Mechanical Modulation, Physiological Roles, and Imaging Innovations of Intercellular Calcium Waves in Living Systems
Simple Summary
Abstract
1. Introduction
2. Functional Interplay Between Mechanical Signals and Calcium Dynamics
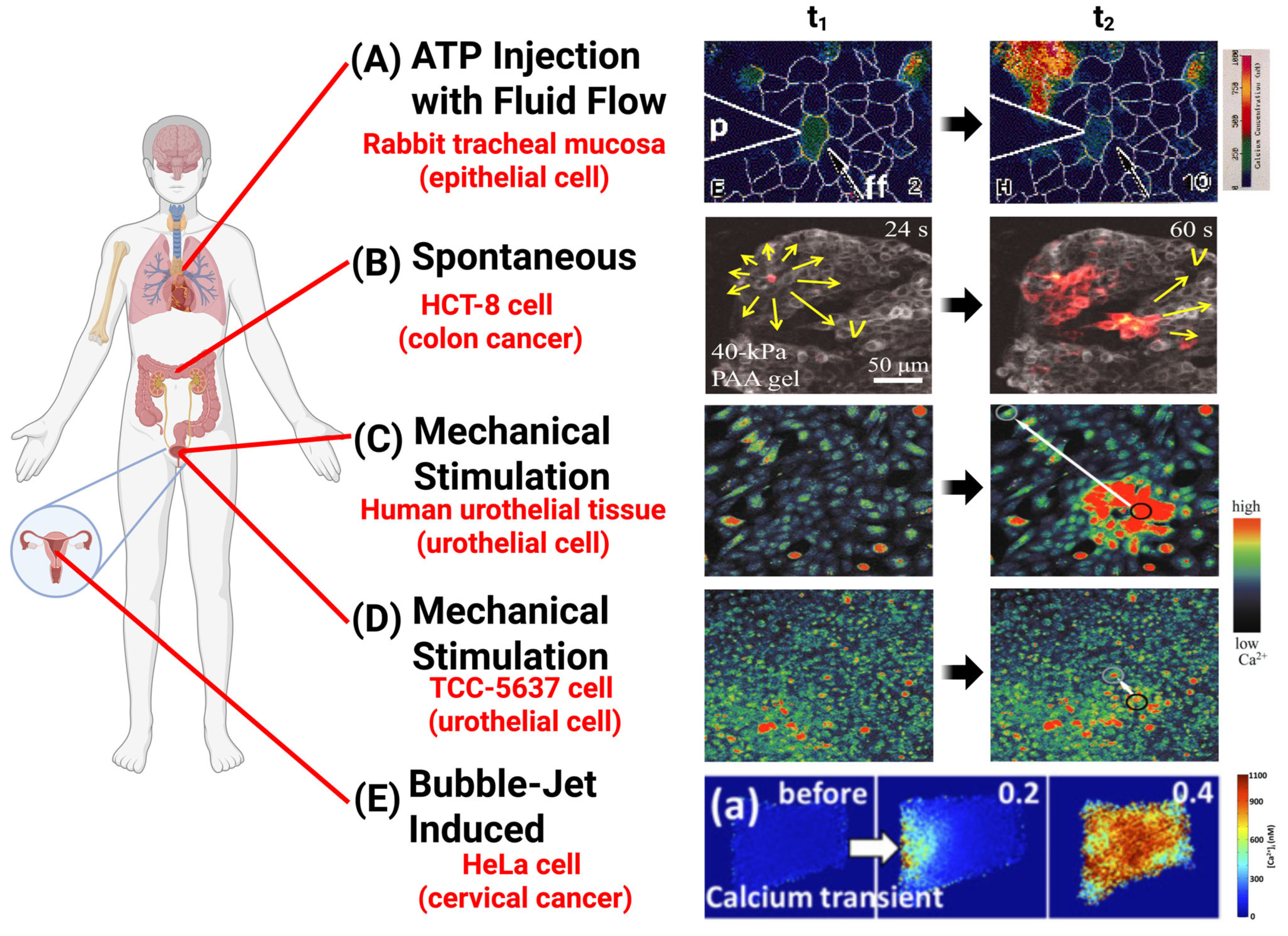
2.1. Mechanically Induced Initiation of ICWs
2.1.1. Mechano-Regulated, Non-Spontaneous ICW Initiation
| Category of Force Types | Methods of Application | Key Parameters | Molecular Mechanisms | Function |
|---|---|---|---|---|
| Molecular-Scale Mechanical Stimulation | Optical Laser Tweezers | Fibronectin (Fn)-coated bead (diameter = 10 μm), force = 300 pN [79] | Induce Ca2+ signals at the plasma membrane and ER via TRPM7 mechanosensitive channels. | ICWs in MSCs depend on TRPM7-mediated calcium signaling, which regulates differentiation. |
| Light-Activated Molecular Machines | Forces = 10−12 to 10−9 N, laser = 400–405 nm (3.2 × 102 to 9.0 × 102 W/cm2), duration = 250 ms (in vitro), 1–2 s (Hydra) [80] | Induce ICWs via IP3-mediated signaling pathways. | MM-induced calcium wave generation can control biological behaviors coordinated in the networks of cells, such as contraction. | |
| Pipette Poking/Probing | Blunt-end glass micropipette, tip diameter = 50 μm [76] | ATP release via mechano-volume-sensitive Cl− anion channels, activating receptors (P2X/P2Y) on wave-receiving cells. | Induced ICWs propagated at velocities of ~15 μm/s and distances of 200–300 μm, transmitting signals to adjacent cells. | |
| Scrape with Pipette Tip | Bent 200 μL micropipette tip [74] | ATP release stimulates calcium waves through purinergic receptor activation. | Induced ICWs regulated intercellular communication. | |
| Glass Micropipette with Micromanipulator | Micropipette tip coupled with 200 ng/mL EGF for 5 min [81] | EGF activates PLC-mediated calcium signaling. | - | |
| Tip diameter = 1 μm, movement = 2–5 μm, controlled by a piezoelectric device [82] | IP3 moves between gap junctions in epithelial respiratory tract cells. | - | ||
| Tip diameter < 1 μm, touching less than 1 % of the cell membrane [83] | Induces a rapid Ca2+ spike and ICWs through gap junctions. | ICWs facilitate intercellular communication, regulate responses to mechanical and metabolic stress, and maintain metabolic homeostasis. | ||
| Tip diameter of 1 μm, moved downward by 10 μm over 0.5 s coupled with 3 M KCl, delivered at 150 hPa for 1 s [84] | Ultrafast wave of calcium, traveling at approximately 15 mm/s. | ICWs synchronize contraction, regulate blood flow, and coordinate rapid vasomotor responses in SMCs. | ||
| Microinjector Capillary | Micrometer precision [85] | ICWs are inhibited by GJ-blocking heptanol, indicating gap junction dependence. | ICWs maintain intercellular communication and coordinated cellular responses in urothelial cells. | |
| Force Probe or 30-Gauge Syringe Needle | Force range = ~2–300 μN, stimulation duration = 20–2000 ms [86] | Not specified. | Induced ICWs regulate endothelial communication, which is critical for immune modulation and tissue healing. | |
| Glass Microelectrode | Tip diameter = 1 μm [87] | Not specified. | - | |
| Subcellular/Cellular-Scales Mechanical Stimulation | Focused Ultrasound (FUS) | Amplitude = 46 MHz (12 Vp–p), pulse repetition frequency = 1 kHz, duty cycle = 5% [88] | PANX1 mechanosensitive channels mediate calcium wave (propagation distance > 1 mm) initiation. | FUS-induced ICWs in PC-3 cells promote ATP release and cytokine/chemokine secretion via PANX1. |
| Bubble-Jetting Methods | RGD-coated beads (6 μm), γ = Sd/Rmax = 1.2 to 2.4 (Sd = 30–60 μm) [89] | Intracellular calcium waves elicited by tandem bubble-induced jetting flow. | The bubble-induced rapid Ca2+ influx showed loss of F-actin stress fibers, cell shrinkage, and apoptosis. | |
| Parallel-Plate Flow Chamber | Shear stress from 100 to 400 μN/cm2 for 3 s [90] | Raising shear stress induced localized ATP release from caveolin-1-rich membrane domains, which activated purinergic receptors and initiated intracellular Ca2+ waves. | The shear stress triggered Ca2+ wave in HPAECs; contributed to cell shear-sensing. | |
| Tissue-Scale Mechanical Stimulation | Mechanical Stretching | Stretching speed at 100 μm/s and distance at 200 μm (17.5% elongation) [91] | Stimulate Piezo1-dependent calcium influx and ATP release. | |
| Applied Mechanical Loading | Diaphragm backpressure = 15 kPa, duration = 300 s [92] | Induces ICWs through physical deformation and ATP release. | ICWs regulate organ growth through calcium spikes, transients, and waves. |
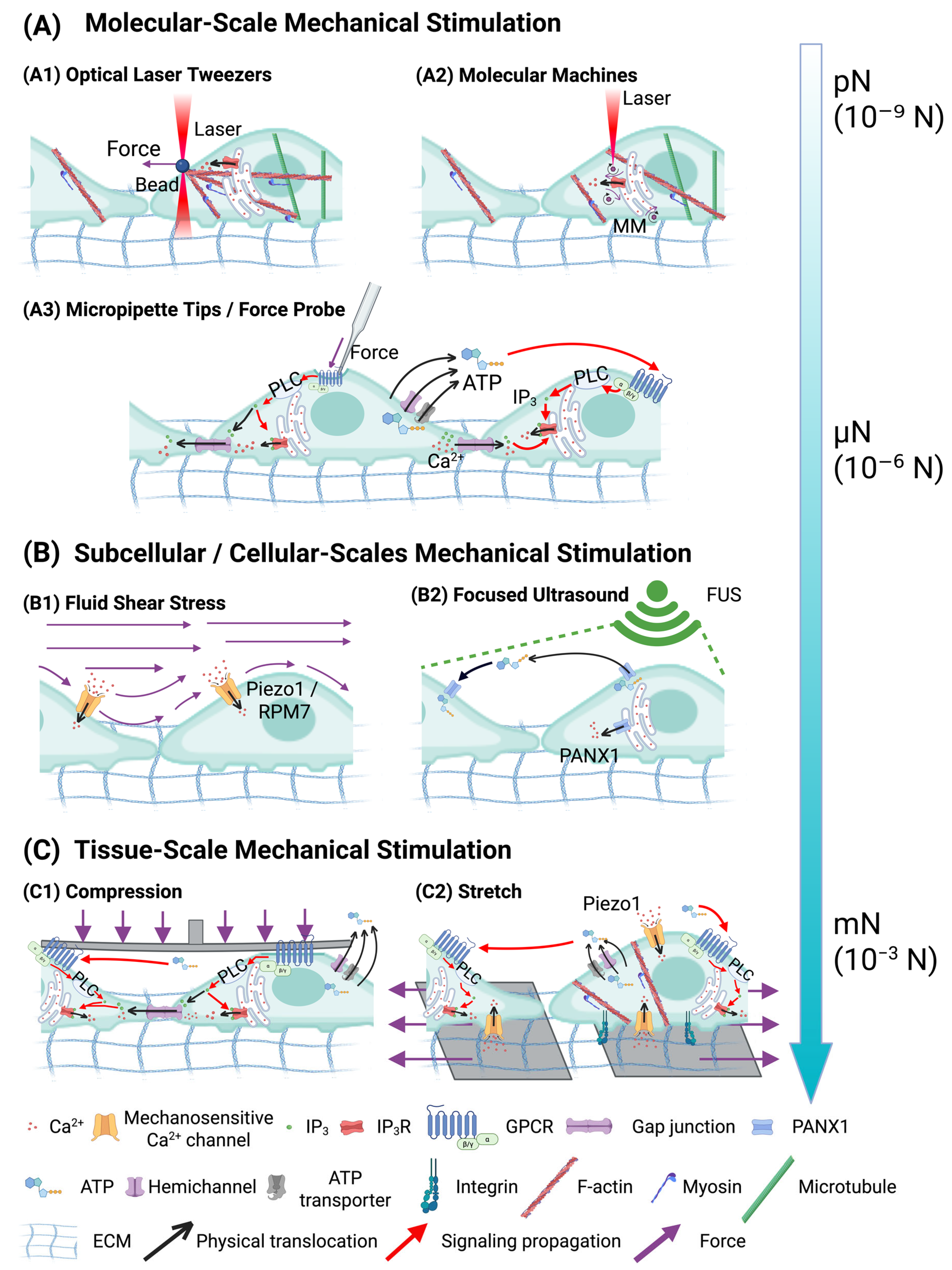
| Mechanical Force Type | Molecular Transducers | Mechanotransduction Pathway |
|---|---|---|
| Shear Stress | Caveolin-1, P2X/P2Y purinergic receptors; Integrins | Shear stress induces ATP release from caveolin-1-enriched membrane domains, which activates P2X/P2Y purinergic receptors and initiates ICWs [90]. Similarly, laser-induced tandem bubble-jetting flow activates integrins, the mechanosensitive ion channel TRPM7, leading to calcium influx and subsequent ER-mediated calcium-induced calcium release [89]. |
| Tension and Stretch | Integrins, Piezo1, Gq-PLC-IP3R pathway | Mechanical stretching or increased ECM stiffness is sensed by integrins and Gq-PLC-IP3R pathway-mediated calcium release [53,94,95,96]; Piezo1 mediates direct calcium influx under stretch-induced membrane tension [97,98,99,100]. |
| Point Stress and Compression | TRPM7, Cl− channels, IP3R, P2X/P2Y receptors, Connexin-based Gap Junctions | Local mechanical indentation activates volume-sensitive Cl− channels and TRPM7, resulting in ATP release and P2X/P2Y purinergic receptor activation [74,76,79]; IP3 is generated and triggers IP3R-mediated calcium release [81,82,86], which propagates through GJ [83,84,85,87]. |
| Membrane Tension | Piezo1 (Force-from-Lipids); PANX1 (ER), IP3R (Ultrasound-induced ER deformation) | In cytoskeleton-deficient conditions, membrane tension activates Piezo1 through a force-from-lipids mechanism, leading to calcium influx [101]; FUS induces ER membrane deformation, activating PANX1 channels and IP3Rs to release calcium [88,102]. |
| Stress Relaxation | IP3R, Inx2 (Gap Junctions in Drosophila) | Mechanical stress release leads to the generation of IP3, which activates IP3Rs and promotes calcium release from the ER and propagation through GJs (Inx2) [92,103]. |
| Nanoscale Molecular Force | IP3R | Light-activated molecular machines deliver nanoscale rotational forces, stimulating Gq-PLC-IP3 pathway and leading to calcium release and ICW generation [80]. |
2.1.2. Spontaneous Initiation Mechanisms of Multiscale ICWs
2.2. Molecular Effectors Underpinning the Mechano-Regulated Initiation of ICWs
2.2.1. Roles of Interacting Cytoskeleton and Mechanosensitive Ion Channels
2.2.2. Roles of IP3 and IP3R in Intracellular Calcium Release
2.3. Multiscale Release, Propagation, and Regeneration of ICWs
2.3.1. Overview of Primary Mechanisms

2.3.2. ATP-Mediated ICW Spread
2.3.3. GJ-Mediated ICW Spread
2.3.4. Combined ATP- and GJ-Mediated Mechanism
2.3.5. Tunneling Nanotubes
2.3.6. Regenerative Wave Spread
3. Physiological Roles of Calcium Waves and ATP Signals
3.1. Cancer Cells
3.2. ICWs in Non-Cancer Cells
3.3. Physiological Conclusions and Therapeutic Targets
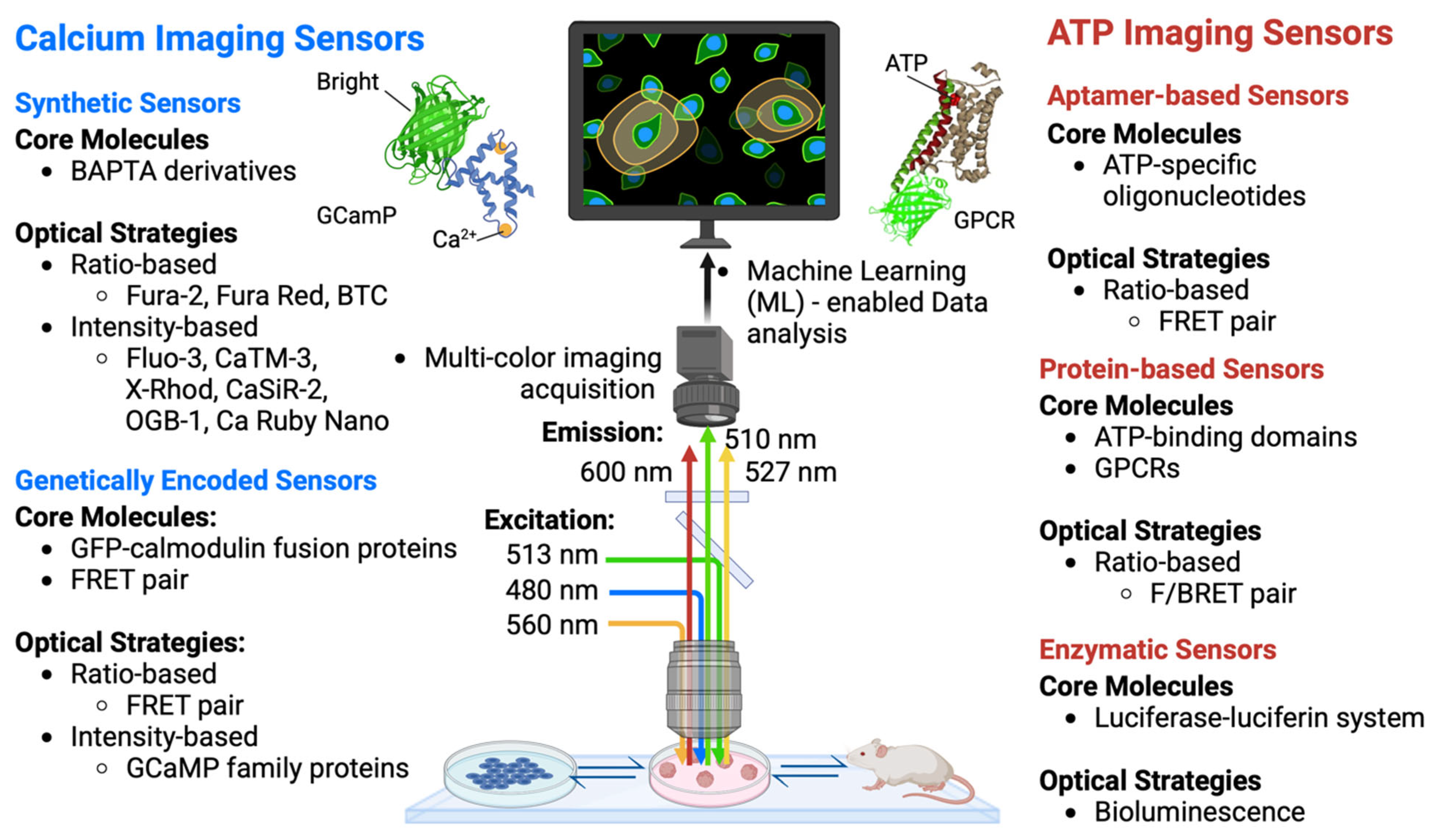
4. Cutting-Edge Technologies for Ca2+ and ATP Imaging
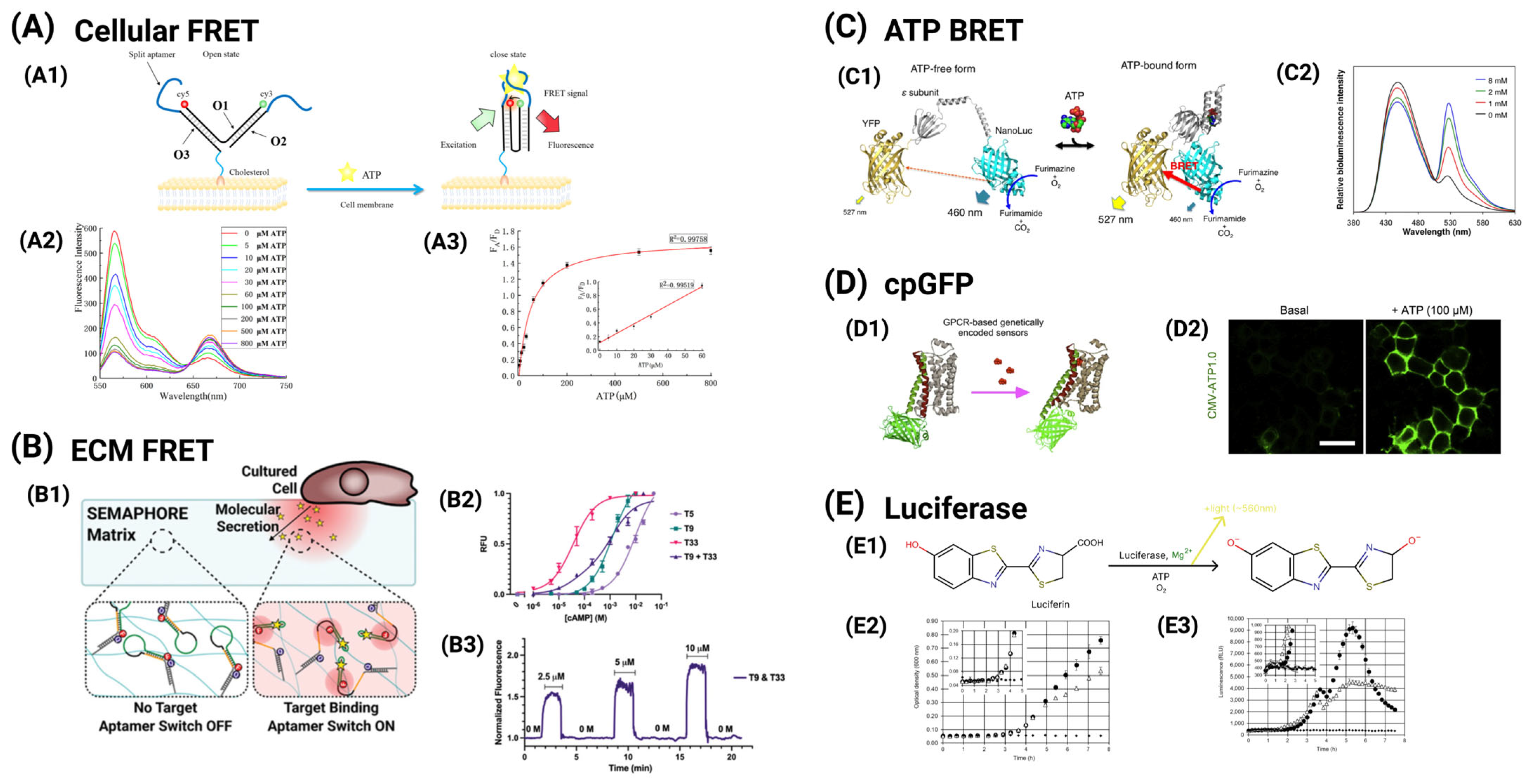
4.1. Functional Imaging of Ca2+ Dynamics
4.2. Functional Imaging of ATP Dynamics
4.3. Artificial Intelligence (AI)/Machine Learning (ML)-Enabled Data Analysis
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xin, Y.; Li, K.; Huang, M.; Liang, C.; Siemann, D.; Wu, L.; Tan, Y.; Tang, X. Biophysics in Tumor Growth and Progression: From Single Mechano-Sensitive Molecules to Mechanomedicine. Oncogene 2023, 42, 3457–3490. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, M.; Li, T.; Li, L.; Sussman, H.; Dai, Y.; Siemann, D.W.; Xie, M.; Tang, X. Towards an integrative understanding of cancer mechanobiology: Calcium, YAP, and microRNA under biophysical forces. Soft Matter 2022, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, H.; Mackey, C.; Chung, M.C.; Guan, J.; Zheng, G.; Roy, A.; Xie, M.; Vulpe, C.; Tang, X. YAP at the Crossroads of Biomechanics and Drug Resistance in Human Cancer. Int. J. Mol. Sci. 2023, 24, 12491. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.R.; Yang, Y.; Zhu, Y.; Yuan, W.; Hoffman, T.; Wu, Y.; Zhu, E.; Zarubova, J.; Shen, J.; et al. Viscoelastic synthetic antigen-presenting cells for augmenting the potency of cancer therapies. Nat. Biomed. Eng. 2024, 8, 1615. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Majedi, F.S.; Zarubova, J.; Thauland, T.J.; Arumugaswami, V.; Hsiai, T.K.; Bouchard, L.-S.; Butte, M.J.; Li, S. Harnessing Biomaterials to Amplify Immunity in Aged Mice through T Memory Stem Cells. ACS Nano 2024, 18, 6908. [Google Scholar] [CrossRef]
- Murthy, S.; Dubin, A.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771. [Google Scholar] [CrossRef]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585. [Google Scholar] [CrossRef]
- Aydin, O.; Passaro, A.P.; Raman, R.; Spellicy, S.E.; Weinberg, R.P.; Kamm, R.D.; Sample, M.; Truskey, G.A.; Zartman, J.; Dar, R.D.; et al. Principles for the design of multicellular engineered living systems. APL Bioeng. 2022, 6, 010903. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kuhlenschmidt, T.B.; Li, Q.; Ali, S.; Lezmi, S.; Chen, H.; Pires-Alves, M.; Laegreid, W.W.; Saif, T.A.; Kuhlenschmidt, M.S. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol. Cancer 2014, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kuhlenschmidt, T.; Zhou, J.; Bell, P.; Wang, F.; Kuhlenschmidt, M.; Saif, T. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys. J. 2010, 99, 2460. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wen, Q.; Kuhlenschmidt, T.; Kuhlenschmidt, M.; Janmey, P.; Saif, T. Attenuation of cell mechanosensitivity in colon cancer cells during in vitro metastasis. PLoS ONE 2012, 7, e50443. [Google Scholar] [CrossRef]
- Naglich, A.; LeDuc, P. Plant Movement Response to Environmental Mechanical Stimulation Toward Understanding Predator Defense. Adv. Sci. 2024, 11, 2404578. [Google Scholar] [CrossRef]
- Radin, I.; Richardson, R.A.; Coomey, J.H.; Weiner, E.R.; Bascom, C.S.; Li, T.; Bezanilla, M.; Haswell, E.S. Plant PIEZO homologs modulate vacuole morphology during tip growth. Science 2021, 373, 586. [Google Scholar] [CrossRef]
- Hamant, O.; Haswell, E.S. Life behind the wall: Sensing mechanical cues in plants. BMC Biol. 2017, 15, 59. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Haick, H. Learning from an Intelligent Mechanosensing System of Plants. Adv. Mater. Technol. 2019, 4, 1800464. [Google Scholar] [CrossRef]
- Gordon, V.D.; Wang, L. Bacterial mechanosensing: The force will be with you, always. J. Cell Sci. 2019, 132, jcs227694. [Google Scholar] [CrossRef]
- Bruni, G.N.; Weekley, R.A.; Dodd, B.J.T.; Kralj, J.M. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc. Natl. Acad. Sci. USA 2017, 114, 9445–9450. [Google Scholar] [CrossRef]
- Persat, A. Bacterial mechanotransduction. Curr. Opin. Microbiol. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an Adaptation to Gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Minc, N.; Peter, M. Cells under pressure: How yeast cells respond to mechanical forces. Trends Microbiol. 2022, 30, 495. [Google Scholar] [CrossRef]
- Matsuda, A.; Mofrad, M.R.K. On the nuclear pore complex and its emerging role in cellular mechanotransduction. APL Bioeng. 2022, 6, 011504. [Google Scholar] [CrossRef]
- Frey, N.; Sönmez, U.M.; Minden, J.; LeDuc, P. Microfluidics for understanding model organisms. Nat. Commun. 2022, 13, 3195. [Google Scholar] [CrossRef]
- Xu, F.; Guo, H.; Zustiak, S.P.; Genin, G.M. Targeting the physical microenvironment of tumors for drug and immunotherapy. Adv. Drug Deliv. Rev. 2023, 196, 114768. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Narumi, R.; Akiyama, R.; Vitiello, E.; Shirai, T.; Tanimura, N.; Kuromiya, K.; Ishikawa, S.; Kajita, M.; Tada, M.; et al. Calcium Wave Promotes Cell Extrusion. Curr. Biol. 2020, 30, 670. [Google Scholar] [CrossRef]
- Tang, X.; Bajaj, P.; Bashir, R.; Saif, T. How far cardiac cells can see each other mechanically. Soft Matter 2011, 7, 6151. [Google Scholar] [CrossRef]
- Bajaj, P.; Tang, X.; Saif, T.A.; Bashir, R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J. Biomed. Mater. Res. A 2010, 95, 1261. [Google Scholar] [CrossRef]
- Dooling, L.J.; Saini, K.; Anlaş, A.A.; Discher, D.E. Tissue mechanics coevolves with fibrillar matrisomes in healthy and fibrotic tissues. Matrix Biol. 2022, 111, 153. [Google Scholar] [CrossRef]
- Coyle, S.; Doss, B.; Huo, Y.; Singh, H.R.; Quinn, D.; Hsia, K.J.; LeDuc, P.R. Cell alignment modulated by surface nano-topography—Roles of cell-matrix and cell-cell interactions. Acta Biomater. 2022, 142, 149. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Chen, C.S. Next-generation engineered microsystems for cell biology: A systems-level roadmap. Trends Cell Biol. 2022, 32, 490. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions. Neuron 2022, 110, 2984–2999. [Google Scholar] [CrossRef]
- Bai, H.; Si, L.; Jiang, A.; Belgur, C.; Zhai, Y.; Plebani, R.; Oh, C.Y.; Rodas, M.; Patil, A.; Nurani, A.; et al. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 2022, 13, 1928. [Google Scholar] [CrossRef]
- Williams, L.N.; LaPlaca, M.C. Editorial overview—Neural engineering: Traumatic brain injury. Curr. Opin. Biomed. Eng. 2023, 27, 100468. [Google Scholar] [CrossRef]
- Narayan, O.P.; Dong, J.; Huang, M.; Chen, L.; Liu, L.; Nguyen, V.; Dozic, A.V.; Liu, X.; Wang, H.; Yin, Q.; et al. Reversible light-responsive protein hydrogel for on-demand cell encapsulation and release. Acta Biomater. 2025, 193, 202. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Z.; Liu, Y.; He, P.; Wang, Y.; Yan, L.; Wang, X.; Gao, S.; Zhou, X.; Yoon, C.W.; et al. Ultrasound Control of Genomic Regulatory Toolboxes for Cancer Immunotherapy. Nat. Commun. 2024, 15, 10444. [Google Scholar] [CrossRef]
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047. [Google Scholar] [CrossRef]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca2+ Waves: Mechanisms of Initiation and Propagation. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 613–618. [Google Scholar]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. In Calcium Signaling; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 827–855. [Google Scholar]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions, Nat. Plants 2020, 6, 750. [Google Scholar]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016, 96, 1261. [Google Scholar] [CrossRef]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca2+ waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359. [Google Scholar] [CrossRef]
- Hashimura, H.; Morimoto, Y.V.; Hirayama, Y.; Ueda, M. Calcium responses to external mechanical stimuli in the multicellular stage of Dictyostelium discoideum. Sci. Rep. 2022, 12, 12428. [Google Scholar] [CrossRef]
- Feske, S. Immunodeficiency due to defects in store-operated calcium entry. Ann. N. Y. Acad. Sci. 2011, 1238, 74. [Google Scholar] [CrossRef]
- Chen, M.; Van Hook, M.J.; Thoreson, W.B. Ca2+ diffusion through endoplasmic reticulum supports elevated intraterminal Ca2+ levels needed to sustain synaptic release from rods in darkness. J. Neurosci. 2015, 35, 11364. [Google Scholar] [CrossRef]
- Monteith, G.R.; McAndrew, D.; Faddy, H.M.; Roberts-Thomson, S.J. Calcium and cancer: Targeting Ca2+ transport. Nat. Rev. Cancer 2007, 7, 519. [Google Scholar] [CrossRef]
- Izu, L.T.; Xie, Y.; Sato, D.; Bányász, T.; Chen-Izu, Y. Ca2+ Waves in the Heart. J. Mol. Cell. Cardiol. 2013, 58, 118–124. [Google Scholar] [CrossRef]
- Monteith, G.R.; Prevarskaya, N.; Roberts-Thomson, S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer 2017, 17, 367. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Q.; Chen, X.; Liu, J.; Tanaka, M.; Wang, S.; Lepler, S.E.; Jin, Z.; Siemann, D.W.; Zeng, B.; et al. Human cancer cells generate spontaneous calcium transients and intercellular waves that modulate tumor growth. Biomaterials 2022, 290, 121823. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sanes, J.R. Neuronal cell-type classification: Challenges, opportunities and the path forward. Nat. Rev. Neurosci. 2017, 18, 530. [Google Scholar] [CrossRef] [PubMed]
- McMillen, P.; Oudin, M.J.; Levin, M.; Payne, S.L. Beyond Neurons: Long Distance Communication in Development and Cancer. Front. Cell Dev. Biol. 2021, 9, 739024. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, L.; Zeng, H.; Tan, P.; Ma, G.; Zheng, S.; Li, Y.; Sun, L.; Dou, F.; Siwko, S.; et al. Engineering of a bona fide light-operated calcium channel. Nat. Commun. 2021, 12, 164. [Google Scholar] [CrossRef]
- Ma, G.; Wei, M.; He, L.; Liu, C.; Wu, B.; Zhang, S.L.; Jing, J.; Liang, X.; Senes, A.; Tan, P.; et al. Inside-out Ca2+ signalling prompted by STIM1 conformational switch. Nat. Commun. 2015, 6, 7826. [Google Scholar] [CrossRef]
- Goldman, D. Potential, impedance, and rectification in membrane. J. Gen. Physiol. 1943, 27, 37. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Charles, A.C.; Boitano, S.; Dirksen, E.R. Mechanisms and function of intercellular calcium signaling. Mol. Cell. Endocrinol. 1994, 98, 173. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Li, Q.; Shi, Z.; Pan, C.; Yan, R.; Fei, D.; Xu, S.; Luo, Y. Mechanical stress facilitates calcium influx and growth of alveolar epithelial cells via activation of the BDKRB1/Ca2+/CaMKII/MEK1/ERK axis. Respir. Res. 2025, 26, 168. [Google Scholar] [CrossRef]
- Zimmermann, B.; Walz, B. Serotonin-induced intercellular calcium waves in salivary glands of the blowfly Calliphora erythrocephala. J. Physiol. 1997, 500, 17. [Google Scholar] [CrossRef]
- Paemeleire, K.; Martin, P.E.M.; Coleman, S.L.; Fogarty, K.E.; Carrington, W.A.; Leybaert, L.; Tuft, R.A.; Evans, W.H.; Sanderson, M.J. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol. Biol. Cell 2000, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Henriques, V.J.; Zonta, M.; Chiavegato, A.; Carmignoto, G.; Gómez-Gonzalo, M.; Losi, G. Calcium Signals in Astrocyte Microdomains, a Decade of Great Advances. Front. Cell. Neurosci. 2021, 15, 673433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, X.; Watsky, M.A. Transient plasma membrane disruption induced calcium waves in mouse and human corneal epithelial cells. PLoS ONE 2024, 19, e0301495. [Google Scholar] [CrossRef]
- Hao, M.M.; Bergner, A.J.; Hirst, C.S.; Stamp, L.A.; Casagranda, F.; Bornstein, J.C.; Boesmans, W.; Berghe, P.V.; Young, H.M. Spontaneous calcium waves in the developing enteric nervous system. Dev. Biol. 2017, 428, 74. [Google Scholar] [CrossRef]
- Zhang, W.; Couldwell, W.T.; Song, H.; Takano, T.; Lin, J.H.C.; Nedergaard, M. Tamoxifen-induced enhancement of calcium signaling in glioma and MCF-7 breast cancer cells. Cancer Res. 2000, 60, 5395. [Google Scholar]
- Haas, B.; Schipke, C.G.; Peters, O.; Söhl, G.; Willecke, K.; Kettenmann, H. Activity-dependent ATP-waves in the Mouse Neocortex are Independent from Astrocytic Calcium Waves. Cereb. Cortex 2006, 16, 237. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Spontaneous long-range calcium waves in developing butterfly wings. BMC Dev. Biol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Ready, D.F.; Chang, H.C. Calcium waves facilitate and coordinate the contraction of endfeet actin stress fibers in Drosophila interommatidial cells. Development 2021, 148, dev199700. [Google Scholar] [CrossRef]
- Rochefort, N.L.; Garaschuk, O.; Milos, R.-I.; Narushima, M.; Marandi, N.; Pichler, B.; Kovalchuk, Y.; Konnerth, A. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc. Natl. Acad. Sci. USA 2009, 106, 15049. [Google Scholar] [CrossRef]
- Jaffe, L.F. Fast calcium waves. Cell Calcium 2010, 48, 102. [Google Scholar] [CrossRef]
- Block, G.J.; DiMattia, G.D.; Prockop, D.J. Stanniocalcin-1 Regulates Extracellular ATP-Induced Calcium Waves in Human Epithelial Cancer Cells by Stimulating ATP Release from Bystander Cells. PLoS ONE 2010, 5, e10237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Feng, L.; Feliu, N.; Guse, A.H.; Parak, W.J. Stimulation of Local Cytosolic Calcium Release by Photothermal Heating for Studying Intra- and Intercellular Calcium Waves. Adv. Mater. 2021, 33, 2008261. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Hescheler, J.; Wartenberg, M. Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am. J. Physiol. Cell Physiol. 2000, 279, C295–C307. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamamoto, T.S.; Ueno, N. Intracellular calcium signal at the leading edge regulates mesodermal sheet migration during Xenopus gastrulation. Sci. Rep. 2018, 8, 2433. [Google Scholar] [CrossRef]
- Kim, T.J.; Joo, C.; Seong, J.; Vafabakhsh, R.; Botvinick, E.L.; Berns, M.W.; Palmer, A.E.; Wang, N.; Ha, T.; Jakobsson, E.; et al. Distinct Mechanisms Regulating Mechanical Force-Induced Ca2+ Signals at the Plasma Membrane and the ER in Human MSCs. eLife 2015, 4, e04876. [Google Scholar] [CrossRef]
- Beckham, J.L.; van Venrooy, A.R.; Kim, S.; Li, G.; Li, B.; Duret, G.; Arnold, D.; Zhao, X.; Li, J.T.; Santos, A.L.; et al. Molecular machines stimulate intercellular calcium waves and cause muscle contraction. Nat. Nanotechnol. 2023, 18, 1051. [Google Scholar] [CrossRef]
- Bryant, J.A.; Finn, R.S.; Slamon, D.J.; Cloughesy, T.F.; Charles, A.C. EGF activates intracellular and intercellular calcium signaling by distinct pathways in tumor cells. Cancer Biol. Ther. 2004, 3, 1243. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Charles, A.C.; Dirksen, E.R. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990, 1, 585. [Google Scholar] [CrossRef]
- Iyyathurai, J.; Himpens, B.; Bultynck, G.; D’hondt, C. Calcium Wave Propagation Triggered by Local Mechanical Stimulation as a Method for Studying Gap Junctions and Hemichannels. In Gap Junction Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 203–211. [Google Scholar]
- Quijano, J.C.; Vianay, B.; Bény, J.-L.; Meister, J.-J. Ultrafast Ca2+ wave in cultured vascular smooth muscle cells aligned on a micropatterned surface. Cell Calcium 2013, 54, 436. [Google Scholar] [CrossRef]
- Leinonen, P.; Aaltonen, V.; Koskela, S.; Lehenkari, P.; Korkiamäki, T.; Peltonen, J. Impaired gap junction formation and intercellular calcium signaling in urinary bladder cancer cells can be improved by Gö6976. Cell Commun. Adhes. 2007, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Junkin, M.; Lu, Y.; Long, J.; Deymier, P.A.; Hoying, J.B.; Wong, P.K. Mechanically induced intercellular calcium communication in confined endothelial structures. Biomaterials 2013, 34, 2049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xing, F.; Zhang, P.; Jiang, P.; Chen, Z.; Yang, J.; Hu, F.; Drevenšek-Olenik, I.; Zhang, X.; Pan, L.; Xu, J. Spatiotemporal Characteristics of Intercellular Calcium Wave Communication in Micropatterned Assemblies of Single Cells. ACS Appl. Mater. Interfaces 2018, 10, 2937. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.S.; Yoon, C.W.; Wang, Q.; Moon, S.; Koo, K.M.; Jung, H.; Chen, R.; Jiang, L.; Lu, G.; Fernandez, A.; et al. Focused Ultrasound Stimulates ER Localized Mechanosensitive PANNEXIN-1 to Mediate Intracellular Calcium Release in Invasive Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 504. [Google Scholar] [CrossRef]
- Li, F.; Yang, C.; Yuan, F.; Liao, D.; Li, T.; Guilak, F.; Zhong, P. Dynamics and mechanisms of intracellular calcium waves elicited by tandem bubble-induced jetting flow. Proc. Natl. Acad. Sci. USA 2018, 115, E353–E362. [Google Scholar] [CrossRef]
- Yamamoto, K.; Furuya, K.; Nakamura, M.; Kobatake, E.; Sokabe, M.; Ando, J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J. Cell Sci. 2011, 124, 3477. [Google Scholar] [CrossRef]
- Kim, T.; Lei, L.; Seong, J.; Suh, J.; Jang, Y.; Jung, S.H.; Sun, J.; Kim, D.; Wang, Y. Matrix Rigidity-Dependent Regulation of Ca2+ at Plasma Membrane Microdomains by FAK Visualized by Fluorescence Resonance Energy Transfer. Adv. Sci. 2019, 6, 1801290. [Google Scholar] [CrossRef]
- Narciso, C.E.; Contento, N.M.; Storey, T.J.; Hoelzle, D.J.; Zartman, J.J. Release of Applied Mechanical Loading Stimulates Intercellular Calcium Waves in Drosophila Wing Discs. Biophys. J. 2017, 113, 491. [Google Scholar] [CrossRef]
- Hansen, M.; Boitano, S.; Dirksen, E.R.; Sanderson, M.J. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J. Cell Sci. 1993, 106, 995. [Google Scholar] [CrossRef]
- Parkash, J.; Asotra, K. Calcium wave signaling in cancer cells. Life Sci. 2010, 87, 587. [Google Scholar] [CrossRef]
- Uray, I.P.; Uray, K. Mechanotransduction at the Plasma Membrane-Cytoskeleton Interface. Int. J. Mol. Sci. 2021, 22, 11566. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, A.-B.; Koenderink, G.H.; Shemesh, M. Intermediate Filaments in Cellular Mechanoresponsiveness: Mediating Cytoskeletal Crosstalk from Membrane to Nucleus and Back. Front. Cell Dev. Biol. 2022, 10, 882037. [Google Scholar] [CrossRef] [PubMed]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on Altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef]
- Miyamoto, T.; Mochizuki, T.; Nakagomi, H.; Kira, S.; Watanabe, M.; Takayama, Y.; Suzuki, Y.; Koizumi, S.; Takeda, M.; Tominaga, M. Functional Role for Piezo1 in Stretch-evoked Ca2+ Influx and ATP Release in Urothelial Cell Cultures. J. Biol. Chem. 2014, 289, 16565. [Google Scholar] [CrossRef]
- Li, R.; Wang, D.; Li, H.; Lei, X.; Liao, W.; Liu, X.-Y. Identification of Piezo1 as a potential target for therapy of colon cancer stem-like cells. Discov. Oncol. 2023, 14, 95. [Google Scholar] [CrossRef]
- Yao, M.; Tijore, A.; Cheng, D.; Li, J.V.; Hariharan, A.; Martinac, B.; Van Nhieu, G.T.; Cox, C.D.; Sheetz, M. Force- and cell state–dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci. Adv. 2022, 8, eabo1461. [Google Scholar] [CrossRef]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.-A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef]
- Weitz, A.C.; Lee, N.S.; Yoon, C.W.; Bonyad, A.; Goo, K.S.; Kim, S.; Moon, S.; Jung, H.; Zhou, Q.; Chow, R.H.; et al. Functional Assay of Cancer Cell Invasion Potential Based on Mechanotransduction of Focused Ultrasound. Front. Oncol. 2017, 7, 161. [Google Scholar] [CrossRef]
- Soundarrajan, D.K.; Huizar, F.J.; Paravitorghabeh, R.; Robinett, T.; Zartman, J.J. From spikes to intercellular waves: Tuning intercellular calcium signaling dynamics modulates organ size control. PLoS Comput. Biol. 2021, 17, e1009543. [Google Scholar] [CrossRef]
- Sabirov, R.Z.; Okada, Y. ATP Release via Anion Channels. Purinergic Signal. 2005, 1, 311–328. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, L.Z.; Yoon, C.W.; Preece, D.; Gomez-Godinez, V.; Lu, S.; Carmona, C.; Woo, S.H.; Chien, S.; Berns, M.W.; et al. Mechanosensor Piezo1 mediates bimodal patterns of intracellular calcium and FAK signaling. EMBO J. 2022, 41, e111799. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.C.; Kodali, S.K.; Tyndale, R.F. Intercellular Calcium Waves in Neurons. Mol. Cell. Neurosci. 1996, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Riera, J.; Hatanaka, R.; Ozaki, T.; Kawashima, R. Modeling the spontaneous Ca2+ oscillations in astrocytes: Inconsistencies and usefulness. J. Integr. Neurosci. 2011, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E.; Giaume, C. Astrocyte calcium waves: What they are and what they do. Glia 2006, 54, 716. [Google Scholar] [CrossRef]
- MacDonald, C.L. Diffusion modeling of ATP signaling suggests a partially regenerative mechanism underlies astrocyte intercellular calcium waves. Front. Neuroeng. 2008, 1, 260. [Google Scholar] [CrossRef]
- Jiang, P.; Xing, F.; Guo, B.; Yang, J.; Li, Z.; Wei, W.; Hu, F.; Lee, I.; Zhang, X.; Pan, L.; et al. Nucleotide transmitters ATP and ADP mediate intercellular calcium wave communication via P2Y12/13 receptors among BV-2 microglia. PLoS ONE 2017, 12, e0183114. [Google Scholar] [CrossRef]
- Seyedbarhagh, M.; Ahmadi, A.; Ahmadi, M. Deep Brain Stimulation on Ca2+ Signalling and Neuro-Glial Network: A Digital Implementation. IEEE Trans. Med. Robot. Bionics 2023, 5, 704. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Cherkas, P.S.; Zuckerman, J.; Smith, D.N.; Spray, D.C.; Hanani, M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010, 6, 43. [Google Scholar] [CrossRef]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 2004, 43, 647. [Google Scholar] [CrossRef]
- Stasiak, S.E.; Jamieson, R.R.; Bouffard, J.; Cram, E.J.; Parameswaran, H. Intercellular communication controls agonist-induced calcium oscillations independently of gap junctions in smooth muscle cells. Sci. Adv. 2020, 6, eaba1149. [Google Scholar] [CrossRef]
- Feng, C.-Y.; Hennig, G.W.; Corrigan, R.D.; Smith, T.K.; von Bartheld, C.S. Analysis of spontaneous and nerve-evoked calcium transients in intact extraocular muscles in vitro. Exp. Eye Res. 2012, 100, 73. [Google Scholar] [CrossRef] [PubMed]
- McHale, N.; Hollywood, M.; Sergeant, G.; Thornbury, K. Origin of spontaneous rhythmicity in smooth muscle. J. Physiol. 2006, 570, 23. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.C.; Olson, E.; Gu, X. Spontaneous calcium transients regulate neuronal plasticity in developing neurons. J. Neurobiol. 1995, 26, 316. [Google Scholar] [CrossRef]
- Gu, X.; Spitzer, N.C. Breaking the Code: Regulation of Neuronal Differentiation by Spontaneous Calcium Transients. Dev. Neurosci. 1997, 19, 33. [Google Scholar] [CrossRef]
- De Faveri, F.; Ceriani, F.; Marcotti, W. In vivo spontaneous Ca2+ activity in the pre-hearing mammalian cochlea. Nat. Commun. 2025, 16, 29. [Google Scholar] [CrossRef]
- Restrepo, S.; Basler, K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat. Commun. 2016, 7, 12450. [Google Scholar] [CrossRef]
- Chevalier, N.R.; Zig, L.; Gomis, A.; Amedzrovi Agbesi, R.J.; El Merhie, A.; Pontoizeau, L.; Le Parco, I.; Rouach, N.; Arnoux, I.; de Santa Barbara, P.; et al. Calcium wave dynamics in the embryonic mouse gut mesenchyme: Impact on smooth muscle differentiation. Commun. Biol. 2024, 7, 1277. [Google Scholar] [CrossRef]
- Balaji, R.; Bielmeier, C.; Harz, H.; Bates, J.; Stadler, C.; Hildebrand, A.; Classen, A.-K. Calcium spikes, waves and oscillations in a large, patterned epithelial tissue. Sci. Rep. 2017, 7, 42786. [Google Scholar] [CrossRef]
- Kim, T.J.; Seong, J.; Ouyang, M.; Sun, J.; Lu, S.; Jun, P.H.; Wang, N.; Wang, Y. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J. Cell. Physiol. 2008, 218, 285. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Jin, F.; Fang, M.; Huang, M.; Yang, C.S.; Chen, T.; Fu, L.; Pan, Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget 2014, 5, 3455. [Google Scholar] [CrossRef]
- Decrock, E.; Hoorelbeke, D.; Ramadan, R.; Delvaeye, T.; De Bock, M.; Wang, N.; Krysko, D.V.; Baatout, S.; Bultynck, G.; Aerts, A.; et al. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1099. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Chen, C.-C. Force from Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels. Front. Cell Dev. Biol. 2022, 10, 886048. [Google Scholar] [CrossRef] [PubMed]
- Putra, V.D.L.; Kilian, K.A.; Tate, M.L.K. Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y.; et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 2021, 5, 1336. [Google Scholar] [CrossRef]
- Mulhall, E.M.; Gharpure, A.; Lee, R.M.; Dubin, A.E.; Aaron, J.S.; Marshall, K.L.; Spencer, K.R.; Reiche, M.A.; Henderson, S.C.; Chew, T.L.; et al. Direct observation of the conformational states of PIEZO1. Nature 2023, 620, 1117. [Google Scholar] [CrossRef]
- Bracho-Sanchez, E.; Rocha, F.G.; Bedingfield, S.K.; Partain, B.D.; Macias, S.L.; Brusko, M.A.; Colazo, J.M.; Fettis, M.M.; Farhadi, S.A.; Helm, E.Y.; et al. Suppression of local inflammation via galectin-anchored indoleamine 2,3-dioxygenase. Nat. Biomed. Eng. 2023, 7, 1156. [Google Scholar] [CrossRef]
- Kwiatkowski, A.J.; Helm, E.Y.; Stewart, J.; Leon, J.; Drashansky, T.; Avram, D.; Keselowsky, B. Design principles of microparticle size and immunomodulatory factor formulation dictate antigen-specific amelioration of multiple sclerosis in a mouse model. Biomaterials 2023, 294, 122001. [Google Scholar] [CrossRef]
- Macias, S.L.; Keselowsky, B.G. Perspectives on immunometabolism at the biomaterials interface. Mol. Asp. Med. 2022, 83, 100992. [Google Scholar] [CrossRef]
- Porras, A.M.; Hutson, H.N.; Berger, A.J.; Masters, K.S. Engineering approaches to study fibrosis in 3-D in vitro systems. Curr. Opin. Biotechnol. 2016, 40, 24. [Google Scholar] [CrossRef]
- Porras, A.M.; van Engeland, N.C.A.; Marchbanks, E.; McCormack, A.; Bouten, C.V.C.; Yacoub, M.H.; Latif, N.; Masters, K.S. Robust Generation of Quiescent Porcine Valvular Interstitial Cell Cultures. J. Am. Heart Assoc. 2017, 6, e005041. [Google Scholar] [CrossRef]
- Del Pino Herrera, A.; Ferrall-Fairbanks, M.C. A war on many fronts: Cross disciplinary approaches for novel cancer treatment strategies. Front. Genet. 2024, 15, 1383676. [Google Scholar] [CrossRef] [PubMed]
- Casson, C.L.; John, S.A.; Ferrall-Fairbanks, M.C. Mathematical modeling of cardio-oncology: Modeling the systemic effects of cancer therapeutics on the cardiovascular system. Semin. Cancer Biol. 2023, 97, 30. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.T.; Parker, I. Termination of Ca2+ puffs during IP3-evoked global Ca2+ signals. Cell Calcium 2021, 100, 102494. [Google Scholar] [CrossRef] [PubMed]
- Ismatullah, H.; Jabeen, I.; Saeed, M.T. Biological Regulatory Network (BRN) Analysis and Molecular Docking Simulations to Probe the Modulation of IP3R Mediated Ca2+ Signaling in Cancer. Genes 2020, 12, 34. [Google Scholar] [CrossRef]
- Woll, K.A.; Van Petegem, F. Calcium-release channels: Structure and function of IP 3 receptors and ryanodine receptors. Physiol. Rev. 2022, 102, 209. [Google Scholar] [CrossRef]
- Van Petegem, F. Ryanodine Receptors: Structure and Function. J. Biol. Chem. 2012, 287, 31624. [Google Scholar] [CrossRef]
- MacMillan, D.; Chalmers, S.; Muir, T.C.; McCarron, J.G. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J. Physiol. 2005, 569, 533. [Google Scholar] [CrossRef]
- Sirko, P.; Gale, J.E.; Ashmore, J.F. Intercellular Ca2+ signalling in the adult mouse cochlea. J. Physiol. 2019, 597, 303. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Flores, C.E.; Urban-Maldonado, M.; Beelitz, M.; Scemes, E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia 2004, 48, 217. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Geist, S.T.; Civitelli, R.; Steinberg, T.H. ATP- and Gap Junction–dependent Intercellular Calcium Signaling in Osteoblastic Cells. J. Cell Biol. 1997, 139, 497. [Google Scholar] [CrossRef]
- Politi, A.; Gaspers, L.D.; Thomas, A.P.; Höfer, T. Models of IP3 and Ca2+ Oscillations: Frequency Encoding and Identification of Underlying Feedbacks. Biophys. J. 2006, 90, 3120. [Google Scholar] [CrossRef] [PubMed]
- Sherer, N.M. Long-distance relationships: Do membrane nanotubes regulate cell–cell communication and disease progression? Mol. Biol. Cell 2013, 24, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.T.; Parker, I.; Smith, I.F. Communication of Ca2+ signals via tunneling membrane nanotubes is mediated by transmission of inositol trisphosphate through gap junctions. Cell Calcium 2016, 60, 266. [Google Scholar] [CrossRef]
- Smith, I.F.; Shuai, J.; Parker, I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys. J. 2011, 100, L37. [Google Scholar] [CrossRef]
- Uhrenholt, T.R.; Schjerning, J.; Vanhoutte, P.M.; Jensen, B.L.; Skøtt, O. Intercellular calcium signaling and nitric oxide feedback during constriction of rabbit renal afferent arterioles. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1124. [Google Scholar] [CrossRef]
- Li, N.; Sul, J.-Y.; Haydon, P.G. A Calcium-Induced Calcium Influx Factor, Nitric Oxide, Modulates the Refilling of Calcium Stores in Astrocytes. J. Neurosci. 2003, 23, 10302. [Google Scholar] [CrossRef]
- Young, R.C.; Schumann, R.; Zhang, P. The signaling mechanisms of long distance intercellular calcium waves (far waves) in cultured human uterine myocytes. J. Muscle Res. Cell Motil. 2002, 23, 279. [Google Scholar] [CrossRef]
- Willmott, N.J.; Wong, K.; Strong, A.J. Intercellular Ca2+ waves in rat hippocampal slice and dissociated glial–neuron cultures mediated by nitric oxide. FEBS Lett. 2000, 487, 239. [Google Scholar] [CrossRef]
- Puebla, M.; Muñoz, M.F.; Lillo, M.A.; Contreras, J.E.; Figueroa, X.F. Control of astrocytic Ca2+ signaling by nitric oxide-dependent S-nitrosylation of Ca2+ homeostasis modulator 1 channels. Biol. Res. 2024, 57, 19. [Google Scholar] [CrossRef]
- Hsiao, M.-Y.; Liao, D.; Xiang, G.; Zhong, P. Intercellular Calcium Waves and Permeability Change Induced by Vertically Deployed Surface Acoustic Waves in a Human Cerebral Microvascular Endothelial Cell Line (hCMEC/D3) Monolayer. Ultrasound Med. Biol. 2023, 49, 1153. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, E.Y.; Skatchkov, S.N.; Ermakov, A.M. Structure and Functions of Gap Junctions and Their Constituent Connexins in the Mammalian CNS. Biochem. (Mosc. ), Suppl. Ser. A Membr. Cell Biol. 2021, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Feng, X.; Chen, D.; Liu, C.; Du, W.; Liu, B.-F. Investigating intercellular calcium waves by microfluidic gated pinched-flow. Sens. Actuators B Chem. 2016, 234, 583. [Google Scholar] [CrossRef]
- Huo, B.; Lu, X.L.; Costa, K.D.; Xu, Q.; Guo, X.E. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 2010, 47, 234. [Google Scholar] [CrossRef]
- Stamatakis, M.; Mantzaris, N.V. Modeling of ATP-mediated signal transduction and wave propagation in astrocytic cellular networks. J. Theor. Biol. 2006, 241, 649. [Google Scholar] [CrossRef]
- Bao, L.; Sachs, F.; Dahl, G. Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 2004, 287, C1389. [Google Scholar] [CrossRef]
- Rain, B.D.; Plourde-Kelly, A.D.; Lafrenie, R.M.; Dotta, B.T. Induction of apoptosis in B16-BL melanoma cells following exposure to electromagnetic fields modeled after intercellular calcium waves. FEBS Open Bio 2024, 14, 515. [Google Scholar] [CrossRef]
- Povstyan, O.V.; Harhun, M.I.; Gordienko, D.V. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br. J. Pharmacol. 2011, 162, 1618. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, M.; Wang, S.; Chu, S.; Zhang, Z.; Chen, N. Tunneling nanotubes: The transport highway for astrocyte-neuron communication in the central nervous system. Brain Res. Bull. 2024, 209, 110921. [Google Scholar] [CrossRef]
- Watkins, S.C.; Salter, R.D. Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity 2005, 23, 309. [Google Scholar] [CrossRef]
- Vargas, J.Y.; Loria, F.; Wu, Y.J.; Córdova, G.; Nonaka, T.; Bellow, S.; Syan, S.; Hasegawa, M.; van Woerden, G.M.; Trollet, C.; et al. The Wnt/Ca2+ pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. 2019, 38, e101230. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: β-Catenin-independent pathways. Cell Calcium 2005, 38, 439. [Google Scholar] [CrossRef] [PubMed]
- Thrasivoulou, C.; Millar, M.; Ahmed, A. Activation of Intracellular Calcium by Multiple Wnt Ligands and Translocation of β-Catenin into the Nucleus. J. Biol. Chem. 2013, 288, 35651. [Google Scholar] [CrossRef]
- Ai, Z.; Fischer, A.; Spray, D.C.; Brown, A.M.C.; Fishman, G.I. Wnt-1 regulation of connexin43 in cardiac myocytes. J. Clin. Investig. 2000, 105, 161. [Google Scholar] [CrossRef]
- Zhang, J.; Chandrasekaran, G.; Li, W.; Kim, D.Y.; Jeong, I.Y.; Lee, S.H.; Liang, T.; Bae, J.Y.; Choi, I.; Kang, H.; et al. Wnt-PLC-IP3-Connexin-Ca2+ axis maintains ependymal motile cilia in zebrafish spinal cord. Nat. Commun. 2020, 11, 1860. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chen, Y.-T.; Chiu, W.-T.; Shen, M.-R. Remodeling of calcium signaling in tumor progression. J. Biomed. Sci. 2013, 20, 23. [Google Scholar] [CrossRef]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3. [Google Scholar] [CrossRef]
- Stewart, T.A.; Yapa, K.T.D.S.; Monteith, G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2502. [Google Scholar] [CrossRef]
- Liang, C.; Zeng, B.; Tanaka, M.; Noy, A.K.; Barrett, M.; Hengartner, E.; Cochrane, A.; Garzon, L.; Litvinov, M.; Siemann, D.; et al. Mechano-Regulated Intercellular Waves Among Cancer Cells. Int. Conf. Comput. Exp. Eng. Sci. 2024, 32, 1. [Google Scholar] [CrossRef]
- Sharma, S.; Kalra, H.; Akundi, R.S. Extracellular ATP Mediates Cancer Cell Migration and Invasion Through Increased Expression of Cyclooxygenase 2. Front. Pharmacol. 2021, 11, 617211. [Google Scholar] [CrossRef]
- Jiang, M.; Kang, Y.; Sewastianik, T.; Wang, J.; Tanton, H.; Alder, K.; Dennis, P.; Xin, Y.; Wang, Z.; Liu, R.; et al. BCL9 provides multi-cellular communication properties in colorectal cancer by interacting with paraspeckle proteins. Nat. Commun. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Chuang, C.-Y.; Saif, M.T.A. Reprogramming cellular phenotype by soft collagen gels. Soft Matter 2014, 10, 8829. [Google Scholar] [CrossRef] [PubMed]
- Harr, M.W.; Distelhorst, C.W. Apoptosis and Autophagy: Decoding Calcium Signals that Mediate Life or Death. Cold Spring Harb. Perspect. Biol. 2010, 2, a005579. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407. [Google Scholar] [CrossRef]
- Danese, A.; Leo, S.; Rimessi, A.; Wieckowski, M.R.; Fiorica, F.; Giorgi, C.; Pinton, P. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 119061. [Google Scholar] [CrossRef]
- Tonelli, F.M.P.; Santos, A.K.; Gomes, D.A.; da Silva, S.L.; Gomes, K.N.; Ladeira, L.O.; Resende, R.R. Stem Cells and Calcium Signaling. In Calcium Signaling. In Calcium Signaling; Springer: Berlin/Heidelberg, Germany, 2012; pp. 891–916. [Google Scholar]
- Sung, Y.-J.; Sung, Z.; Ho, C.-L.; Lin, M.-T.; Wang, J.-S.; Yang, S.-C.; Chen, Y.-J.; Lin, C.-H. Intercellular calcium waves mediate preferential cell growth toward the wound edge in polarized hepatic cells. Exp. Cell Res. 2003, 287, 209. [Google Scholar] [CrossRef]
- Ghilardi, S.J.; O’Reilly, B.M.; Sgro, A.E. Intracellular signaling dynamics and their role in coordinating tissue repair. WIREs Syst. Biol. Med. 2020, 12, e1479. [Google Scholar] [CrossRef]
- Chang-Graham, A.L.; Perry, J.L.; Engevik, M.A.; Engevik, K.A.; Scribano, F.J.; Gebert, J.T.; Danhof, H.A.; Nelson, J.C.; Kellen, J.S.; Strtak, A.C.; et al. Rotavirus induces intercellular calcium waves through ADP signaling. Science 2020, 370, eabc3621. [Google Scholar] [CrossRef]
- Perry, J.L.; Scribano, F.J.; Gebert, J.T.; Engevik, K.A.; Ellis, J.M.; Hyser, J.M. Host IP3 R channels are dispensable for rotavirus Ca2+ signaling but critical for intercellular Ca2+ waves that prime uninfected cells for rapid virus spread. mBio 2024, 15, e02145-23. [Google Scholar] [CrossRef]
- Fei, W. The Multidimensional Impact of Oral Diseases on Overall Health. Front. Med. Sci. Res. 2024, 6, 90–97. [Google Scholar]
- Macey, R.; Thiruvenkatachari, B.; O’Brien, K.; Batista, K.B.S.L. Do malocclusion and orthodontic treatment impact oral health? A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 738. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Permatasari, N. The Role of Stem Cell on Orthodontic Tooth Movement Induced-Alveolar Bone Remodeling. Res. J. Pharm. Technol. 2023, 16, 123–128. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. , J. Biol. Chem. 2019, 294, 17693. [Google Scholar] [CrossRef]
- Sayedyahossein, S.; Thines, L.; Sacks, D.B. Ca2+ signaling and the Hippo pathway: Intersections in cellular regulation. Cell Signal. 2023, 110, 110846. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Kong, K.; Chang, Y.; Hu, Y.; Qiao, H.; Zhao, C.; Rong, K.; Zhang, P.; Zhang, J.; Zhai, Z.; Li, H. TiO2 Nanotubes Promote Osteogenic Differentiation Through Regulation of Yap and Piezo1. Front. Bioeng. Biotechnol. 2022, 10, 872088. [Google Scholar] [CrossRef]
- Sun, Y.; Leng, P.; Guo, P.; Gao, H.; Liu, Y.; Li, C.; Li, Z.; Zhang, H. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol. Med. 2021, 27, 96. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Wang, Y.; Ye, R.; Feng, X.; Jing, Z.; Zhao, Z. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015, 85, 87. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, X.; Zhai, Q.; Xin, L.; Tan, H.; He, X.; Li, X.; Yang, G.; Song, J.; Zheng, L. Heavy mechanical force decelerates orthodontic tooth movement via Piezo1-induced mitochondrial calcium down-regulation. Genes Dis. 2025, 12, 101434. [Google Scholar] [CrossRef]
- Kumari, A.; Veena, S.M.; Luha, R.; Tijore, A. Mechanobiological Strategies to Augment Cancer Treatment. ACS Omega 2023, 8, 42072. [Google Scholar] [CrossRef]
- Yoon, C.W.; Pan, Y.; Wang, Y. The application of mechanobiotechnology for immuno-engineering and cancer immunotherapy. Front. Cell Dev. Biol. 2022, 10, 1064484. [Google Scholar] [CrossRef] [PubMed]
- Ng, X.Y.; Peh, G.; Morales-Wong, F.; Gabriel, R.; Soong, P.L.; Lin, K.-H.; Mehta, J.S. Towards Clinical Application: Calcium Waves for In Vitro Qualitative Assessment of Propagated Primary Human Corneal Endothelial Cells. Cells 2024, 13, 2012. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019, 55, 629. [Google Scholar] [CrossRef]
- Tijore, A.; Yao, M.; Wang, Y.H.; Hariharan, A.; Nematbakhsh, Y.; Doss, B.L.; Lim, C.T.; Sheetz, M. Selective killing of transformed cells by mechanical stretch. Biomaterials 2021, 275, 120866. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Wang, T.; Pang, G.; Zhang, Y.; Zhang, C.; Yu, P.; Chang, J. Sonogenetic nanosystem activated mechanosensitive ion channel to induce cell apoptosis for cancer immunotherapy. Chem. Eng. J. 2021, 407, 127173. [Google Scholar] [CrossRef]
- Liang, C.; Huang, M.; Tanaka, M.; Lightsey, S.; Temples, M.; Lepler, S.E.; Sheng, P.; Mann, W.P.; Widener, A.E.; Siemann, D.W.; et al. Functional Interrogation of Ca2+ Signals in Human Cancer Cells In Vitro and Ex Vivo by Fluorescent Microscopy and Molecular Tools. In Microfluidic Systems for Cancer Diagnosis; Springer: New York, NY, USA, 2023; pp. 95–125. [Google Scholar]
- Farhi, S.L.; Parot, V.J.; Grama, A.; Yamagata, M.; Abdelfattah, A.S.; Adam, Y.; Lou, S.; Kim, J.J.; Campbell, R.E.; Cox, D.D.; et al. Wide-Area All-Optical Neurophysiology in Acute Brain Slices. J. Neurosci. 2019, 39, 4889. [Google Scholar] [CrossRef]
- Werley, C.A.; Boccardo, S.; Rigamonti, A.; Hansson, E.M.; Cohen, A.E. Multiplexed Optical Sensors in Arrayed Islands of Cells for multimodal recordings of cellular physiology. Nat. Commun. 2020, 11, 3881. [Google Scholar] [CrossRef]
- Combs, D.J.; Moult, E.M.; England, S.K.; Cohen, A.E. Mapping Uterine Calcium Dynamics during the Ovulatory Cycle in Live Mice. PNAS Nexus 2024, 3, pgae446. [Google Scholar] [CrossRef]
- Zhou, X.; Belavek, K.J.; Miller, E.W. Origins of Ca2+ Imaging with Fluorescent Indicators. Biochemistry 2021, 60, 3547. [Google Scholar] [CrossRef]
- Collatz, M.B.; Rüdel, R.; Brinkmeier, H. Intracellular calcium chelator BAPTA protects cells against toxic calcium overload but also alters physiological calcium responses. Cell Calcium 1997, 21, 453. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, J.; Ryu, B.; Lee, S.; Oh, M.; Baek, J.; Ren, X.; Canavero, S.; Kim, C.; Chung, H.M. BAPTA, a calcium chelator, neuroprotects injured neurons in vitro and promotes motor recovery after spinal cord transection in vivo. CNS Neurosci. Ther. 2021, 27, 919. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gupta, S.; Zheng, W.S.; Si, K.; Zhu, J.J. Genetically encoded sensors enable micro- and nano-scopic decoding of transmission in healthy and diseased brains. Mol. Psychiatry 2021, 26, 443. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Martínez, N.; Silva, W. Measurement of the Intracellular Calcium Concentration with Fura-2 AM Using a Fluorescence Plate Reader. Bio Protoc. 2017, 7, e2411. [Google Scholar] [CrossRef]
- Wendt, E.R.; Ferry, H.; Greaves, D.R.; Keshav, S. Ratiometric Analysis of Fura Red by Flow Cytometry: A Technique for Monitoring Intracellular Calcium Flux in Primary Cell Subsets. PLoS ONE 2015, 10, e0119532. [Google Scholar] [CrossRef]
- Hyrc, K.L.; Bownik, J.M.; Goldberg, M.P. Ionic selectivity of low-affinity ratiometric calcium indicators: Mag-Fura-2, Fura-2FF and BTC. Cell Calcium 2000, 27, 75. [Google Scholar] [CrossRef]
- Meng, G.; Pan, L.; Li, C.; Hu, F.; Shi, X.; Lee, I.; Drevenšek-Olenik, I.; Zhang, X.; Xu, J. Temperature-induced labelling of Fluo-3 AM selectively yields brighter nucleus in adherent cells. Biochem. Biophys. Res. Commun. 2014, 443, 888. [Google Scholar] [CrossRef]
- Baudon, A.; Clauss-Creusot, E.; Darbon, P.; Patwell, R.; Grinevich, V.; Charlet, A. Calcium imaging and BAPTA loading of amygdala astrocytes in mouse brain slices. STAR Protoc. 2022, 3, 101159. [Google Scholar] [CrossRef]
- Hendel, T.; Mank, M.; Schnell, B.; Griesbeck, O.; Borst, A.; Reiff, D.F. Fluorescence Changes of Genetic Calcium Indicators and OGB-1 Correlated with Neural Activity and Calcium In Vivo and In Vitro. J. Neurosci. 2008, 28, 7399. [Google Scholar] [CrossRef]
- Zhang, Y.; Rózsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 2023, 615, 884. [Google Scholar] [CrossRef]
- Inoue, M. Genetically encoded calcium indicators to probe complex brain circuit dynamics in vivo. Neurosci. Res. 2021, 169, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Schleifenbaum, J. Genetically Encoded Calcium Indicators: A New Tool in Renal Hypertension Research. Front. Med. 2019, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Kanemaru, K.; Iino, M. Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging. Biophys. J. 2016, 111, 1119. [Google Scholar] [CrossRef]
- Piljić, A.; de Diego, I.; Wilmanns, M.; Schultz, C. Rapid Development of Genetically Encoded FRET Reporters. ACS Chem. Biol. 2011, 6, 685. [Google Scholar] [CrossRef]
- Kostyuk, A.I.; Demidovich, A.D.; Kotova, D.A.; Belousov, V.V.; Bilan, D.S. Circularly Permuted Fluorescent Protein-Based Indicators: History, Principles, and Classification. Int. J. Mol. Sci. 2019, 20, 4200. [Google Scholar] [CrossRef]
- Oheim, M.; van ’t Hoff, M.; Feltz, A.; Zamaleeva, A.; Mallet, J.-M.; Collot, M. New red-fluorescent calcium indicators for optogenetics, photoactivation and multi-color imaging. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2284. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Monakhov, M.V.; Zhang, Q.; Canton-Josh, J.E.; Kumar, M.; Chen, M.; Matlashov, M.E.; Li, X.; Yang, W.; Nie, L.; et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat. Biotechnol. 2021, 39, 368. [Google Scholar] [CrossRef]
- Tang, S.; Reddish, F.; Zhuo, Y.; Yang, J.J. Fast kinetics of calcium signaling and sensor design. Curr. Opin. Chem. Biol. 2015, 27, 90. [Google Scholar] [CrossRef]
- Zhang, Y.; Looger, L.L. Fast and sensitive GCaMP calcium indicators for neuronal imaging. J. Physiol. 2024, 602, 1595. [Google Scholar] [CrossRef]
- Sun, X.R.; Badura, A.; Pacheco, D.A.; Lynch, L.A.; Schneider, E.R.; Taylor, M.P.; Hogue, I.B.; Enquist, L.W.; Murthy, M.; Wang, S.S.-H. Fast GCaMPs for improved tracking of neuronal activity. Nat. Commun. 2013, 4, 2170. [Google Scholar] [CrossRef]
- Kerruth, S.; Coates, C.; Dürst, C.D.; Oertner, T.G.; Török, K. The kinetic mechanisms of fast-decay red-fluorescent genetically encoded calcium indicators. J. Biol. Chem. 2019, 294, 3934. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Bates, M.; Zhuang, X. Super-Resolution Fluorescence Microscopy. Annu. Rev. Biochem. 2009, 78, 993. [Google Scholar] [CrossRef]
- Yuan, J.; Deng, Z.; Liu, H.; Li, X.; Li, J.; He, Y.; Qing, Z.; Yang, Y.; Zhong, S. Cell-Surface-Anchored Ratiometric DNA Nanoswitch for Extracellular ATP Imaging. ACS Sens. 2019, 4, 1648. [Google Scholar] [CrossRef]
- Xia, T.; Yuan, J.; Fang, X. Conformational Dynamics of an ATP-Binding DNA Aptamer: A Single-Molecule Study. J. Phys. Chem. B 2013, 117, 14994. [Google Scholar] [CrossRef]
- Yao, W.; Wang, L.; Wang, H.; Zhang, X.; Li, L. An aptamer-based electrochemiluminescent biosensor for ATP detection. Biosens. Bioelectron. 2009, 24, 3269. [Google Scholar] [CrossRef]
- Zhou, Z.-M.; Yu, Y.; Zhao, Y.-D. A new strategy for the detection of adenosine triphosphate by aptamer/quantum dot biosensor based on chemiluminescence resonance energy transfer. Analyst 2012, 137, 4262. [Google Scholar] [CrossRef]
- Park, C.H.; Thompson, I.A.P.; Newman, S.S.; Hein, L.A.; Lian, X.; Fu, K.X.; Pan, J.; Eisenstein, M.; Soh, H.T. Real-Time Spatiotemporal Measurement of Extracellular Signaling Molecules Using an Aptamer Switch-Conjugated Hydrogel Matrix. Adv. Mater. 2024, 36, 2306704. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, L.; Yuan, D.; Li, J.; Yang, G.; Song, D.; Miao, H.; Shu, L.; Mo, X.; Xu, X.; et al. A genetically encoded fluorescent biosensor for monitoring ATP in living cells with heterobifunctional aptamers. Biosens. Bioelectron. 2022, 198, 113827. [Google Scholar] [CrossRef]
- Yoshida, T.; Kakizuka, A.; Imamura, H. BTeam, a Novel BRET-based Biosensor for the Accurate Quantification of ATP Concentration within Living Cells. Sci. Rep. 2016, 6, 39618. [Google Scholar] [CrossRef]
- Wu, Z.; He, K.; Chen, Y.; Li, H.; Pan, S.; Li, B.; Liu, T.; Xi, F.; Deng, F.; Wang, H.; et al. A sensitive GRAB sensor for detecting extracellular ATP in vitro and in vivo. Neuron 2022, 110, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Dong, H.; Lischinsky, J.E.; Zhou, J.; Deng, F.; Zhuang, C.; Miao, X.; Wang, H.; Li, G.; Cai, R.; et al. Monitoring norepinephrine release in vivo using next-generation GRABNE sensors. Neuron 2024, 112, 1930. [Google Scholar] [CrossRef]
- Lobas, M.A.; Tao, R.; Nagai, J.; Kronschläger, M.T.; Borden, P.M.; Marvin, J.S.; Looger, L.L.; Khakh, B.S. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 2019, 10, 711. [Google Scholar] [CrossRef]
- Marvin, J.S.; Kokotos, A.C.; Kumar, M.; Pulido, C.; Tkachuk, A.N.; Yao, J.S.; Brown, T.A.; Ryan, T.A. iATPSnFR2: A high-dynamic-range fluorescent sensor for monitoring intracellular ATP. Proc. Natl. Acad. Sci. USA 2024, 121, e2314604121. [Google Scholar] [CrossRef]
- Mu, X.; Evans, T.D.; Zhang, F. ATP biosensor reveals microbial energetic dynamics and facilitates bioproduction. Nat. Commun. 2024, 15, 5299. [Google Scholar] [CrossRef]
- Tsuyama, T.; Kishikawa, J.; Han, Y.-W.; Harada, Y.; Tsubouchi, A.; Noji, H.; Kakizuka, A.; Yokoyama, K.; Uemura, T.; Imamura, H. In Vivo Fluorescent Adenosine 5′-Triphosphate (ATP) Imaging of Drosophila melanogaster and Caenorhabditis elegans by Using a Genetically Encoded Fluorescent ATP Biosensor Optimized for Low Temperatures. Anal. Chem. 2013, 85, 7889. [Google Scholar] [CrossRef]
- Ihssen, J.; Jovanovic, N.; Sirec, T.; Spitz, U. Real-time monitoring of extracellular ATP in bacterial cultures using thermostable luciferase. PLoS ONE 2021, 16, e0244200. [Google Scholar] [CrossRef]
- Morciano, G.; Sarti, A.C.; Marchi, S.; Missiroli, S.; Falzoni, S.; Raffaghello, L.; Pistoia, V.; Giorgi, C.; Di Virgilio, F.; Pinton, P. Use of luciferase probes to measure ATP in living cells and animals. Nat. Protoc. 2017, 12, 1542. [Google Scholar] [CrossRef]
- Wang, C.; Huang, C.-Y.C.; Lin, W.-C. Optical ATP biosensor for extracellular ATP measurement. Biosens. Bioelectron. 2013, 43, 355. [Google Scholar] [CrossRef]
- Abeles, M.; Goldstein, M.H. Multispike train analysis. Proc. IEEE 1977, 65, 762. [Google Scholar] [CrossRef]
- Gerstein, G.L.; Clark, W.A. Simultaneous Studies of Firing Patterns in Several Neurons. Science 1964, 143, 1325. [Google Scholar] [CrossRef] [PubMed]
- Nenadic, Z.; Burdick, J.W. Spike Detection Using the Continuous Wavelet Transform. IEEE Trans. Biomed. Eng. 2005, 52, 74. [Google Scholar] [CrossRef]
- Quiroga, R.Q.; Nadasdy, Z.; Ben-Shaul, Y. Unsupervised Spike Detection and Sorting with Wavelets and Superparamagnetic Clustering. Neural Comput. 2004, 16, 1661. [Google Scholar] [CrossRef]
- Adamos, D.A.; Kosmidis, E.K.; Theophilidis, G. Performance evaluation of PCA-based spike sorting algorithms. Comput. Methods Programs Biomed. 2008, 91, 232. [Google Scholar] [CrossRef]
- Gibson, S.; Judy, J.W.; Markovic, D. Technology-Aware Algorithm Design for Neural Spike Detection, Feature Extraction, and Dimensionality Reduction. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 469. [Google Scholar] [CrossRef]
- Chah, E.; Hok, V.; Della-Chiesa, A.; Miller, J.J.H.; O’Mara, S.M.; Reilly, R.B. Automated spike sorting algorithmbased on Laplacian eigenmaps and k -means clustering. J. Neural Eng. 2011, 8, 016006. [Google Scholar] [CrossRef]
- İnan, Z.H.; Kuntalp, M. A study on fuzzy C-means clustering-based systems in automatic spike detection. Comput. Biol. Med. 2007, 37, 1160. [Google Scholar] [CrossRef]
- Souza, B.C.; Lopes-dos-Santos, V.; Bacelo, J.; Tort, A.B.L. Spike sorting with Gaussian mixture models. Sci. Rep. 2019, 9, 3627. [Google Scholar] [CrossRef]
- Wood, E.; Fellows, M.; Donoghue, J.R.; Black, M.J. Automatic Spike Sorting for Neural Decoding. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 4009–4012. [Google Scholar]
- Xu, Z.; Wang, T.; Cao, J.; Bao, Z.; Jiang, T.; Gao, F. BECT Spike Detection Based on Novel EEG Sequence Features and LSTM Algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1734. [Google Scholar] [CrossRef]
- Saif-ur-Rehman, M.; Lienkämper, R.; Parpaley, Y.; Wellmer, J.; Liu, C.; Lee, B.; Kellis, S.; Andersen, R.; Iossifidis, I.; Glasmachers, T.; et al. SpikeDeeptector: A deep-learning based method for detection of neural spiking activity. J. Neural Eng. 2019, 16, 056003. [Google Scholar] [CrossRef]
- Rácz, M.; Liber, C.; Németh, E.; Fiáth, R.; Rokai, J.; Harmati, I.; Ulbert, I.; Márton, G. Spike detection and sorting with deep learning. J. Neural Eng. 2020, 17, 016038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, H.; Zhang, X.; Xia, G.; Gong, X.; Yue, D.; Fan, Y.; Wang, B.; Wang, G.; Li, Y.; et al. Deep learning models for image and data processes of intracellular calcium ions. Cell Signal 2022, 91, 110225. [Google Scholar] [CrossRef] [PubMed]
- Dotti, P.; Fernandez-Tenorio, M.; Janicek, R.; Márquez-Neila, P.; Wullschleger, M.; Sznitman, R.; Egger, M. A deep learning-based approach for efficient detection and classification of local Ca2+ release events in Full-Frame confocal imaging. Cell Calcium 2024, 121, 102893. [Google Scholar] [CrossRef]
- Tenneti, S.V.; Vaidyanathan, P.P. Nested Periodic Matrices and Dictionaries: New Signal Representations for Period Estimation. IEEE Trans. Signal Process. 2015, 63, 3736. [Google Scholar] [CrossRef]
- Tosic, I.; Frossard, P. Dictionary Learning. IEEE Signal Process. Mag. 2011, 28, 27. [Google Scholar] [CrossRef]
- English, K.; Shepherd, A.; Uzor, N.-E.; Trinh, R.; Kavelaars, A.; Heijnen, C.J. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol. Commun. 2020, 8, 36. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Tacer, K.F.; Bezprozvanny, I. Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer’s Disease Treatment. J. Neurosci. 2016, 36, 11837. [Google Scholar] [CrossRef]
- Wegierski, T.; Kuznicki, J. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium 2018, 74, 102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackey, C.; Feng, Y.; Liang, C.; Liang, A.; Tian, H.; Narayan, O.P.; Dong, J.; Tai, Y.; Hu, J.; Mu, Y.; et al. Mechanical Modulation, Physiological Roles, and Imaging Innovations of Intercellular Calcium Waves in Living Systems. Cancers 2025, 17, 1851. https://doi.org/10.3390/cancers17111851
Mackey C, Feng Y, Liang C, Liang A, Tian H, Narayan OP, Dong J, Tai Y, Hu J, Mu Y, et al. Mechanical Modulation, Physiological Roles, and Imaging Innovations of Intercellular Calcium Waves in Living Systems. Cancers. 2025; 17(11):1851. https://doi.org/10.3390/cancers17111851
Chicago/Turabian StyleMackey, Cole, Yuning Feng, Chenyu Liang, Angela Liang, He Tian, Om Prakash Narayan, Jiawei Dong, Yongchen Tai, Jingzhou Hu, Yu Mu, and et al. 2025. "Mechanical Modulation, Physiological Roles, and Imaging Innovations of Intercellular Calcium Waves in Living Systems" Cancers 17, no. 11: 1851. https://doi.org/10.3390/cancers17111851
APA StyleMackey, C., Feng, Y., Liang, C., Liang, A., Tian, H., Narayan, O. P., Dong, J., Tai, Y., Hu, J., Mu, Y., Vo, Q., Wu, L., Siemann, D., Pan, J., Yang, X., Huang, K., George, T., Guan, J., & Tang, X. (2025). Mechanical Modulation, Physiological Roles, and Imaging Innovations of Intercellular Calcium Waves in Living Systems. Cancers, 17(11), 1851. https://doi.org/10.3390/cancers17111851







