Machine Learning for Predicting the Low Risk of Postoperative Pancreatic Fistula After Pancreaticoduodenectomy: Toward a Dynamic and Personalized Postoperative Management Strategy
Simple Summary
Abstract
1. Introduction
- -
- We conducted a large-scale comparative analysis involving twenty-four machine learning algorithms to predict POPF after pancreaticoduodenectomy, leveraging a structured and temporally aligned dataset including preoperative, intraoperative, and early postoperative variables.
- -
- We implemented a task-specific model selection strategy based on the performance of each algorithm using the Matthews Correlation Coefficient (MCC), a metric particularly well-suited for imbalanced clinical classification problems.
- -
- We included a classical multivariate logistic regression model as a comparator to assess the added value of modern ML approaches. This allowed us to demonstrate the performance gains offered by non-linear, ensemble-based methods such as GradientBoostingClassifier, which consistently outperformed the baseline in multiple tasks.
- -
- We integrated explainable AI (XAI) techniques, specifically SHAP (SHapley Additive exPlanations), to provide interpretability of model outputs. This enabled the identification of the most influential clinical features contributing to model decisions, enhancing transparency and supporting clinical insight generation.
- -
- We focused on the early prediction of POPF risk within the first three postoperative days, aligning our modeling approach with real-world clinical decision points, such as the timing of drain removal.
2. Materials and Methods
2.1. Study Design, Population, and Variables
2.2. Outcome and POPF Definition
2.3. Statistical Analysis and Machine Learning Procedure
2.4. Data Preprocessing
2.5. Machine Learning Training
2.6. Algorithms Considered
2.7. Evaluation of the Best Model
2.8. Explainable Artificial Intelligence (XAI)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Ensemble Methods: AdaBoostClassifier, ExtraTreesClassifier, GradientBoostingClassifier, HistGradientBoostingClassifier, RandomForestClassifier, XGBClassifier, XGBRFClassifier.
- Naive Bayes Classifiers: BernoulliNB, GaussianNB.
- Tree-Based Methods: DecisionTreeClassifier, ExtraTreeClassifier.
- Linear Models: RidgeClassifier, PassiveAggressiveClassifier, Perceptron, SGDClassifier.
- Support Vector Machines: SVC, LinearSVC.
- Nearest Neighbors: KneighborsClassifier.
- Neural Networks: MLPClassifier.
- Discriminant Analysis: LinearDiscriminantAnalysis, QuadraticDiscriminantAnalysis.
- Gaussian Processes: GaussianProcessClassifier.
- Semi-Supervised Methods: LabelPropagation, LabelSpreading.
References
- Del Chiaro, M.; Sugawara, T.; Karam, S.D.; Messersmith, W.A. Advances in the management of pancreatic cancer. BMJ 2023, 383, e073995. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, B.; Pendharkar, S.A.; Connor, S.; Koea, J.; Sarfati, D.; Dennett, E.; Pandanaboyana, S.; Windsor, J.A. Patient volume and clinical outcome after pancreatic cancer resection: A contemporary systematic review and meta-analysis. Surgery 2022, 172, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Uttinger, K.; Niezold, A.; Weimann, L.; Plum, P.S.; Baum, P.; Diers, J.; Brunotte, M.; Rademacher, S.; Germer, C.T.; Seehofer, D.; et al. Weekday effect of surgery on in-hospital outcome in pancreatic surgery: A population-based study. Langenbecks Arch. Surg. 2024, 410, 4. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Amini, N.; Pasha, S.A.; Demyan, L.; Newman, E.; King, D.A.; DePeralta, D.; Gholami, S.; Deutsch, G.B.; Melis, M.; et al. Impact of postoperative pancreatic fistula on outcomes in pancreatoduodenectomy: A comprehensive analysis of American College of Surgeons National Surgical Quality Improvement Program data. J. Gastrointest. Surg. 2024, 28, 1406–1411. [Google Scholar] [CrossRef]
- Smits, F.J.; Henry, A.C.; Besselink, M.G.; Busch, O.R.; van Eijck, C.H.; Arntz, M.; Bollen, T.L.; van Delden, O.M.; van den Heuvel, D.; van der Leij, C.; et al. Algorithm-based care versus usual care for the early recognition and management of complications after pancreatic resection in the Netherlands: An open-label, nationwide, stepped-wedge cluster-randomised trial. Lancet 2022, 399, 1867–1875. [Google Scholar] [CrossRef]
- Callery, M.P.; Pratt, W.B.; Kent, T.S.; Chaikof, E.L.; Vollmer, C.M., Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J. Am. Coll. Surg. 2013, 216, 1–14. [Google Scholar] [CrossRef]
- Shehab, M.; Abualigah, L.; Shambour, Q.; Abu-Hashem, M.A.; Shambour, M.K.Y.; Alsalibi, A.I.; Gandomi, A.H. Machine learning in medical applications: A review of state-of-the-art methods. Comput. Biol. Med. 2022, 145, 105458. [Google Scholar] [CrossRef]
- Devnath, L.; Summons, P.; Luo, S.; Wang, D.; Shaukat, K.; Hameed, I.A.; Aljuaid, H. Computer-Aided Diagnosis of Coal Workers’ Pneumoconiosis in Chest X-ray Radiographs Using Machine Learning: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 6439. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial intelligence in surgery: Promises and perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef]
- Guarrasi, V.; Aksu, F.; Caruso, C.M.; Di Feola, F.; Rofena, A.; Ruffini, F.; Soda, P. A systematic review of intermediate fusion in multimodal deep learning for biomedical applications. Image Vis. Comput. 2025, 158, 105509. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, B.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.S.; Acharya, R. External validation and comparison of the original, alternative and updated-alternative fistula risk scores for the prediction of postoperative pancreatic fistula after pancreatoduodenectomy. Pancreatology 2020, 20, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Meara, J.G.; Leather, A.J.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Surgery 2015, 158, 3–6. [Google Scholar] [CrossRef]
- PancreasGroup.org Collaborative. Pancreatic surgery outcomes: Multicentre prospective snapshot study in 67 countries. Br. J. Surg. 2024, 111, znad330. [Google Scholar] [CrossRef]
- Stoop, T.F.; Marra, A.; Eskens, F.A.; van Dijk, D.P.; Boenink, T.; van der Vliet, W.J.; Vogel, J.A.; van Hooft, J.E.; Besselink, M.G.; Wilmink, J.W.; et al. Pancreatic cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef]
- McMillan, M.T.; Mavros, M.N.; Asbun, H.J.; Barreto, S.G.; Bassi, C.; Beane, J.D.; Hollis, R.H.; Kent, T.S.; Miller, B.C.; Sprys, M.H.; et al. Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery 2016, 159, 1013–1022. [Google Scholar] [CrossRef]
- Marchegiani, G.; Bassi, C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br. J. Surg. 2021, 108, 602–604. [Google Scholar] [CrossRef]
- Caputo, D.; Coppola, A.; La Vaccara, V.; Passa, R.; Carbone, L.; Ciccozzi, M.; Angeletti, S.; Coppola, R. Validations of new cut-offs for surgical drains management and use of computerized tomography scan after pancreatoduodenectomy: The DALCUT trial. World J. Clin. Cases 2022, 10, 4836–4842. [Google Scholar] [CrossRef]
- Nebbia, M.; Capretti, G.; Nappo, G.; Zerbi, A. Updates in the management of postoperative pancreatic fistula. Int. J. Surg. 2024, 110, 6135–6144. [Google Scholar] [CrossRef]
- de Ponthaud, C.; Gaujoux, S. Algorithm-based care for early recognition and management of complications after pancreatic resection: Toward standardization of postoperative care. Hepatobiliary Surg. Nutr. 2022, 11, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Kantor, O.; Talamonti, M.S. Using the NSQIP Pancreatic Demonstration Project to derive a modified fistula risk score for preoperative risk stratification in patients undergoing pancreaticoduodenectomy. J. Am. Coll. Surg. 2017, 224, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Mungroop, T.H.; van Rijssen, L.B.; van Klaveren, D.; Smits, F.J.; van Woerden, V.; Linnemann, R.J.; de Pastena, M.; Klompmaker, S.; Marchegiani, G.; Ecker, B.L.; et al. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and international external validation. Ann. Surg. 2019, 269, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, F.; Tronchin, L.; Wu, Z.; Chen, W.; Soda, P.; Shen, L.; Guarrasi, V. Multi-Dataset Multi-Task Learning for COVID-19 Prognosis. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2024, Proceedings of the 27th International Conference on Medical Image Computing and Computer-Assisted Intervention, Marrakesh, Morocco, 6–10 October 2024; Lecture Notes in Computer Science; Springer Nature Switzerland: Cham, Switzerland, 2024; Volume 15012, pp. 251–261. [Google Scholar]
- Chen, K.A.; Berginski, M.E. Differential performance of machine learning models in prediction of procedure-specific outcomes. J. Gastrointest. Surg. 2022, 26, 1732–1742. [Google Scholar] [CrossRef]
- Yang, F.; Windsor, J.A.; Fu, D.L. Optimizing prediction models for pancreatic fistula after pancreatectomy: Current status and future perspectives. World J. Gastroenterol. 2024, 30, 1329–1345. [Google Scholar] [CrossRef]
- Ingwersen, E.W.; Stam, W.T. Machine learning versus logistic regression for the prediction of complications after pancreatoduodenectomy. Surgery 2023, 174, 435–440. [Google Scholar] [CrossRef]
- Coppola, A.; La Vaccara, V. Postoperative procalcitonin is a biomarker for excluding the onset of clinically relevant pancreatic fistula after pancreaticoduodenectomy. J. Gastrointest. Oncol. 2023, 14, 1077–1086. [Google Scholar] [CrossRef]
- Marchegiani, G.; Dervenis, C.; Bassi, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Falconi, M.; Besselink, M.G.; Salvia, R.; Wolfgang, C.L.; et al. Postpancreatectomy acute pancreatitis (PPAP): Definition and grading from the International Study Group for Pancreatic Surgery (ISGPS). Ann. Surg. 2022, 275, 663–672. [Google Scholar] [CrossRef]
- Tang, B.J.; Li, S.J.; Wang, P.F.; Xiang, C.H.; Zeng, J.P.; Shi, J.; Dong, J.H.; Wang, X.D. Predictive value of postoperative serum lipase level for postoperative pancreatic fistula after pancreaticoduodenectomy. Hepatobiliary Pancreat. Dis. Int. 2025, 24, 197–205. [Google Scholar] [CrossRef]
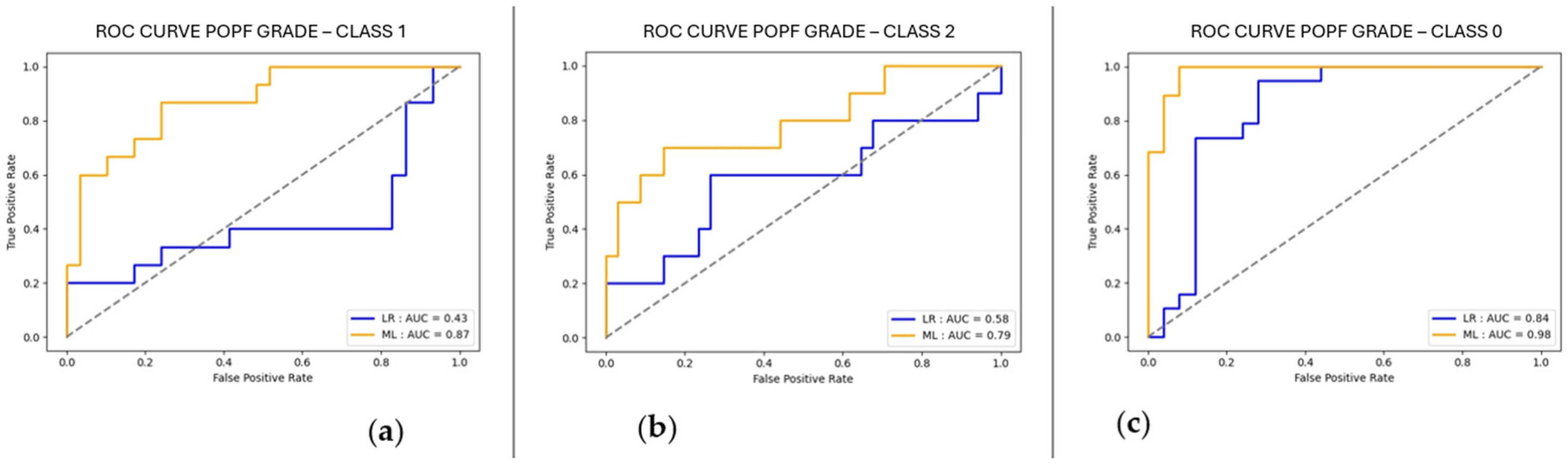

| Variable | N = 216 |
|---|---|
| Age (median [IQR]), years | 71 [65–76] |
| Sex (%) | |
| Male | 103 (47.7) |
| Female | 113 (52.3) |
| BMI (median [IQR]), kg/m2 | 23.94 [21.30–26.83] |
| Cardiovascular comorbidity (%) | 67 (31) |
| Pulmonary comorbidity (%) | 30 (13.9) |
| Diabetes (%) | 38 (17.6) |
| Smoker (%) | 45 (20.8) |
| Hypertension (%) | 104 (48.1) |
| ASA classification (%) | |
| 1 | 9 (4.2) |
| 2 | 97 (44.9) |
| 3–4 | 110 (51) |
| Neoadjuvant therapy (%) | 25 (11.5) |
| Positive bile culture (%) | 128 (59.3) |
| Surgical approach: Open (%) | 206 (95.4) |
| Whipple (%) | 75 (34.7) |
| Traverso (%) | 141 (65.3) |
| Texture (%) | |
| Soft | 130 (60.19) |
| Firm | 86 (39.81) |
| Diameter of MPD (%) | |
| <3 mm | 85 (39.4) |
| >3 mm | 131 (60.6) |
| Surgery time (median [IQR]), min | 363.00 [321.00, 420.00] |
| Intraoperative bool loss (median [IQR]), mL | 260.00 [170.00, 390.00] |
| Vascular resection (%) | 42 (19.4) |
| Pathology: PDAC (%) | 170 (78.7) |
| Variable | N = 216 |
|---|---|
| Amylase right-drain PODIII (median [IQR]), UI/L | 156 [23–676.5] |
| Amylase left-drain PODIII (median [IQR]), UI/L | 227.5 [23.5–1223] |
| Blood lipase PODI (median [IQR]), UI/L | 101.95 [30.6–403.75] |
| Blood lipase PODII (median [IQR]), UI/L | 52 [22.25–193.05] |
| Blood lipase PODII (median [IQR]), UI/L | 32.6 [20.61–72.55] |
| PCT PODIII (median [IQR]), UI/L | 0.375 [0.195–0.845] |
| Klebsiella pneumoniae (%) | 47 (21.8) |
| Klebsiella oxytoca (%) | 19 (8.8) |
| Enterobacter (%) | 22 (10.2) |
| Pseudomonas aeuriginosa (%) | 12 (5.5) |
| Citrobacter (%) | 18 (8.3) |
| Enterococcus faecalis (%) | 62 (28.7) |
| Enterococcus faecium (%) | 58 (26.9) |
| Streptococcus (%) | 23 (10.6) |
| Candida albicans (%) | 14 (6.5) |
| Variable | No Fistula (n = 94) | Grade A (n = 71) | Grade B-C (n = 51) | p Value |
|---|---|---|---|---|
| Sex | 0.026 | |||
| Male | 35 (37.2%) | 39 (54.9%) | 29 (56.9%) | |
| Female | 59 (62.8%) | 32 (45.1%) | 22 (43.1%) | |
| Cardiovascular Diseases | 0.125 | |||
| No | 63 (67%) | 55 (77.5%) | 31 (60.8%) | |
| Yes | 31 (33%) | 16 (22.5%) | 20 (39.2%) | |
| Respiratory Diseases | 0.456 | |||
| No | 78 (83%) | 62 (87.3%) | 46 (90.2%) | |
| Yes | 16 (17%) | 9 (12.7%) | 5 (9.8%) | |
| Smoking | 0.258 | |||
| Non-smoker | 63 (67%) | 53 (74.6%) | 31 (60.8%) | |
| Smoker | 31 (33%) | 18 (25.4%) | 20 (39.2%) | |
| Hypertension | 0.042 | |||
| No | 41 (43.6%) | 45 (63.4%) | 26 (51%) | |
| Yes | 53 (56.4%) | 26 (36.6%) | 25 (49%) | |
| Diabetes | 0.016 | |||
| No | 70 (74.5%) | 65 (91.5%) | 43 (84.3%) | |
| Yes | 24 (25.5%) | 6 (8.5%) | 8 (15.7%) | |
| Histology | 0.059 | |||
| PDAC | 81 (86.2%) | 51 (71.8%) | 38 (74.5%) | |
| Other Types | 13 (13.8%) | 20 (28.2%) | 13 (25.5%) | |
| Neoadjuvant Treatment | 0.031 | |||
| No | 77 (81.9%) | 66 (93.7%) | 48 (94.1%) | |
| Yes | 17 (18.1%) | 5 (6.3%) | 3 (5.9%) | |
| ASA Classification | 0.421 | |||
| ASA 1 | 5 (5.3%) | 1 (1.4%) | 3 (5.9%) | |
| ASA 2 | 41 (43.6%) | 37 (52.1%) | 19 (37.3%) | |
| ASA 3 | 45 (47.9%) | 28 (39.4%) | 25 (49%) | |
| ASA 4 | 3 (3.2%) | 5 (7%) | 4 (7.8%) | |
| MPD Diameter | <0.001 | |||
| <3 mm | 21 (22.3%) | 33 (46.5%) | 31 (60.8%) | |
| ≥3 mm | 73 (77.7%) | 38 (53.5%) | 20 (39.2%) | |
| Vascular Resection | 0.026 | |||
| No | 68 (72.3%) | 61 (85.9%) | 45 (88.2%) | |
| Yes | 26 (27.7%) | 10 (14.1%) | 6 (11.8%) | |
| Pancreatic Texture | 0.033 | |||
| Soft | 46 (49%) | 26 (36.6%) | 14 (27.5%) | |
| Firm | 48 (51%) | 45 (63.4%) | 37 (72.5%) | |
| Surgical Approach | 0.131 | |||
| Open | 91 (96.8%) | 69 (97.2%) | 46 (90.2%) | |
| Minimally Invasive | 3 (3.2%) | 2 (2.8%) | 5 (9.8%) | |
| Whipple | 35 (37.2%) | 17 (23.9%) | 23 (45.1%) | 0.042 |
| Traverso | 59 (62.8%) | 54 (76.1%) | 28 (54.9%) | |
| Bile Culture | 0.161 | |||
| Positive | 45 (47.9%) | 24 (33.8%) | 19 (37.3%) | |
| Negative | 49 (52.1%) | 47 (66.2%) | 32 (62.7%) | |
| Age (Median [IQR], years) | 70 [63–76] | 71 [66–75] | 71 [66–75] | 0.395 |
| BMI (Median [IQR], kg/m²) | 23.4 [21.1–26.8] | 24.2 [22.2–26.3] | 24.7 [22.8–27.7] | 0.395 |
| Surgical Time (Median [IQR], min) | 358.5 [311–418] | 360 [312–423] | 395 [347–466] | 0.0169 |
| Intraoperative Blood Loss (Median [IQR], mL) | 200 [170–390] | 260 [200–390] | 250 [200–390] | 0.9028 |
| Variable | No Fistula (n = 94) | Grade A (n = 71) | Grade B-C (n = 51) | p Value |
|---|---|---|---|---|
| Klebsiella Pneumoniae | 0.633 | |||
| No | 76 (80.9%) | 53 (74.6%) | 40 (78.4%) | |
| Yes | 18 (19.1%) | 18 (25.4%) | 11 (21.6%) | |
| Klebsiella Oxytoca | 0.676 | |||
| No | 87 (92.6%) | 65 (91.5%) | 45 (88.2%) | |
| Yes | 7 (7.4%) | 6 (8.5%) | 6 (11.8%) | |
| Enterobacter | 0.476 | |||
| No | 84 (89.4%) | 66 (93%) | 44 (86.3%) | |
| Yes | 10 (10.6%) | 5 (7%) | 7 (13.7%) | |
| Pseudomonas Aeuriginosa | 0.154 | |||
| No | 92 (97.9%) | 65 (91.5%) | 47 (92.2%) | |
| Yes | 2 (2.1%) | 6 (8.5%) | 4 7.8%) | |
| Citrobacter | 0.886 | |||
| No | 87 (92.6%) | 65 (91.5%) | 46 (90.2%) | |
| Yes | 7 (7.4%) | 6 (8.5%) | 5 (9.8%) | |
| Enterococcus Faecalis | 0.600 | |||
| No | 70 (74.5%) | 50 (70.4%) | 34 (66.7%) | |
| Yes | 24 (25.5%) | 21 (29.6%) | 17 (33.3%) | |
| Enterococcus Faecium | 0.032 | |||
| No | 75 (79.8%) | 44 (62.0%) | 39 (76.5%) | |
| Yes | 19 (20.2%) | 27 (38%) | 12 (23.5%) | |
| Streptococcus | 0.205 | |||
| No | 80 (85.1%) | 66 (93%) | 47 (92.2%) | |
| Yes | 14 (14.9%) | 5 (7%) | 4 (7.8%) | |
| Candida Albicans | 0.529 | |||
| No | 86 (91.5%) | 67 (94.4%) | 49 (96.1%) | |
| Yes | 8 (8.5%) | 4 (5.6%) | 2 (3.9%) | |
| Amylase right-drain PODIII (median [IQR]), UI/L | 17.5 [11–45] | 402 [160–964] | 877 [222–1940] | <0.001 |
| Amylase left-drain PODIII (median [IQR]), UI/L | 18.5 [9–82] | 585 [222–1652] | 1758 [336–5580] | <0.001 |
| Blood lipase PODI (median [IQR]), UI/L | 30 [17.5–88] | 329 [68.9–1004] | 237.2 [104.7–618] | <0.001 |
| Blood lipase PODII (median [IQR]), UI/L | 22 [16.4–44.97] | 157 [45.9–404.9] | 164 [50.2–409.8] | <0.001 |
| Blood lipase PODIII (median [IQR]), UI/L | 21.65 [15.1–34] | 61.5 [30–131] | 46.93 [23.7–226] | <0.001 |
| PCT PODIII (median [IQR]), UI/L | 0.23 [0.13–0.52] | 0.36 [0.22–0.73] | 0.97 [0.46–3.2] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammarata, R.; Ruffini, F.; Catamerò, A.; Melone, G.; Costa, G.; Angeletti, S.; Seghetti, F.; La Vaccara, V.; Coppola, R.; Soda, P.; et al. Machine Learning for Predicting the Low Risk of Postoperative Pancreatic Fistula After Pancreaticoduodenectomy: Toward a Dynamic and Personalized Postoperative Management Strategy. Cancers 2025, 17, 1846. https://doi.org/10.3390/cancers17111846
Cammarata R, Ruffini F, Catamerò A, Melone G, Costa G, Angeletti S, Seghetti F, La Vaccara V, Coppola R, Soda P, et al. Machine Learning for Predicting the Low Risk of Postoperative Pancreatic Fistula After Pancreaticoduodenectomy: Toward a Dynamic and Personalized Postoperative Management Strategy. Cancers. 2025; 17(11):1846. https://doi.org/10.3390/cancers17111846
Chicago/Turabian StyleCammarata, Roberto, Filippo Ruffini, Alberto Catamerò, Gennaro Melone, Gianluca Costa, Silvia Angeletti, Federico Seghetti, Vincenzo La Vaccara, Roberto Coppola, Paolo Soda, and et al. 2025. "Machine Learning for Predicting the Low Risk of Postoperative Pancreatic Fistula After Pancreaticoduodenectomy: Toward a Dynamic and Personalized Postoperative Management Strategy" Cancers 17, no. 11: 1846. https://doi.org/10.3390/cancers17111846
APA StyleCammarata, R., Ruffini, F., Catamerò, A., Melone, G., Costa, G., Angeletti, S., Seghetti, F., La Vaccara, V., Coppola, R., Soda, P., Guarrasi, V., & Caputo, D. (2025). Machine Learning for Predicting the Low Risk of Postoperative Pancreatic Fistula After Pancreaticoduodenectomy: Toward a Dynamic and Personalized Postoperative Management Strategy. Cancers, 17(11), 1846. https://doi.org/10.3390/cancers17111846









