | ||||
| Access, n (%) | <0.001 | <0.001 | 1.000 | 0.818 | ||||
| Transperitoneal | 99 (100) | 36 (35.0) | 54 (100) | 30 (36.6) | ||||
| Extraperitoneal | 0 (0.0) | 67 (65.0) | 0 (0.0) | 52 (63.4) | ||||
| Operative Time min, median (IQR) | 288 (83.5) | 241 (56.0) | <0.001 | 290 (74.0) | 256 (72.3) | <0.001 | 0.084 | 0.098 |
| Lymphadenectomy, n (%) | 0.026 | <0.001 | 0.024 | 0.846 | ||||
| No | 24 (24.2) | 40 (38.8) | 5 (9.3) | 33 (40.2) | ||||
| Yes | 75 (75.8) | 63 (61.2) | 49 (90.7) | 49 (59.8) | ||||
| EBL ml, median (IQR) | 150 (150) | 100 (150) | 0.008 | 150 (188) | 100 (180) | 0.052 | 0.352 | 0.210 |
| Intraoperative complications, n (%) | 0.584 | 0.381 | 0.251 | 0.074 | ||||
| No | 98 (99.0) | 101 (98.1) | 52 (96.3) | 76 (92.7) | ||||
| Yes | 1 (1.0) | 2 (1.9) | 2 (3.7) | 6 (7.3) | ||||
| LOS hours, median (IQR) | 34 (22) | 16 (21) | 0.785 | 35 (17) | 18 (20.8) | 0.002 | 0.389 | 0.332 |
| SDD, n (%) | <0.001 | <0.001 | 0.643 | 0.477 | ||||
| No | 78 (78.8) | 40 (38.8) | 45 (83.3) | 37 (45.1) | ||||
| Yes | 21 (21.2) | 63 (61.2) | 9 (16.7) | 45 (54.9) | ||||
| PONV, n (%) | 0.040 | 0.776 | 0.112 | |||||
| No | 70 (70.7) | 98 (95.1) | 41 (75.9) | 73 (89.1) | ||||
| Yes | 29 (29.3) | 5 (4.9) | 13 (24.1) | 9 (10.9) | ||||
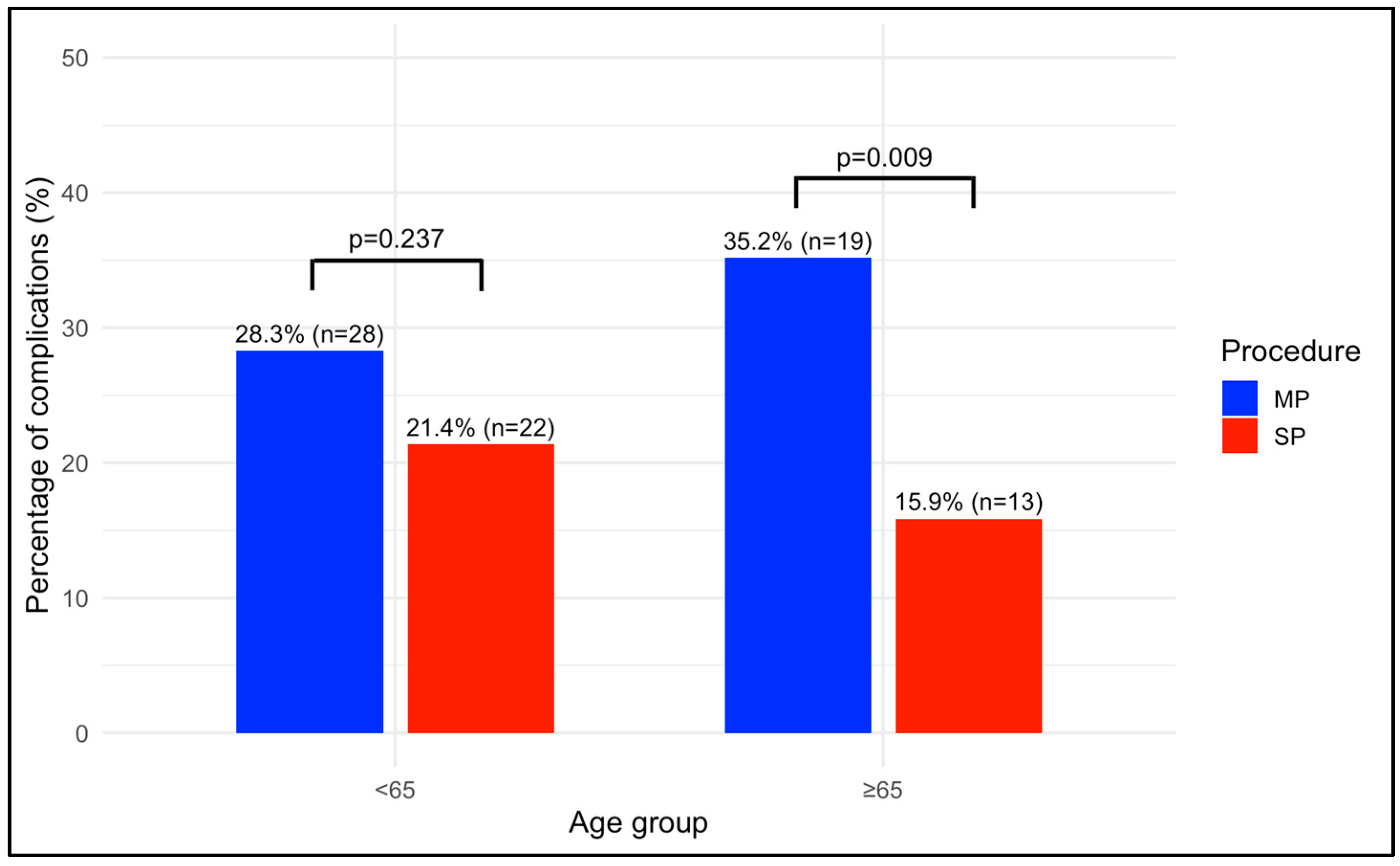
| 30 days postoperative complications, n (%) | 0.237 | 0.009 | 0.398 | 0.342 | ||||
| No | 71 (71.7) | 81 (78.6) | 35 (64.8) | 69 (84.1) | ||||
| Yes | 28 (28.3) | 22 (21.4) | 19 (35.2) | 13 (15.9) | ||||
| 30 days CD, n (%) | 0.222 | 0.557 | 0.590 | 0.833 | ||||
| 1 | 11 (39.3) | 6 (27.4) | 6 (31.7) | 3 (23.1) | ||||
| 2 | 10 (35.7) | 14 (63.6) | 7 (36.8) | 8 (61.5) | ||||
| 3 | 5 (17.9) | 1 (4.5) | 5 (26.3) | 2 (15.4) | ||||
| 4 | 2 (7.1) | 1 (4.5) | 0 (0.0) | 0 (0.0) | ||||
| 5 | 0 (0.0) | 0 (0.0) | 1 (5.2) | 0 (0.0) | ||||
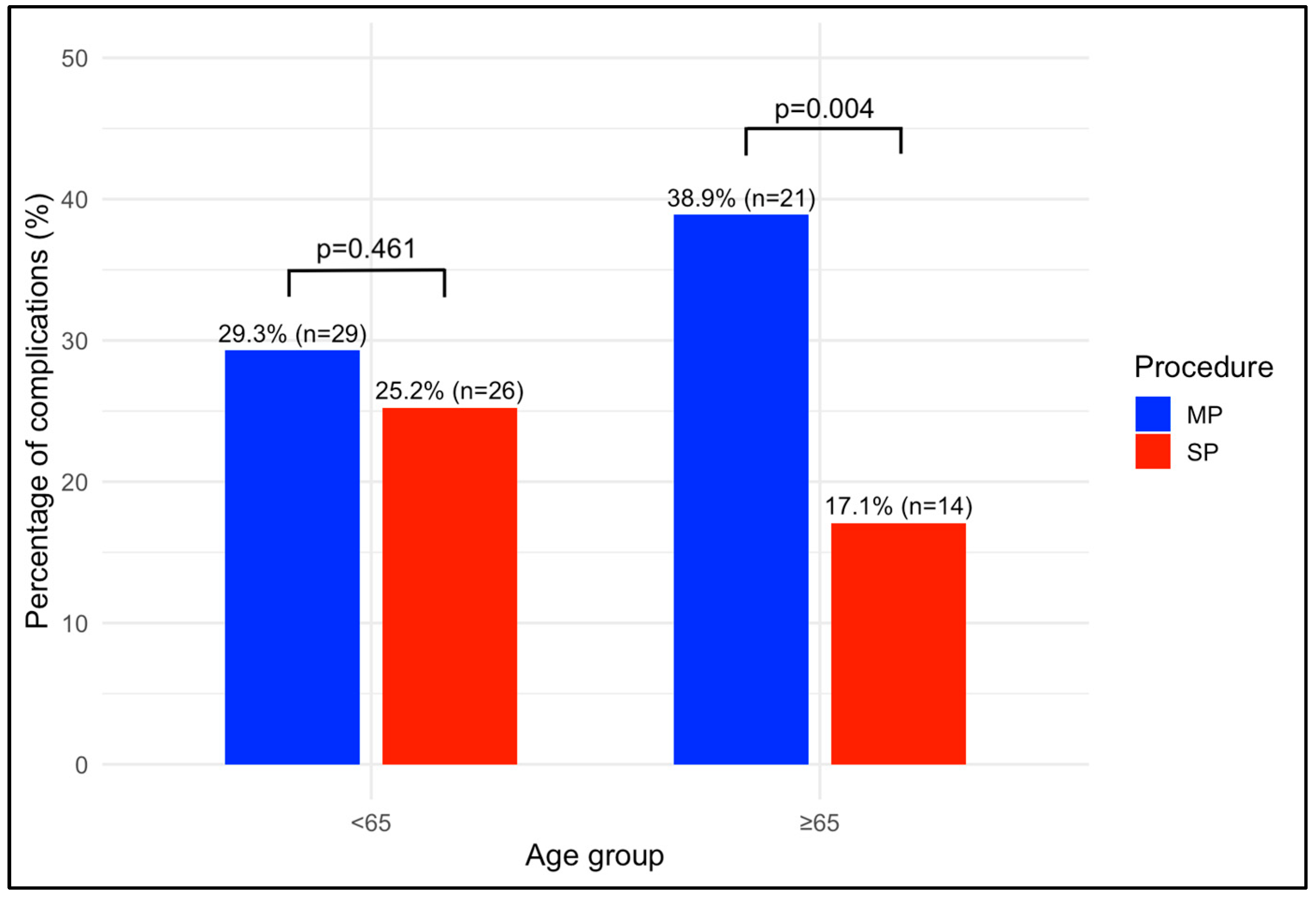
| 90 days postoperative complications, n (%) | 0.461 | 0.004 | 0.260 | 0.180 | ||||
| No | 70 (70.7) | 77 (74.8) | 33 (61.1) | 68 (82.9) | ||||
| Yes | 29 (29.3) | 26 (25.2) | 21 (38.9) | 14 (17.1) | ||||
| 90 days CD, n (%) | 0.387 | 0.511 | 0.387 | 0.511 | ||||
| 1 | 11 (37.9) | 7 (26.9) | 7 (33.3) | 4 (28.6) | ||||
| 2 | 11 (37.9) | 14 (53.8) | 8 (38.1) | 8 (57.1) | ||||
| 3 | 5 (17.3) | 3 (11.6) | 5 (23.8) | 2 (14.3) | ||||
| 4 | 2 (6.9) | 2 (7.7) | 0 (0.0) | 0 (0.0) | ||||
| 5 | 0 (0.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | ||||
| ISUP, n (%) | 0.051 | 0.083 | 0.105 | |||||
| 1 | 9 (9.1) | 5 (4.9) | 0 (0.0) | 4 (4.9) | ||||
| 2 | 49 (49.4) | 61 (59.2) | 27 (50.0) | 35 (42.7) | ||||
| 3 | 27 (27.3) | 22 (21.4) | 14 (25.9) | 25 (30.5) | ||||
| 4 | 6 (6.1) | 6 (5.8) | 3 (5.5) | 12 (14.6) | ||||
| 5 | 8 (8.1) | 9 (8.7) | 10 (18.6) | 6 (7.3) | ||||
| pT stage, n (%) | 0.725 | 0.282 | 0.622 | 0.431 | ||||
| T1 | 2 (2.1) | 1 (1.0) | 0 (0.0) | 2 (2.4) | ||||
| T2 | 51 (51.5) | 58 (56.3) | 32 (59.3) | 39 (47.6) | ||||
| T3 | 45 (45.4) | 43 (41.7) | 22 (40.7) | 41 (50.0) | ||||
| T4 | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | ||||
| pN stage, n (%) | 0.313 | 0.074 | 0.561 | 0.530 | ||||
| N0/Nx | 89 (89.9) | 90 (87.4) | 50 (92.5) | 69 (84.1) | ||||
| N1 | 10 (10.1) | 13 (12.6) | 4 (7.4) | 13 (15.9) | ||||
| Biochemical recurrence, n (%) | 0.203 * | 0.040 * | 0.856 * | 0.175 * | ||||
| No | 76 (76.8) | 86 (83.5) | 42 (77.8) | 74 (90.2) | ||||
| Yes | 23 (23.2) | 17 (16.5) | 12 (22.2) | 8 (9.8) | ||||
| Logistic Regression for Postoperative Complications at 30 Days in Patients of Group B | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p Value |
| Procedure (SP) | 0.41 | 0.15, 0.97 | 0.027 |
| LOS | 1.02 | 1.01, 1.13 | 0.046 |
| Access (Retroperitoneal) | 0.45 | 0.23, 1.14 | 0.082 |
| Intercept | 0.23 | 0.02, 0.68 | 0.001 |
| Logistic Regression for Postoperative Complications at 90 Days in Patients of Group B | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p Value |
| Procedure (SP) | 0.38 | 0.17, 0.88 | 0.024 |
| Lymphadenectomy | 1.56 | 0.55, 4.44 | 0.405 |
| LOS | 1.01 | 0.99, 1.02 | 0.217 |
| Access (Retroperitoneal) | 0.52 | 0.21, 1.12 | 0.087 |
| Intercept | 0.17 | 0.03, 0.86 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzi, F.M.; Santarelli, V.; Avesani, G.; Aljoulani, M.; Haberal, H.B.; Torres Anguiano, J.R.; Morgantini, L.A.; Calvo, R.S.; Biasatti, A.; Fuschi, A.; et al. Single-Port Versus Multi-Port Robotic Radical Prostatectomy in Elderly Patients. Cancers 2025, 17, 1857. https://doi.org/10.3390/cancers17111857
Valenzi FM, Santarelli V, Avesani G, Aljoulani M, Haberal HB, Torres Anguiano JR, Morgantini LA, Calvo RS, Biasatti A, Fuschi A, et al. Single-Port Versus Multi-Port Robotic Radical Prostatectomy in Elderly Patients. Cancers. 2025; 17(11):1857. https://doi.org/10.3390/cancers17111857
Chicago/Turabian StyleValenzi, Fabio Maria, Valerio Santarelli, Giulio Avesani, Muhannad Aljoulani, Hakan Bahadir Haberal, Juan R. Torres Anguiano, Luca Alfredo Morgantini, Ruben Sauer Calvo, Arianna Biasatti, Andrea Fuschi, and et al. 2025. "Single-Port Versus Multi-Port Robotic Radical Prostatectomy in Elderly Patients" Cancers 17, no. 11: 1857. https://doi.org/10.3390/cancers17111857
APA StyleValenzi, F. M., Santarelli, V., Avesani, G., Aljoulani, M., Haberal, H. B., Torres Anguiano, J. R., Morgantini, L. A., Calvo, R. S., Biasatti, A., Fuschi, A., Pastore, A. L., & Crivellaro, S. (2025). Single-Port Versus Multi-Port Robotic Radical Prostatectomy in Elderly Patients. Cancers, 17(11), 1857. https://doi.org/10.3390/cancers17111857








